Abstract
Colon cancer is one of the major prevailing types of cancer worldwide. It has been the most important public health difficulty. Thus, we planned phytoconstituents arbitrated synthesis of gold nanoparticles (AuNPs) and examined their curative efficacy against the colon cancer (HCT-116) cells. In this current study, we formulated the AuNPs by using Albizia lebbeck (AL) aqueous leaf extract by the green method and synthesized AL-AuNPs were distinguished by UV-visible spectroscopy (UV-vis), energy dispersive X-ray diffraction (XRD), selected area (electron) diffraction (SAED) pattern, Fourier transform infrared spectroscopy (FTIR) and high-resolution transmission electron microscopy (HR-TEM). Synthesized AL-AuNPs confirmed by the UV absorption highest at 535 nm and the crystal structure of AL-AuNPs was additionally established by XRD and SAED pattern. HR-TEM images explained the size and morphology allocation of nanoparticles. FTIR analysis confirmed the presence of alkynes, aromatic compounds, and alkenes of biomolecules in AL-AuNPs. Furthermore, AL-AuNPs induced cytotoxicity at the IC50 concentration 48 µg/ml and also induced apoptosis by enhanced ROS production, decreased ΔΨm, apoptotic morphological changes by AO/EtBr and altering pro and anti-apoptotic protein expressions were analyzed in HCT-116 colon cancer cells. The findings of this investigation proved that the AL-AuNPs were revealed the potential anticancer activity against colon cancer (HCT-116) cells.
Introduction
Cancer is a cluster of diseases described by the uncontrolled development and spread of anomalous cells. If the development of the cells is not controlled, it will consequence in death [Citation1]. Colon cancer is one of the majority of prevailing forms of cancer worldwide. It has been a major public health difficulty [Citation2]. Colon carcinogenesis is separated into three distinctive stages similar to all types of carcinogens containing initiation, promotion, and progression, during which the common colonic epithelium undergoes a pathological conversion into the hyperproliferative epithelium and followed by an adenoma, carcinoma in situ and ultimately invasive and metastatic cancer [Citation3].
The nanotechnology field is one of the primary energetic areas of study in present science. The green approach to the production of nanoparticles with plant resources such as capping and reducing agents could be considered gorgeous in nanobiotechnology. When evaluating with mechanical strategy, this technology is easy, environmentally friendly, non-toxic, safe and efficient and also offers effective single-pot responses without the need for extra surfactants or capping mediators [Citation4]. The green arbitrated synthesis and categorization of nanoparticles have become known as a significant division of nanotechnology from the last decade, predominantly from genial metals containing gold, platinum, palladium, and silver. Amongst these metals, recently, gold nanoparticles (AuNPs) have been broadly examined because of their possible purposes in catalysis, optics, and electronics [Citation5].
Chemotherapy agents can recover the value of life in cancer patients, but drug resistance and severe undesirable side effects such as bone marrow suppression, liver function damage, and neurotoxicity are significant difficulties that diminish treatment associated compliance and tolerance leading to therapeutic failure [Citation6]. Therefore, there is an imperative need to extend novel drugs that have the advantages of being more efficient, causing fewer side effects, and overcoming drug resistance in all types of cancers. In modern years, there has been improved attention in the natural products from plants that play essential roles in research and improvement of therapeutic agents for cancer [Citation7]. Therapeutic plants play a significant function in the invention of new drugs used in contemporary remedies [Citation8]. Conventional drugs have been used for several centuries by a significant proportion of the people of India. India has a wealth of traditional information and is home to many essential and time-honoured methods of health care, such as Siddha, Unani and Ayurveda [Citation9]. Herbal drugs have the capability to change body systems. The effects are reliant on the chemical composition, distribution in the plant [Citation10].
