Abstract
Photothermal therapy (PTT) is a promising approach for effective cancer treatment because of its non-invasive procedure, low toxicity to normal tissues, and high tumour ablation efficiency. Developing a PTT agent with precise tumour imaging capabilities is an essential prerequisite for effective PTT. In this study, we developed a bifunctional near-infra-red (NIR) fluorescent conjugate consisting of chitosan oligosaccharide lactate (COL) and the ZW800-1 NIR fluorophore (COL-ZW). We demonstrate that this conjugate is easy to use and that it is an effective theranostic agent for fluorescence-guided photothermal treatment. The temperature of COL-ZW increased by 62.3 °C after NIR laser irradiation (1.1 W/cm2) for 5 min in HT-29 tumour-bearing mice. The HT-29 tumours targeted by COL-ZW showed a remarkable decrease in tumour volume until a week after photothermal treatment. These in vivo results demonstrate that the bifunctional COL-ZW generates strong fluorescence and light-triggered PTT in tumour sites, indicating successful fluorescence-guided PTT. Importantly, no tumour recurrence or treatment-induced toxicity was observed after a single dose of COL-ZW with laser irradiation. Therefore, a combinatorial treatment with COL-ZW and NIR laser irradiation could serve as a promising strategy for photothermal cancer therapy.
Introduction
To date, a number of technologies for cancer treatments have been developed including chemotherapy, radiotherapy, photodynamic therapy, photothermal therapy (PTT), and combination therapy. Among these options, PTT has great potential for effectively treating cancers because of its non-invasive procedure, low toxicity to normal tissues, and high tumour ablation efficiency [Citation1–7]. Photothermal therapeutic agents that exhibit strong absorbance in the near-infra-red (NIR) region can convert light energy into heat energy to induce hyperthermia (>48 °C) and kill tumour cells [Citation8–12]. Although various materials including inorganic and polymeric nanoparticles have been explored as photothermal agents, many such materials are associated with certain disadvantages that limit their use, such as non-biodegradability, insufficient photothermal conversion efficiency, and unsolved biosafety issues [Citation13–16].
Chitosan oligosaccharide lactate (COL) is a natural, biodegradable, non-toxic, cationic carbohydrate polymer, which is the depolymerised product of chitosan with a lower degree of polymerisation (≈ 30) and an average molecular weight (MW ≈ 5000 Da). Because of its high water solubility, short chain length, low viscosity, and antitumor properties, COL is suitable for pharmaceutical and biomedical applications [Citation17,Citation18]. The potential of COL as a carrier for imaging agents and anticancer drugs has been suggested due to its efficient intracellular and site-specific delivery [Citation19–24]. Due to its tumour targetability, the biocompatible COL could be successfully used for PTT when combined with NIR fluorophores to act as an imaging as well as photothermal agent.
ZW800-1, a zwitterionic NIR fluorophore, is a promising candidate for photothermal cancer therapy because it shows remarkable optical properties including a high molar extinction coefficient, relatively high quantum yield, and good photostability in warm serum [Citation25,Citation26]. Moreover, ZW800-1 itself exhibits no serum binding, has ultralow non-specific tissue uptake, and is rapidly excreted from the body. These properties are more desirable than those of the FDA-approved NIR dye indocyanine green (ICG) and other commercial dyes like Cy5.5 and IRDye800CW [Citation25,Citation27]. As it is very important to preserve the tumour targetability of COL after conjugation with an NIR fluorophore, ZW800-1 could serve as an optimal NIR photothermal agent based on its optical properties and in vivo performance.
In this study, we prepared an NIR fluorescent agent COL-ZW by conjugating the ZW800-1 NIR fluorophore to COL for tumour-targeted imaging and photothermal treatments in vivo. Bifunctional COL-ZW was capable of targeting the tumour tissue and allowed for the monitoring of time-dependent accumulation of COL-ZW in the tumour, consequently enabling the determination of the optimal timing for photothermal treatment. We thus demonstrated that COL-ZW may be a safe and reliable theranostic modality for fluorescence-guided photothermal cancer therapy in clinical applications. To our knowledge, this is the first report of using COL in combination with the ZW800-1 NIR fluorophore as a simple and effective method for targeted photothermal treatment of cancer.
