?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study deals with facile and rapid synthesis of silver nanoparticles (AgNPs) and Gold nanoparticles (AuNPs) using Mentha longifolia leaves extracts (MLE). The synthesized AgNPs and AuNPs were characterized by UV-visible spectroscopy (UV-Vis), Fourier transformed infra-red spectroscopy (FT-IR), atomic force microscopy (AFM) and transmission electron microscopy (TEM) techniques. The phytochemical analysis showed the presence of bioactive secondary metabolites, which are involved in the synthesis of nanoparticles (NPs). The surface plasmon resonance (SPR) observed at 435 and 550 nm, confirmed the green synthesis of AgNPs and AuNPs, respectively. The TEM images showed poly dispersed and round oval shapes of Ag and Au NPs with an average particles size of 10.23 ± 2 nm and 13.45 ± 2 nm, respectively. TEM results are in close agreements with that of AFM analysis. The FT-IR spectroscopy revealed the presence of OH, –NH2 and C = O groups, which involved in the synthesis of NPs. The MLE and their AgNPs and AuNP exhibited good in vitro antibacterial and anti-oxidant activities. Moreover, MLE and NPs also showed in vivo analgesic activities in mice, and excellent sedative properties in open field test paradigm.
Introduction
Nanotechnology is considered as a significant tool to unveil the hidden secrets of nature, such as natural products coated metallic nanoparticles (MNPs) are nowadays considered for their extensive use in scientific arena to decode the natural enigmas. Green synthesis of MNPs has gained momentum in the last decade due to its cost-effectiveness. Moreover, it is environmentally friendly, and easily scalable for commercial purposes [Citation1–5]. Various types of molecules are used for the green synthesis of MNPs, among them natural products are considered as the best candidates, as they can be used effectively for reduction as well as stabilization of the MNPs [Citation6–8]. Moreover, natural products coated MNPs have significant medicinal and pharmaceutical applications [Citation9–13]. A large number of medicinal plants have been used for the synthesis of AgNPs and AuNPs, including leaves of Dodonaea genus [Citation14] Cinnamomum camphora, [Citation15] Aloe vera [Citation16] and Amla (Emblica officinalis) [Citation17]. Triangular synthesis of AuNPs has been performed using extract of Aloe vera plant [Citation16]. Magnolia kobus and Diopyros kaki leaf extract has also been used for successful synthesis of metallic AuNPs [Citation18]. Sun dried Cinnamomum camphora leaves [Citation15] and purified apiin compound from henna leaves [Citation19] are famous for photosynthetic synthesis of AuNPs. Vijayan et al. have reported green synthesis of AgNPs and AuNPs from Bauhinia purpurea and examined their potential against lung carcinoma cell line A549 in a dose-dependent manner with IC50 values of 27.97 µg/mL and 36.39 µg/mL, respectively. Synthesized NPs showed a significant increase in anticancer in contrast to plant extract [Citation20,Citation21]. Later Ghramh and co-worker used Euphorbia peplus leaves extract for MNPs synthesis and tested their anticancer activity against Hela and HepG2 cell lines, antimicrobial, haemolytic and insecticidal activities [Citation22]. Different fractions of Musa acuminata colla flower extract were used to synthesise AgNPs and AuNPs, and their antibactrial activity against spectrum beta-lactamase (ESBL) gene-producing bacteria and anticancer efficacy considerable after NPs formation [Citation23,Citation24]. AgNPs exhibit immense application in agriculture and medicine as antibacterial, antifungal and antioxidants [Citation21,Citation25].
In the current studies, MLE was used for the green synthesis of AgNPs and AuNPs [Citation26,Citation27]. Mentha longifolia is distributed in southern Africa, Botswana, Namibia, Zimbabwe [Citation28], Europe, Western and Central Asia. M. longifolia is a fast-growing perennial herb with a peppermint-scented fragrance. It has a creeping rhizome, with a stem 40–120 cm tall. The leaves vary from oblong-elliptical to soft, lanceolate (long and narrow with a sharp point) 5–10 cm long and 1.5–3 cm broad, and the colour varies from green to greyish-green above and white below. The purple and white colour flowers are produced at the tip of the branched, tapering and tall spikes in the form of cluster. Flowering starts from mid to late summer. It also forms colonies by scattering through rhizome [Citation29].
Mentha longifolia like other members of this family is used as a domestic herbal remedy, the whole plant is used as a medicine and sometimes rhizomes of the plant is also used [Citation30]. A tea made from the leaves has traditionally been used to treat of fevers, headaches, digestive disorders and various minor ailments [Citation31]. Moreover, the essential oil of this plant has numerous applications like anti-microbial activity, decongestant, anti-spasmodic effects, etc. The medicinal properties include diuretic effects [Citation32], carminative, stomachic, digestive health improver and anti-inflammatory agent in folk medicines [Citation33]. The essential oils and methanol extract of M. longifolia L. have reported for their antimicrobial and antioxidant properties [Citation34]. The essential oil of M. longifolia L. also possess calcium channel blocking activity [Citation35], hepatoprotective [Citation36] and insecticidal activities [Citation37]. Literature showed that two main classes, terpenoids and flavonoids, have been reported from M. longifolia [Citation38–40]. The antimicrobial activities for these two class have also been reported [Citation41]. Javed et al. synthesized AgNPs of M. longifolia to check their inhibitory potential against various bacterial strains [Citation42]. Here, we report the rapid green synthesis of AgNPs and AuNPs using M. longifolia leaves extracts and examined their in vitro and in vivo pharmacological activities and also study their analgesic activities in mice.