Albizia lebbeck (AL) is a deciduous tree with composite leaves, round cream-coloured seeds, flat an oblong fruit and grows up wild. The plant is identifying all over India, Bangladesh, Africa, tropical and subtropical Asia [Citation11]. Beneficially bark is used in bronchitis; root in hemicranias; seeds and bark in piles; flowers in bronchitis, asthma and tropical pulmonary eosinophilia [Citation12]. It has been informed that the AL has analgesic, anti-asthmatic and anti-anaphylactic, nootropic, anti-inflammatory, anxiolytic, antibacterial, antioxidant and anticonvulsant properties [Citation13]. There are numerous researches that emphasize the uses of herbal agents using the plant extracts of Albizia in hepatoprotection [Citation14]. These explanations have drawn interest in the current study, which is a challenge to investigate the anticarcinogenic effect of A. lebbeck gold nanoparticles (AL-AuNPs) on HCT-116 colon carcinoma cells.
Materials and methods
Chemicals and reagents
Dulbecco’s Modified Eagles Medium (DMEM), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), Foetal Bovine Serum (FBS), Ethidium Bromide (EtBr), Phosphate Buffered Saline (PBS) and Dimethyl Sulfoxide (DMSO) were obtained from Himedia Lab Ltd., Mumbai, India. Gold(III) chloride trihydrate (HAuCl3) (99.9%) are procured from Sigma Aldrich (St Louis, MO, USA). The primary antibodies for Bcl-2, Bax, Bid and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All the further chemicals were used in analytical grade.
Cell culture
HCT-116 cells were purchased from the National Centre for Cell Sciences (NCCS), Pune, India. The HCT-116 cells were cultured using DMEM with 10% FBS and 1% antibiotic antimycotic solution. They were maintained at sterilized condition in a CO2 incubator (5%) at a temperature of 37 °C. The cells get 80% of confluence were allowed to subcultured using trypsin solution and used for further research.
Preparation of AL leaf extract
The freshly AL leaves were collected from the local farm. They were washed well with double distilled water and shadow dried until the water fully dries. Subsequently, 30 g of leaves were cut into small pieces, mixed along with 30 ml of sterile double distilled water and centrifuged for the removal of ungrounded particles. The understandable homogenous extract was further used for the preparation of AL-AuNPs.
Synthesis of gold nanoparticles
The AL-AuNPs were biosynthesized and optimized in the aqueous extract A. lebbeck leaves. Briefly, the 45 ml of 1 mM HAuCl3 solution was mixed with the different volumes of AL aqueous leaf extract (5, 10, 15, 20 and 25 ml) to reduce the Au3+ ions to Au0. The synthesis of AL-AuNPs was observed by the colour change of the solution from yellow to ruby red which further confirmed by UV-spectroscopic analysis and characterized by using the different methods.
Characterizxation of AuNPs
The UV spectrum was documented using a UV-vis double beam spectrophotometer (UV-1601, Shimadzu, Japan) at a wavelength vary from 300–700nm. X-ray diffraction (XRD) investigation (PhilpsX’Pert Pro X-ray diffractometer) was executed by preparing a thin film of powdered AL-AuNPs. Fourier transform infrared spectroscopy (FT-IR) was carried out by a spectrum RX1 instrument in diffuse reflectance mode operated at a resolution of 4 cm−1 of the wavelength of about 4000–600 cm−1. The studies on morphology, size and composition of the AL-AuNPs were executed by the method of high resolution-transmission electron microscopy (HR-TEM) (PHILIPS TECNAI 10). The selected area (electron) diffraction (SAED) pattern was assessed by using a Malvern Zetasizer instrument for the crystalline of structure. The SAED samples were prepared with Millipore filtered water for avoiding contamination reaction and SAED patterns were calculated the distance between the planes.
Anticancer activity of AL-AuNPs
Cell viability by MTT assay
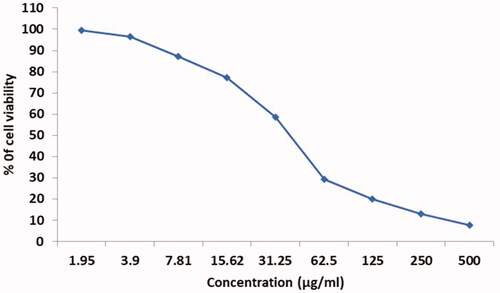
The HCT-116 colon cancer cell lines were seeded in a medium at a level of 5 × 104 cell/well in 96-well plates and were kept for 24 h. The AL-AuNPs with a dosage ranging from 1.95 to 500µg/ml were added into all wells. After being incubated for 24 h, the numbers of viable cells were measured by the MTT test [Citation15].