Materials and methods
Materials
All chemicals and solvents were of American Chemical Society grade or HPLC purity. COL (average MW ≈ 5000 Da, > 90% deacetylated form) was purchased from Sigma-Aldrich (St. Louis, USA). ZW800-1 NHS ester was prepared as described previously [Citation27]. All chemicals were used as received without further purification.
Conjugation of COL to ZW800-1 NIR fluorophore (COL-ZW)
COL (1 µmol, 5 mg) was conjugated to the ZW800-1 NHS ester (1 µmol, 1 mg) in phosphate-buffered saline (PBS; 2 ml, pH 8) at room temperature for 6 h. The reaction mixture was purified using a gel-filtration chromatography (GFC) system with Econo-Pac P6 cartridges (Bio-Rad, Hercules, USA) and a flow rate of 1 ml/min (PBS, pH 7.4). Size distribution and diameter of COL and COL-ZW in water were measured by dynamic light scattering (DLS, Nano ZS Zetasizer, Malvern Instruments Ltd., UK).
Optical property measurement
Optical measurements were carried out at 37 °C in PBS, pH 7.4. Absorption and fluorescence spectra of COL-ZW conjugate were detected using a fibre optic flame spectrophotometer (Ocean Optics, Dunedin, USA). NIR excitation was generated by 5 mW of 655 nm red laser pointer (Opcom Inc., Xiamen, China) connected with a 400 µm NA 0.22 fibre (Ocean Optics) [Citation28].
In vitro cancer cell binding assay
The human colorectal adenocarcinoma cell line, HT-29 and the human breast adenocarcinoma cell lines, MCF-7 and MDA-MB-231 were purchased from the American Type Culture Collection (ATCC, Manassas, USA). The cancer cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% foetal bovine serum (FBS, Gibco BRL, Paisley, UK) and an antibiotic-antimycotic solution (100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B; Welgene, Daegu, South Korea) in a humidified 5% CO2 atmosphere at 37 °C. The final concentration of 2 μM COL-ZW was added when the cells attained 50 ∼ 60% confluence. After 1 h incubation at 37 °C, the cells were washed with PBS and imaged using a Nikon Eclipse Ti-U inverted microscope system (Nikon, Seoul, South Korea) [Citation28].
In vitro photothermal cytotoxicity
Calcein-AM (green for live cells) and propidium iodide (red for dead cells) fluorescent stains were used to visualise the PTT-induced cell death in COL-ZW treated cells. Initially, HT-29 cells (1 × 104 per well) were seeded into a 24-well plate. After 24 h, the cells were treated with 2 μM COL-ZW for 1 h and washed with PBS. Then, PTT was performed under 808 nm laser at 1.1 W/cm2 for 5 min. After PTT, the cells were allowed to incubate for another 3 h and then costained with calcein-AM and propidium iodide for 30 min. After washing twice with PBS, the stained cells were observed under a fluorescent microscope (Nikon).
HT-29 xenograft mouse model
Animal experiments were carried out in accordance with protocols approved by Chonnam National University Animal Research Committee (CNU IACUC-H-2017-64). Male NCRNU nude mice (6 weeks old, ∼25 g) were obtained from Orient (Seongnam, South Korea). HT-29 cancer cells were harvested in 100 μL PBS with 1 × 106 cells per mouse and subcutaneously inoculated into the right flank of each mouse. The COL-ZW conjugate was intravenously injected when tumours attained a size of 1 cm in diameter. Animals were anaesthetized and imaged over the entire time period [Citation28].
In vivo NIR fluorescence tumour imaging
In vivo NIR fluorescence imaging was carried out using a FOBI imaging system (NeoScience, Suwon, South Korea). Tumour-to-background ratios (TBR) as fluorescence/background signals were analysed by ImageJ software. The background means the fluorescence signal of a region neighbouring to the tumour during the imaging time period. To validate in vivo antitumor effects, tumour volumes were observed over the entire time period and determined by the following formula: V = 0.5 × longest diameter × (shortest diameter)2 [Citation28].
Evaluation of in vivo photothermal effect
HT-29 tumour-bearing mice were subjected to intravenous injection of PBS or COL-ZW. After 4 h injection, mice were anaesthetized and the tumour sites were exposed to the laser irradiation (1.1 W/cm2, λ = 808 nm) for 5 min. Tumour temperature was confirmed using a FLIR® thermal imager (FLIR Systems., Wilsonville, USA), and temperature changes in the tumour sites were observed every 1 min from the initial stage of the laser irradiation over the entire time period. After 24 h irradiation, tumours were collected from the laser-treated mice for histological examination using haematoxylin and eosin (H&E) staining [Citation28].