Measurement of intracellular reactive oxygen species (ROS)
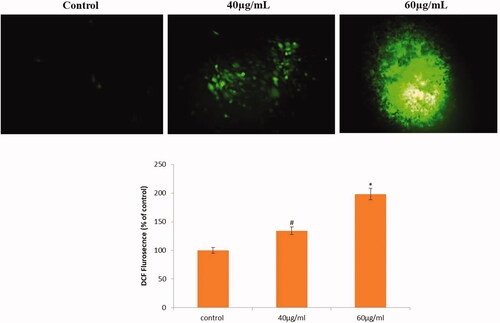
The HCT-116 cells were seeded at a concentration of 2 × 105 cells/well in a 6 well plate and incubated for 24 h [Citation16]. After the incubation, cells were added with different concentrations of AL-AuNPs (40 and 60 µg/ml) and the control cells left untreated with AL-AuNPs and received only the medium, then maintained at 37 °C in 5% CO2 and 95% air. Afterward, cells from every well were collected, washed two times with PBS and re-suspended in 500 μl of DCFH-DA (10 μM) for ROS assessment at 37 °C in a dark room. The samples were then evaluated immediately using flow cytometry.
Measurement of mitochondrial membrane potential (ΔΨm)
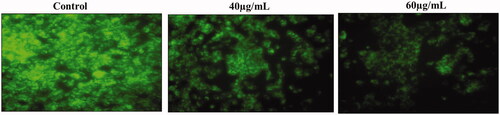
The HCT-116 cells were treated at a concentration of 2 × 105 cells/well in a 6 well plate and kept for 24 h [Citation17]. After the incubation, cells were added with various dosages of AL-AuNPs (40 and 60 µg/ml) and the control cells received only the medium without the AL-AuNPs supplementation and sustained at 37 °C in 5% CO2 and 95% air. Subsequently, the cells from every well were collected, washed two times with PBS and re-suspended in the Rh-123 dye for 30 min at 37 °C in a dark room. The samples were then evaluated immediately using flow cytometry.
Analysis of dual staining (AO/EtBr)
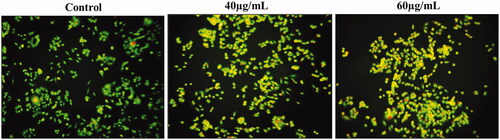
The HCT-116 cells were stained with AO/EtBr double staining and evidently indicated between the live and dead cells described previously [Citation18]. The cells were treated in a 6-well plate at the levels of 3 × 104cells/well and added with different dosages of AL-AuNPs (40 and 60 µg/ml) for 24 h. Control cells were excluded from the AL-AuNPs treatment. The cells were predetermined in methanol:glacial acetic acid (3:1) at 4 °C for 30 min and the cells were stained by 1:1 ratio of AO/EtBr for 30 min at 37 °C.
Assay of caspase proteins
The activity of caspase-9 and caspase-3 in HCT-116 cells were treated with AL-AuNPs (40 and 60 µg/ml), alongside control cells that were left untreated with AL-AuNPs and determined after 24 h using a colorimetric assay kit (BD Biosciences) according to the manufacturer’s information.
Western blotting analysis
HCT-116 cells were administrated by AL-AuNPs at various concentrations (40 and 60 µg/ml) and lysed with suitable lysis buffer. Control cells were excluded from the AL-AuNPs treatment. Proteins were estimated, separated by using SDS-PAGE gel electrophoresis, transferred onto a PVDF membrane and blocked with a blocking buffer containing 5% BSA. The membranes were kept with specific primary antibodies for Bcl-2, Bax, and Bid at 4 °C overnight. They were washed with tris buffered saline and kept with suitable secondary antibodies. The protein bands were identified by an enhanced chemiluminescence technique using an ECL kit (GenScript ECL kit, Piscataway, NJ).
Statistical analysis
The statistical investigation was employed by using SPSS software 17 (SPSS Inc., Chicago, IL) version. The one-way ANOVA was employed for the expressing significance of the current study. Statistical significance was established at a level of p < .05.
Results
Visual observation of synthesized AL-AuNPs
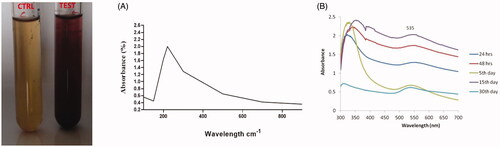
After the 24 h reaction, the suspension was changed its colour from yellow to ruby red, which indicates the formation of AL-AuNPs. When compared to other volumes, the 5 ml of the plant extract gives better results in the AuNPs generation. The bio-reduction of gold ions results in the formation of AuNPs in the reaction mixture.
Characterization of AL-AuNPs
Uv-visible spectroscopy analysis of AL-AuNPs
The production and stability of biosynthesized AL-AuNPs were evaluated using UV-Vis spectroscopic analysis (). The colour changes were observed from yellow to ruby red during the synthesis of the AL-AuNPs is due to the excitation of surface plasmon vibrations, which is stated as a characteristic feature of synthesized nanoparticles and various time periods of 24 h, 48 h, 5 days, 15 days and 30 days. The high absorption peak was observed at 535 nm which authenticated that the presence of AuNPs.
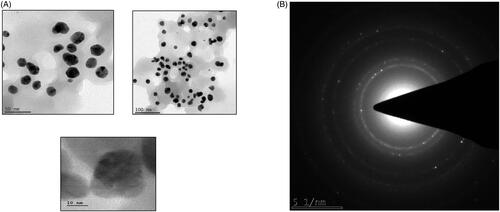
SAED pattern analysis of AL-AuNPs
The structure of the biosynthesized AL-AuNPs was further confirmed by their consequent SAED pattern analysis showed in . The SAED pattern of AL-AuNPs with the characteristic ring-shaped pattern demonstrating the crystalline nature was confirmed.
HR-TEM analysis of AL-AuNPs
The HR-TEM images of AL-AuNPs were illustrated in . HR-TEM imaging uses a narrow beam of electrons listening carefully to the sample and measures the size of the particle perfectly. This image showed AL-AuNPs as a dark spot against a light background. The size and spherical shape of the biosynthesized AL-AuNPs were measured with a size of 20 and 30 nm.
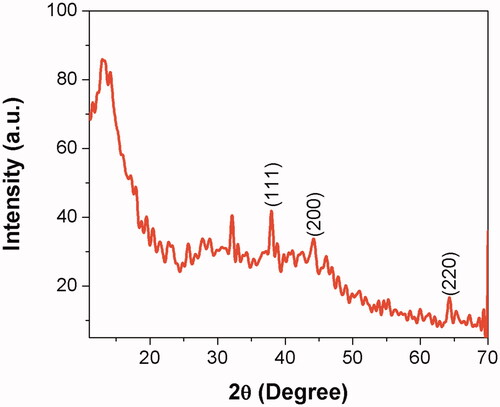
XRD pattern analysis of AL-AuNPs
The crystalline nature of the synthesized AL-AuNPs was observed using XRD assessments. The representative XRD pattern of the AL-AuNPs was revealed as shown in . The pattern demonstrates a number of Bragg reflections, which can be categorized into the face-centered cubic (FCC) structure of gold. The diffraction peaks identified at 2θ = 38.18° (111), 44.38° (200) and 66.57° (220) are matched to those statements for the standard gold metal (Au0). Therefore, the XRD pattern indicates that the AL-AuNPs were effectively crystalline.