Statistical analysis
Statistical analysis was carried out using a one-way ANOVA followed by Tukey’s multiple comparisons test. Statistically significant differences were considered to be at a level of p < .05. Results are expressed as mean ± SD and curve fitting was carried out using the Prism software (GraphPad, San Diego, USA) [Citation28].
Histological examination
Collected tumours were fixed in 2% paraformaldehyde and flash frozen in liquid nitrogen after embedding in optimal cutting temperature (OCT) compound. Frozen tumour tissues were cryosectioned with a 10 µm thickness followed by staining with H&E. Histological examination was carried out using a Nikon Eclipse Ti-U inverted microscope system (Nikon) [Citation28].
Results and discussion
Preparation and characterisation of the COL-ZW conjugate
The procedure for COL-ZW synthesis is shown in . The amine groups of COL were covalently conjugated to the ZW800-1 NHS ester through amide bond formation via a condensation reaction performed in PBS, pH 8 at room temperature for 6 h. To preserve the tumour targetability of COL, the conjugation reaction was initiated a molar ratio of 1:1 of COL to ZW800-1. The advantage of using the ZW800-1 NIR fluorophore is that there is no need to consider any potential changes to the properties of COL after the conjugation reaction because the charge-balanced ZW800-1 is optimised for labelling peptides, especially small molecules, as demonstrated previously [Citation27]. The COL-ZW conjugate was purified in a GFC system as shown in and demonstrated successful conjugation and favourable characteristics for further in vitro and in vivo studies. In addition, the size distribution and diameter of COL and COL-ZW conjugate were examined by DLS measurements (). As expected, the COL was found with nanoaggregates in the size range with diameter 150–200 nm in water, because chitosan is known to form aggregation having a hydrophobic core and a hydrophilic surface in aqueous solutions. Interestingly, the size of COL-ZW conjugates was measured in the 1–1.5 nm size range and much smaller than COL nanoaggregates. This indicates that a COL-ZW conjugate may not form the same assembly process that of the COL in water, because the charge-balanced ZW800-1 plays an important role by preventing the self-assembly of COL.
Figure 1. (a) Synthesis scheme, (b) GFC purification (mobile phase: PBS, pH 7.4), (c) DLS analysis, and (d) optical properties of the COL-ZW conjugate. Size distribution and diameter of COL and COL-ZW were obtained at a concentration of 1 μM in water. Optical measurements were performed at 37 °C in PBS, pH 7.4. The molar extinction coefficient and quantum yield of Chitosan-ZW800 were based on that of the ZW800-1 fluorophore [Citation26].
![Figure 1. (a) Synthesis scheme, (b) GFC purification (mobile phase: PBS, pH 7.4), (c) DLS analysis, and (d) optical properties of the COL-ZW conjugate. Size distribution and diameter of COL and COL-ZW were obtained at a concentration of 1 μM in water. Optical measurements were performed at 37 °C in PBS, pH 7.4. The molar extinction coefficient and quantum yield of Chitosan-ZW800 were based on that of the ZW800-1 fluorophore [Citation26].](/cms/asset/80427c61-3911-4a21-ae0f-d02a08042575/ianb_a_1817054_f0001_c.jpg)
As the COL-ZW conjugate has high water solubility, the molar extinction coefficient and quantum yield (ε = 2,46,000 M−1cm−1, Φ = 13.5%) are significantly higher than those of ICG (ε = 111,060 M−1cm−1, Φ = 1.7%) in aqueous conditions [Citation26,Citation29]. shows the optical spectra of COL-ZW in PBS. COL-ZW showed an absorbance peak at 768 nm and yielded the highest fluorescence at 789 nm. Although the absorption peak of COL-ZW did not match the 808 nm laser diode used for photothermal treatments, the photothermal effect can still be triggered by the 808 nm laser diode due to the high molar extinction coefficient of the ZW800-1 NIR fluorophore.