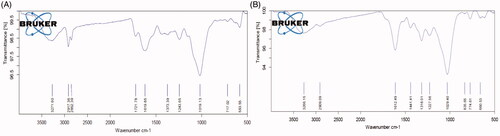
FTIR spectroscopy analysis of AL-AuNPs
The FTIR investigation of AuNPs from the AL leaves extract is shown in . The interaction of nanoparticles with biomolecules of AL-AuNPs demonstrated intensive peaks at 3333, 2129, 1636 and 729 cm−1. The FTIR peaks were determined the bonds important for the presence of alkynes with C–H stretch (3333 cm−1), alkynes C≡C bond stretch (2129 cm−1), alkenes with C=C stretch (1636 cm−1) and aromatic compounds (729 cm−1).
Anticancer activity of AL-AuNPs
Effect of AL-AuNPs on MTT assay
The MTT assay was used to observe the cytotoxic effect of synthesized AL-AuNPs shown in . The HCT-116 cell lines were added in a concentration ranging from 1.95–500 µg/ml for 24 h with AL-AuNPs. The cell viability was identified to be reduced with rising concentrations of the biosynthesized AL-AuNPs. The inhibitory concentration (IC50) level of the HCT-116 cells was identified as 48 µg/ml and we have selected for 40 and 60 μg concentrations of AL-AuNPs for further study.
Effect of AL-AuNPs on the generation of ROS
The effect of AL-AuNPs on the production of ROS was shown in . The HCT-116 cells were added to AL-AuNPs for different concentrations (40 and 60 µg/ml) and ROS levels were assessed. A significant rise in the intracellular ROS level was experienced in the AL-AuNPs treated HCT-116 cells as compared to the normal control. It was found that AL-AuNPs treatment noticeably improved the ROS levels in HCT-116 cells.
Effect of AL-AuNPs on alteration of ΔΨm
The effect of AL-AuNPs on the level of ΔΨm was shown in . The HCT-116 cells were administrated with AL-AuNPs for different dosages (40 and 60 µg/ml) and ΔΨm levels were evaluated. A significant decrease of ΔΨm level was experienced in the AL-AuNPs treated cells as compared to the normal control. Moreover, AL-AuNPs significantly reduced ΔΨm level in 24 h administration in HCT-116 cells as compared to control.
Effect of AL-AuNPs on status of AO/EtBr
The apoptotic morphological changes were determined by AO/EtBr staining shown in . The EtBr, a red fluorescence dye selectively penetrated into fragmented nuclei of apoptotic cells. Whereas, the green fluorescent dye of AO had uptake only in the non-apoptotic healthy cells. Taken together, the present findings revealed that untreated cells had a deep green fluorescence nucleus, which indicates live cells. On the other hand, various dosages of AL-AuNPs (40 and 60 µg/ml) treated HCT-116 cells demonstrated orange (early apoptosis) and red-stained (late apoptosis) apoptotic cells for 24 h.
Effect of AL-AuNPs on caspase protein levels
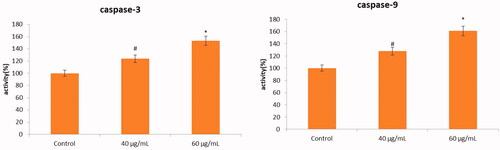
The apoptotic effect of AL-AuNPs was observed by measuring caspase-3 and caspase-9 levels relative to varying dosages (40 and 60 µg/ml) of protein content in cells treated with AL-AuNPs. The increased levels of caspase-3 and caspase-9 were observed in AL-AuNPs treated HCT-116 cells when compared with normal control cells as shown in .
Figure 9. The anticancer effect of AL-AuNPs on apoptotic signalling protein levels in HCT-116 colon cancer cell lines were determined by colorimetric method. Cells were treated with AL-AuNPs (40 and 60 μg) for 24 h and dose-dependent changes in the levels of caspase-3 and caspase-9 were analyzed. Values were presented as mean ± SD of three independent experiments (one-way ANOVA) followed by DMRT. Asterisks indicate statistically different from control: *,# p < 0.05.
Effect of AL-AuNPs on the Western blotting protein expression
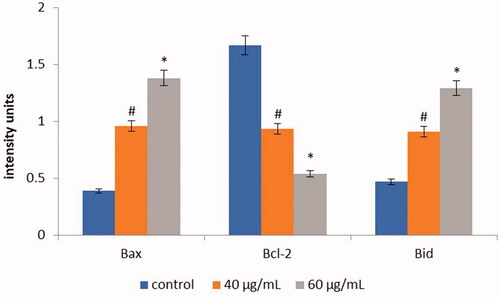
Apoptotic protein expression of control and AL-AuNPs treated HCT-116 cells were shown in . The control cells showed augmented expression of Bcl-2, whereas, low expression of Bax and Bid were observed. Conversely, AL-AuNPs (40 and 60 µg/ml) treated cells demonstrated down-regulate the Bcl-2 and improved the expression of Bax and Bid when compared with untreated control cells.
Figure 10. The anticancer effect of AL-AuNPs on apoptotic signalling proteins in HCT-116 colon cancer cell lines were examined by western blotting technique. The cells were treated with AL-AuNPs (40 and 60 μg) for 24 h and the protein expressions of Bcl-2, Bax and Bid were determined. β-actin was used as a loading control. Values were presented as mean ± SD of three independent experiments (one-way ANOVA) followed by DMRT. Asterisks indicate statistically different from control: *,# p < 0.05.
Discussion
The synthesis and categorization of AuNPs gained many interests for extraordinary properties such as high surface to level ratio and biocompatibility [Citation19]. The use of AuNPs revealed tremendous physio-chemical effects and the green synthesis of AuNPs is considered as an economical and eco-friendly approach. These nanoparticles could offer an energetic coating of biological deposits on the surface of the particle and also evade the usage of chemicals [Citation20]. Thus, we used green synthesis of AuNPs from AL leaf extract and it was characterized by UV-vis spectroscopy, SAED pattern, FTIR, HR-TEM, and XRD. From our findings, we observed the colour changes of the synthesized nanoparticles from yellow to ruby red with a maximum absorbance of 535 nm for bioreduction, crystalline nature by SAED and XRD, size and shape by HR-TEM and presence of different biomolecules by analyzing the FTIR technique of the synthesized AL-AuNPs. Similarly, Wu et al. [Citation21] reported that the Abies spectabilis plant extract was synthesized and characterized the AuNPs and tested their potential on bladder cancer cell lines.
The IC50 values of the AL-AuNPs showed to be fundamentally effective against the HCT-116 cells due to the presence of active biomolecules. Kumar et al. [Citation22] reported that methanolic seed extracts of AL demonstrated cytotoxic effects on HepG2 cells with IC50 values 50 µg/ml. The plant-based AuNPs normally induce reactive oxygen species (ROS) leading to cellular deaths. The overproduction of ROS was disturbing the signal transduction pathways which also leading cellular apoptosis. The production of peroxide (H2O2) radicals modifies the trans-membrane potential of mitochondria causing uncoupling of the respiration system [Citation23]. The ΔΨm was confirmed by Rh-123 staining that the HCT-116 cell lines treated with the AL-AuNPs for 24 h revealed reduced green colour emission results in the loss of ΔΨm, which leads to the initiation of apoptosis, whereas the untreated normal control cells had intact mitochondria with normal morphology and emitted an elevated green fluorescence. The long-term retention of the nanoparticles augmented the levels of ROS with reduced the membrane potential of the mitochondria and the possibility of cell death [Citation24]. The present results indicated that AL-AguNPs significantly improved the formation of ROS and reduced the ΔΨ min HCT-116 cancer cells in a dose-response manner, given that a strong indication towards the role of ROS mediated ΔΨm modification in cell death. Our results agree with earlier studies wherein flavonoids such as 5, 7-DMF have been informed to generate reactive oxygen intermediates in cancer cells [Citation25]. The increased production of intracellular ROS leads to the disruption of the ΔΨm, the release of cytochrome c into the cytosol with following activation of the caspase cascade and Bcl-2 member proteins and eventually leading to apoptosis [Citation26,Citation27]. Therefore, the previous study also reported that Alternanthera sessilis leaf extract induced apoptosis by altering the Bcl-2 family proteins such as Bax, Bcl-2, and Bid is concordant with the present investigations [Citation28].