Assessment of in vitro photothermal effect
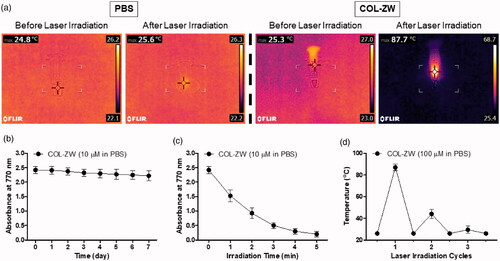
For real-time photothermal imaging, a FLIR® thermal imager equipped with an 808 nm laser diode (0–2 W) was used to measure the photothermal conversion efficiency of the COL-ZW conjugate. The photothermal effect was confirmed in vitro by monitoring the following solutions: 100 μM COL-ZW prepared in PBS and PBS alone, during irradiation with an 808 nm NIR laser (1.1 W/cm2) for 1 min. The concentration of COL-ZW used was equivalent to the 0.4 mg/kg single dose of ZW800-1 NIR fluorophore used previously for in vivo studies [Citation26]. As the irradiation time of the COL-ZW solution increased, the colour of the photothermal images dramatically changed from dark purple (indicating low temperature) to bright yellow (indicating high temperature) within 1 min, compared to minimal colour change in PBS alone (). The temperature of the COL-ZW solution increased rapidly from ambient temperature (25.3 °C) to 87.7 °C, while no obvious change was observed for PBS alone at 1 min post-irradiation. These results demonstrated that the COL-ZW solution can absorb the 808 nm laser light and convert the absorbed energy into a considerable amount of thermal energy in a time-dependent manner. In terms of stability, the COL-ZW conjugate presents stable physicochemical and optical properties in PBS for one week, as proven by the constant absorbance values (). However, the photostability of COL-ZW solution gradually decreased during the 5 min of laser irradiation, which indicates the ZW800-1 NIR fluorophore degraded after showing the photothermal conversion performance (). To further evaluate the photothermal stability under repeated laser irradiation, three cycles of irradiations were performed as shown in . As expected, the temperature of COL-ZW solutions rapidly elevated in the first cycle and significantly decreased in the second and third cycles under repeated laser irradiation. This indicates that the COL is unable to protect ZW800-1 from photodegradation. Furthermore, an important consideration is that the photothermal conversion efficiency in vivo is highly dependent on the tumour targetability of COL-ZW conjugate; which is unlike the case for in vitro photothermal effects.
Figure 2. (a) In vitro photothermal images of COL-ZW solution dissolved in PBS (10 nmol based on the absorbance value of ZW800-1; 100 μM concentration is equivalent to a 0.4 mg/kg single dose of ZW800-1) and PBS alone (100 μL) before and after exposure to an 808 nm laser (1.1 W/cm2) for 1 min. The maximum temperature was automatically recorded as a function of the irradiation time using an infra-red thermal camera. (b) Long-term stability of COL-ZW solutions stored at 37 °C in PBS, pH 7.4. Absorbance changes of 10 μM COL-ZW solutions were measured at 770 nm for 7 days. (c) Photostability of COL-ZW solutions under the laser irradiation. Absorbance changes of 10 μM COL-ZW solutions were measured at 770 nm during the 5 min of laser irradiation. (d) Photothermal stability curve of 100 μM COL-ZW solutions after repeated laser irradiation (1 cycle = 1 min of laser irradiation). Data points are expressed as mean ± SD of the three independent experiments.
In vitro cancer cell binding
To identify the binding affinity of the COL-ZW conjugate to cancer cells, 2 μM COL-ZW solution was incubated with either HT-29, MCF-7, or MDA-MB-231 human cancer cell lines for 1 h at 37 °C. COL-ZW conjugates localised to the cell boundaries with high fluorescence intensities in all three cancer cell lines (). These results confirm that the COL-ZW can adhere to cell membranes, thereby delivering thermal energy directly to the cell membrane. As ZW800-1 alone showed no cellular uptake under any conditions [Citation27], COL may play a crucial role in targeting COL-ZW to cancer cells. The higher cellular uptake of COL-ZW conjugates is most likely due to the positive charge of COL, which allows the COL-ZW conjugate adhere to the negatively charged outer surfaces of cell membranes.