Apoptosis is coordinated by a relation of cysteine proteases well-known as caspases. The major effectors of apoptotic proteins include proteases from the caspase family, which exist in as dormant precursors in mainly nucleated animal cells. The caspase-3 pathway activation has been known, which are either independent or dependent of leave go of mitochondrial cytochrome c and caspase-9 role. Caspase-3 activation is the hallmark of apoptosis and is essential for apoptotic DNA fragmentation and chromatin condensation in all cell types. Therefore, caspase-3 is important for convincing processes related to the dismantling of the cell and the development of apoptotic bodies [Citation29]. The dual staining of microscopic examination demonstrated that the common viable cells become known with green fluorescence for the reason that of the dispersion of acridine orange (AO) into the cell membrane, while apoptotic cells were shown as orange colored-bodies due to the nuclear shrinkage and blebbing. On the other hand, the necrotic cells with red fluorescence due to their decrease of membrane integrity by AL-AuNPs induced cytotoxicity [Citation30]. It was found that the total number of apoptotic cells increased when the cells were treated with their various dosages of AL-AguNPs when compared to the control. The AL-AuNPs treated HCT-116 cells improved the activity of caspase-9 and caspase-3 in a dose-dependent manner is well agreeing with previous reports that the seaweed aqueous extracts [Citation4].
Conclusions
In this present study, we concluded that the synthesis of AuNPs from AL was characterized. It was primarily found with the development of the ruby red colour solution and UV absorption spectra also verified the highest absorbance at 535 nm. The crystal nature of AL-AuNPs was further confirmed by SAED and XRD. The HR-TEM images illustrated the size and morphological structures of nanoparticles. The alkynes, aromatic compounds and alkenes groups of biomolecules distribution in the AL-AuNPs were identified by FTIR. Furthermore, AL-AuNPs induce cytotoxicity at a concentration of 48 µg/ml in HCT-116 colon cancer cells and also induce apoptosis through pro- and anti-apoptotic protein members. This green synthesis of AuNPs from the AL approach was eco-friendly, large-scaled up and easy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Luzzatto L, Pandolfi PP. Causality and chance in the development of cancer. N Engl J Med. 2015;373(1):84–88.
- Zhao Y, Hu H, Zuo X, et al. Chemopreventive effects of some popular phytochemicals on human colon cancer: a review. Food Funct. 2018;9(9):4548–4568.
- Kasdagly M, Radhakrishnan S, Reddivari L, et al. Colon carcinogenesis: influence of Western diet-induced obesity and targeting stem cells using dietary bioactive compounds. Nutrition. 2014;30(11–12):1242–1256.
- Namvar F, Rahman HS, Mohamad R, et al. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomedicine. 2014;9:2479–2488.
- Jin Y, Kannan S, Wu M, et al. Toxicity of luminescent silica nanoparticles to living cells. Chem Res Toxicol. 2007;20(8):1126–1133.
- Ye B, Li J, Li Z, et al. Anti-tumor activity and relative mechanism of ethanolic extract of Marsdenia tenacissima (Asclepiadaceae) against human hematologic neoplasm in vitro and in vivo. J Ethnopharmacol. 2014;153(1):258–267.
- Wang X, Yan Y, Chen X, et al. The antitumor activities of Marsdenia tenacissima. Front Oncol. 2018;8:473.
- Sheela DJ, Jeeva S, Shamila IR, et al. Antimicrobial activity and phytochemical analysis of Sanseiveria roxburghiana leaf. Asian J Plant Sci Res. 2012;2:41–44.
- Prema R, Sekar DS, Sekhar KB, et al. In vitro cytotoxicity study on combined plants extracts (Cissus quadrangularis and Aegle marmelos). Eur J Exp Biol. 2012;2:882–888.
- Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2(1):32–50.
- Saha A, Ahmed M. The analgesic and anti-inflammatory activities of the extract of Albizia lebbeck in animal model. Pak J Pharm Sci. 2009;22:74–77.
- Rahul C, Lincy J, Methew G, et al. Pharmacognostic standardization and phytochemical screening of Albizzia lebbeck. J Chem Pharm Res. 2010;2:432–443.
- Chaddha V, Nayak S, Solanki S. Preliminary phytochemical screening on bark & pods of Albizzia lebbeck L. PharmacieGlobale. 2011;11:1–3.
- Verma VK, Sarwa KK, Kumar A, et al. Comparison of hepatoprotective activity of Swertia chirayita and Andrographis paniculata plant of North-East India against CCl4 induced hepatotoxic rats. J Pharm Res. 2013;7(7):647–653.
- Leong CO, Suggitt M, Swaine DJ, et al. In vitro, in vivo, and in silico analyses of the antitumor activity of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazoles. Mol Cancer Ther. 2004;3(12):1565–1575.
- Jesudason EP, Masilamoni J, Jebaraj CE, et al. Efficacy of DL-alpha lipoic acid against systemic inflammation-induced mice: antioxidant defense system. Mol Cell Biochem. 2008;313(1-2):113–123.
- Johnson LV, Walsh ML, Chen LB. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci USA. 1980;77(2):990–994.
- Karthikeyan S, Kanimozhi G, Prasad NR, et al. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol in Vitro. 2011;25(7):1366–1375.
- Yeh YC, Creran B, Rotello VM. Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale. 2012;4(6):1871–1880.
- Kunoh T, Takeda M, Matsumoto S, et al. Green synthesis of gold nanoparticles coupled with nucleic acid oxidation. ACS Sustainable Chem Eng. 2018;6(1):364–373.
- Wu T, Duan X, Hu C, et al. Synthesis and characterization of gold nanoparticles from Abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif Cells Nanomed Biotechnol. 2019;47(1):512–523.
- Kumar A, Mo. Pai M, Rai N. In-vitro hepatoprotective activity of Albizia lebbeck, cassia occidentalis and swertiachirata on hepg2 cells. Asian J Pharm Clin Res. 2016;9:276–280.
- Hembram KC, Kumar R, Kandha L, et al. Therapeutic prospective of plant-induced silver nanoparticles: application as antimicrobial and anticancer agent. Artif Cells Nanomed Biotechnol. 2018;46(3):S38–S51.
- Cui W, Li J, Zhang Y, et al. Effects of aggregation and the surface properties of gold nanoparticles on cytotoxicity and cell growth. Nanomedicine. 2012;8(1):46–53.
- Li H, Zhang X, Wang W. Anticancer activity of 5,7-dimethoxyflavone against Liver cancer cell line hepg2 involves apoptosis, ros generation and cell cycle Arrest. Afr J Tradit Complement Altern Med. 2017;14(4):213–220.
- Zou W, Liu X, Yue P, et al. c-Jun NH2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate in human lung cancer cells. Cancer Res. 2004;64(20):7570–7578.
- Yang JF, Cao JG, Tian L, et al. 5, 7-Dimethoxyflavone sensitizes TRAIL-induced apoptosis through DR5 upregulation in hepatocellular carcinoma cells. Cancer Chemother Pharmacol. 2012;69(1):195–206.
- Qian L, Su W, Wang Y, et al. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif Cells Nanomed Biotechnol. 2019;47(1):1173–1180.
- Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64(4):821–846.
- Prasannaraj G, Sahi SV, Ravikumar S, et al. Enhanced cytotoxicity of biomolecules loaded metallic silver nanoparticles against human liver (HepG2) and prostate (PC3) cancer cell lines. J Nanosci Nanotechnol. 2016;16(5):4948–4959.