Figure 3. (a) Live binding of COL-ZW to HT-29, MCF-7, and MDA-MB-231 cells. Phase contrast and NIR fluorescence images of each cell line were obtained using 2 μM COL-ZW. The sample concentration is calculated using the absorbance value of the ZW800-1 NIR fluorophore. Green pseudo-colour is used for phase-fluorescence merged images. (b) Fluorescence images of HT-29 cells after photothermal treatment under the 808 nm laser irradiation (1.1 W/cm2) for 5 min. HT-29 cells were then costained with calcein-AM (green for live cells) and propidium iodide (red for dead cells). Images are representative of three independent experiments. All fluorescence images have been subjected to identical exposure time and normalisation. Scale bars = 100 μm.
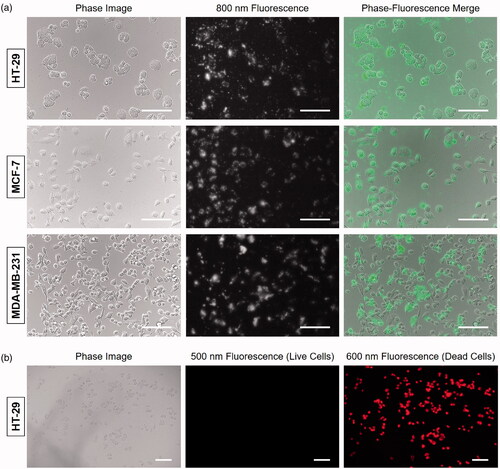
In order to visually demonstrate the laser-induced cellular photothermal effect, HT-29 cells were costained with calcein-AM and propidium iodide to identify live and dead cells, respectively, after photothermal treatment under the 808 nm laser irradiation (1.1 W/cm2) for 5 min (). As expected, COL-ZW induced massive cell death and showed intense homogeneous red fluorescence from propidium iodide, while no green fluorescence from calcein-AM was detected after the laser irradiation. This result suggests that the COL-ZW under laser irradiation could generate thermal energy and induce cell death.
In vivo NIR fluorescence imaging for tumour targetability
Accumulation of the COL-ZW conjugate in the tumours of HT-29 tumour-bearing mice was monitored in real time at different time points after intravenous injection for 24 h (). NIR fluorescence intensities of the tumour tissue increased within 2 h, and then gradually declined by 24 h post-injection. Based on TBR values, the photothermal treatments were performed at 4 h post-injection to prevent damage to normal tissues in the vicinity of tumour tissues due to high fluorescence levels in the skin at earlier time points (). These observations indicate that the COL-ZW conjugate could be useful for targeted tumour imaging, and that it can provide fluorescence-based guidance in real-time for effective photothermal treatments.
Figure 4. In vivo HT-29 tumour targeting efficiency of COL-ZW and ZW800-1. (a) NIR fluorescence imaging for 24 h post-injection of COL-ZW. (b) Time course of tumour-to-background ratio (TBR) of tumour sites targeted by COL-ZW. TBR was calculated as the fluorescence intensity of tumour tissue versus the signal intensity of neighbouring tissue obtained at different time points. (C) NIR fluorescence imaging at 2 h and 4 h post-injection of ZW800-1 alone. (d) Biodistribution and resected organs imaged at 4 h post-injection of COL-ZW. Tumour-bearing mice were intravenously injected with COL-ZW or ZW800-1 (10 nmol based on the absorbance value of ZW800-1; 100 μM concentration is equivalent to a 0.4 mg/kg single dose of ZW800-1) and imaged for 24 h. Tumour site is indicated by arrowhead. Du: duodenum; He: heart; In: intestine; Ki: kidneys; Li: liver; Lu: lungs; Mu: muscle; Pa: pancreas; Sp: spleen; Tu: tumour; PI: post-injection. Scale bars = 1 cm. Images are representative of three independent experiments. All NIR fluorescence images have identical exposure and normalizations. Data points are expressed as mean ± SD of the three independent experiments.
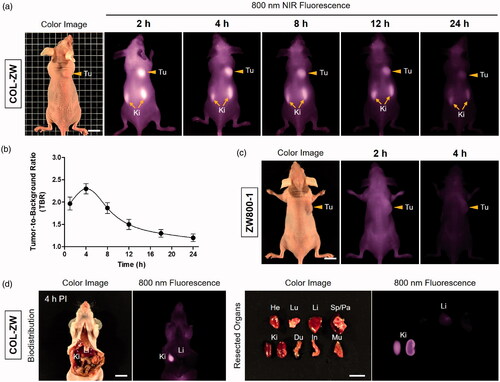
As shown in , tumours showed no uptake of ZW800-1 alone for up to 4 h after injection. This result corresponds to those from a previous study [Citation3,Citation30]. Additionally, we confirmed the biodistribution of the COL-ZW conjugate by imaging fluorescence in major organs resected from mice (). COL-ZW mainly shows rapid renal clearance at 4 h post-injection without significant uptake in other tissues. As the COL-ZW conjugate has high tumour targetability and low non-specific tissue uptake within a short period of time, COL-ZW could be useful for addressing unmet clinical needs.
Assessment of in vivo photothermal effect
Based on the high photothermal conversion efficiency of COL-ZW in vitro, the PTT capability of COL-ZW in vivo was further studied using the HT-29 tumour-bearing mouse model. Importantly, the optimal power density of an 808 nm laser was investigated in a PBS-treated group to prevent the photothermal effect generated by only laser power without the photothermal agent. With the increase of the laser power from 1.1 W/cm2 to 1.2 W/cm2, temperatures in tumour sites continuously increased by ∼50 °C during the 5 min of laser irradiation, which is dependent to the laser power density (). Although a power density of 1.0 W/cm2 is still available in this study, the power density of 1.1 W/cm2 was optimally selected to maximise the PTT efficiency of COL-ZW.
Figure 5. (a) Photothermal curves of PBS-injected mice at tumour sites irradiated with different power densities (1.0 W/cm2, 1.1 W/cm2, and 1.2 W/cm2) for 5 min. (b) Temperature changes in tumour sites in each treatment group were monitored during the 808 nm laser irradiation (1.1 W/cm2) for 5 min. Data points represent mean ± SD of three independent experiments. (c) Whole-body photothermal images of tumour-bearing mice at 4 h post-injections of PBS and COL-ZW, respectively, on irradiation with an 808 nm laser (1.1 W/cm2) for 5 min. Maximum tumour temperatures were automatically recorded using an infra-red thermal camera as a function of irradiation time.
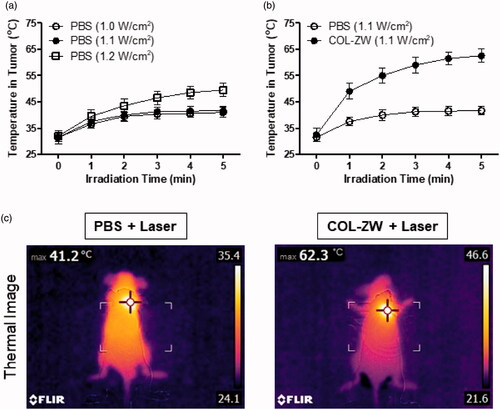
Mice were intravenously injected with COL-ZW (100 μM based on the ZW800-1 NIR fluorophore) at 4 h before laser irradiation. Tumour sites were then irradiated with an 808 nm laser with a power density of 1.1 W/cm2 for 5 min. As shown in , the tumour temperature showed a rapid increase to ∼56 °C within 2 min after laser irradiation, and the photothermal treatment was maintained for an additional 3 min for effective tumour ablation. This demonstrates that the temperature change in tumours was generated by the strong photothermal effects of COL-ZW. Additionally, the real-time temperature variation at the tumour boundary was monitored using a FLIR® thermal imager (). After laser irradiation, only a mild temperature increase to ∼41.2 °C was observed in case of tumours treated with PBS alone, while a rapid temperature increase to ∼62.3 °C was observed in tumours treated with COL-ZW. These results indicate that the increased tumour temperature is sufficient to kill cancer cells without damaging the adjacent normal tissue.
In vivo PTT efficacy
To further confirm the therapeutic effects of the COL-ZW conjugate, HT-29 tumour-bearing mice were carefully monitored for a week after photothermal treatment (). The tumour sizes in PBS- or COL-ZW-treated groups were measured every other day. Tumour volumes in the COL-ZW-injected group gradually decreased within 5 days after laser irradiation, and ultimately only the black scars on the initial tumour sites remained. In contrast, tumours in the PBS group markedly increased over time without any apparent effects of laser irradiation (). These results suggest that a combination of COL-ZW injection and laser irradiation can suppress tumour growth. Moreover, no tumour recurrence or treatment-induced toxicity was observed in the COL-ZW group for 10 days after photothermal treatment. We therefore demonstrated that the COL-ZW conjugate has an excellent PTT efficacy from a single-dose treatment.
Figure 6. (a) In vivo NIR photothermal therapeutic efficacy. Representative photos of tumour size changes after 808 nm laser irradiation (1.1 W/cm2) for 5 min at 4 h post-injection with PBS and COL-ZW. (b) Tumour growth rates in each treatment group were monitored for 7 days. Data points represent mean ± SD of three independent experiments. Tu: tumour. Scale bars = 1 cm. (c) Tumour H&E-stained slices of PBS and COL-ZW injected mice at 24 h after laser irradiation. Scale bars = 100 μm.
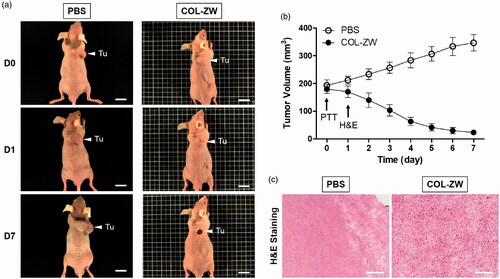
The tumours were resected at 24 h after laser irradiation, and examined using H&E staining for histological analysis. As expected, evidence of cell damage, including nuclear damage and cell shrinkage, were observed in tumours treated with COL-ZW and laser irradiation, while tumour tissues in the PBS and laser-treated group contained intact cells and exhibited normal patterns of cell proliferation without photothermal effects (). This result confirms the high hyperthermic therapeutic efficacy of COL-ZW in vivo and indicates that the COL-ZW conjugate is a biocompatible and effective PTT agent that can be safely used in cancer treatment.
Conclusions
Although many different types of PTT nanomaterials have been reported to solve the longstanding biosafety problems, the issues of uniformity, reproducibility and practical synthesis still remain for many PTT nanomaterials as they move from laboratories to clinical trials. In the present study, we showed that COL-ZW, consisting of natural COL and the biocompatible ZW800-1 NIR fluorophore, can be prepared by a simple one-step process without the requirement of organic solvents. Furthermore, tumour targeted COL-ZW was successfully utilised as a theranostic modality for fluorescence-guided photothermal treatment. COL-ZW showed strong absorbance in the NIR region, and exhibited tumour-specific targeting, high photothermal conversion efficiency, and effective tumour ablation. Photothermal tumour ablation could be induced by increasing the temperature of COL-ZW by triggering it with an 808 nm laser. COL-ZW is especially effective for photothermal cancer treatment due to the high tumour targetability of COL and the excellent photothermal property of the ZW800-1 NIR fluorophore. Therefore, COL-ZW is a promising potential PTT agent for enhancing the NIR fluorescence signal in tumour sites in order to provide more accurate guidance for photothermal cancer treatments. Overall, the biocompatible, bifunctional, COL-ZW conjugate has great potential for use in the development of next-generation cancer treatments.
Disclosure statement
The authors declare that there are no conflicts of interest. The authors are responsible for the conduction of experiments and writing of the paper.
Additional information
Funding
References
- Song XJ, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8(2):340–354.
- Zhang H, Li Q, Liu R, et al. A versatile prodrug strategy to in situ encapsulate drugs in MOF nanocarriers: a case of cytarabine‐IR820 prodrug encapsulated ZIF‐8 toward chemo‐photothermal therapy. Adv Funct Mater. 2018;28(35):1802830.
- Lee S, Jung JS, Jo G, et al. Near-infrared fluorescent sorbitol probe for targeted photothermal cancer therapy. Cancers. 2019;11:1286.
- Nouri S, Mohammadi E, Mehravi B, et al. NIR triggered glycosylated gold nanoshell as a photothermal agent on melanoma cancer cells. Artif Cells Nanomed Biotechnol. 2019;47(1):2316–2324.
- Zhang J, Miao Y, Ni W, et al. Cancer cell membrane coated silica nanoparticles loaded with ICG for tumour specific photothermal therapy of osteosarcoma. Artif Cells Nanomed Biotechnol. 2019;47(1):2298–2305.
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41(12):1842–1851.
- Riley RS, Day ES. Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment. WIREs Nanomed Nanobiotechnol. 2017;9(4):e1449.
- Ghaznavi H, Hosseini-Nami S, Kamrava SK, et al. Folic acid conjugated PEG coated gold-iron oxide core-shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif Cells Nanomed Biotechnol. 2018;46(8):1594–1604.
- Yu H, Cui Z, Yu P, et al. pH- and NIR light-responsive micelles with hyperthermia-triggered tumor penetration and cytoplasm drug release to reverse doxorubicin resistance in breast cancer. Adv Funct Mater. 2015;25(17):2489–2500.
- Zhu H, Cheng P, Chen P, et al. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater Sci. 2018;6(4):746–765.
- Tan X, Luo S, Wang D, et al. A NIR heptamethine dye with intrinsic cancer targeting, imaging and photosensitizing properties. Biomaterials. 2012;33(7):2230–2239.
- Ma Y, Zhang M, Li P, et al. Multifunctional small molecule fluorophore for long-duration tumor-targeted monitoring and dual modal phototherapy. Part Part Syst Charact. 2017;34(7):1700076.
- Cheng L, Wang C, Feng L, et al. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114(21):10869–10939.
- Song J, Yang X, Jacobson O, et al. Ultrasmall gold nanorod vesicles with enhanced tumor accumulation and fast excretion from the body for cancer therapy. Adv Mater Weinheim. 2015;27(33):4910–4917.
- Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 2010;5(4):523–528.
- Devarajan PV, Patravale VB. Nanomedicine-prospects and challenges. Drug Deliv Transl Res. 2013;3(5):381.
- Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97.
- Lee EH, Lim SJ, Lee MK. Chitosan-coated liposomes to stabilize and enhance transdermal delivery of indocyanine green for photodynamic therapy of melanoma. Carbohydr Polym. 2019;224:115143.
- Ignjatović NL, Sakač M, Kuzminac I, et al. Chitosan oligosaccharide lactate coated hydroxyapatite nanoparticles as a vehicle for the delivery of steroid drugs and the targeting of breast cancer cells. J Mater Chem B. 2018;6(43):6957–6968.
- Jadidi-Niaragh F, Atyabi F, Rastegari A, et al. CD73 specific siRNA loaded chitosan lactate nanoparticles potentiate the antitumor effect of a dendritic cell vaccine in 4T1 breast cancer bearing mice. J Control Release. 2017;246:46–59.
- Manivasagan P, Jun SW, Nguyen VT, et al. A multifunctional near-infrared laser-triggered drug delivery system using folic acid conjugated chitosan oligosaccharide encapsulated gold nanorods for targeted chemo-photothermal therapy. J Mater Chem B. 2019;7(24):3811–3825.
- Bharathiraja S, Bui NQ, Manivasagan P, et al. Multimodal tumor-homing chitosan oligosaccharide-coated biocompatible palladium nanoparticles for photo-based imaging and therapy. Sci Rep. 2018;8(1):500
- Manivasagan P, Bharathiraja S, Santha Moorthy M, et al. Biocompatible chitosan oligosaccharide modified gold nanorods as highly effective photothermal agents for ablation of breast cancer cells. Polymers. 2018;10(3):232.
- Manivasagan P, Hoang G, Santha Moorthy M, et al. Chitosan/fucoidan multilayer coating of gold nanorods as highly efficient near-infrared photothermal agents for cancer therapy. Carbohydr Polym. 2019;211:360–369.
- Choi HS, Nasr K, Alyabyev S, et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem Int Ed Engl. 2011;50(28):6258–6263.
- Hyun H, Bordo MW, Nasr K, et al. cGMP-Compatible preparative scale synthesis of near-infrared fluorophores. Contrast Media Mol Imaging. 2012;7(6):516–524.
- Choi HS, Gibbs SL, Lee JH, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol. 2013;31(2):148–153.
- Yoo Y, Jo G, Jung JS, et al. Multivalent sorbitol probes for near‐infrared photothermal cancer therapy. Part Part Syst Charact. 2020;37(2):1900490.
- Thavornpradit S, Usama SM, Park GK, et al. QuatCy: a heptamethine cyanine modification with improved characteristics. Theranostics. 2019;9(10):2856–2867.
- Lee S, Lim W, Jo D, et al. Near-infrared fluorescent sorbitol probe for tumor diagnosis in vivo. J Ind Eng Chem. 2018;64:80–84.