Material and methods
All solvents used were HPLC grade and purchased from Sigma-Aldrich. Silver nitrate (AgNO3) and gold chloride (H[AuCl4]) were purchased from Merck. Sodium hydroxide (NaOH), hydrochloric acid (HCl), and sodium citrate were purchased from Sigma Aldrich. Double-distilled water was used throughout the experiment. The pH effect was checked using a pH metre (Oakton, Eutech) equipped with glass working electrode.
Collection of plant material and extraction
Fresh leaves of M. longifolia were collected from different location of Barawal, District Upper Dir, KPK, Pakistan at attitude of 5000–7000 m (35° 00′ 45.27ʺN and 71° 50 40.14ʺE). Shade dried leaves of M. longifolia were ground with a grinder. The dried leaves (1kg) were then soaked in methanol for 5 days. The obtained extract was filtered and concentrated by using negative pressure in rotary evaporator at 40 °C to get a dark green extract (22.5 gm).
Phytochemical screening
The extracted plants crudes were analysed for the presence of secondary metabolites (alkaloids, tannins, anthraquinones, reducing sugars, glycosides, saponins, flavonoids, phlobatanins, steroids, terpenoids, coumarin, emodins, anthocyanin, betacyanins and carbohydrates) using standard procedures [Citation43,Citation44].
Preparation of stock and salt solutions
A 0.1 g MLE was dissolved in 100 ml of methanol to prepare a stock solution. Salt solutions having 1 mM concentration were separately prepared by using AgNO3 and H[AuCl4] in deionised water. Both salt solutions were then placed in the refrigerator.
Synthesis of AuNPs and AgNPs
Around 1 mM solution of AgNO3 and H[AuCl4] were prepared by dissolving 42.46 mg and 84.94 mg in 250 ml of double-distilled water, respectively. Similarly, 0.1 g methanolic extract of plants M. longifolia was dissolved in 100 ml of methanol and dilute the solution up to 500 ml by adding of water. The extract solution was filtered to remove undissolved extract component. Reaction was optimised using different metal to extract ratio. After mixing plant extract and metal at 70 °C at different ratios, reaction mixture was kept on stirring. Slight change in solution colour (greenish for AgNPs and reddish for AuNPs) was noticed at early moments (2–3 min) and reaction colour become more intense over constant stirring with the passage of time. Change in solution colour is primary indication of NPs formation. Later NPs syntheses was further confirmed via recording UV-Vis spectra of AuNPs and AgNPs. The ratio which gives best absorbance peak was used to synthesise NPs. The AgNPs and AuNPs were synthesized by mixing separately salt solution with a stock solution in 1:1 ratio at 70 °C; a colour change indicated the reduction process showing the synthesis of AgNPs and AuNPs. In contrast to previous studies, we synthesized AuNPs and AgNPs using M. longifolia plant extract without altering reaction pH. More importantly NPs syntheses were carried out at lower salt concentration in comparison to previous studies where higher concentration of silver salt was used. Synthesized NPs were centrifuged at 1500 rpm for 20 min, followed by washing with double-distilled water and vacuum-dried. Vacuum-dried NPs were used for further characterization, that is, TEM, AFM and FT-IR spectroscopy.
Characterization of AgNPs and AuNPs
The synthesized AgNPs and AuNPs were characterized by UV-Vis spectroscopy (Shimadzu UV-240, Hitachi U-3200, Japan), FT-IR (Prestige-21 Shimadzu, Japan), AFM (Agilent Technologies 5500, USA) Agilent 5500 operated in tapping mode and TEM (TEM-1400 Electron Microscope, JEOL), operated at an accelerating voltage of 110 kV. Few drops of AuNPs and AgNPs solution were placed on the carbon grid and allowed to dry prior to measurement.
Antioxidant activity
The antioxidant activity was performed by the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, through previously reported protocol [Citation45]. The electron donation abilities of the corresponding crude extracts, synthesized NPs and standards (n-Propyl gallate) were measured by changing the purple-coloured methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH). About 9.5 mg DPPH was dissolved in 50 ml methanol to make a solution. The stock solution of plant crude extract was prepared by dissolving 25 mg of the extract in 50 ml methanol, while NPs solution was prepared by adding 5 mg of NPs in 5 ml methanol. Different concentrations were made from a stock solution of crude extract and from NPs, that is, (10, 20, 40, 80, 100, 150, and 250 µg/mL) and were mixed with 1 ml of DPPH solution, while the control has only methanol and DPPH solution. The solutions were kept for 30 min in the dark then absorbance was recorded at 517 nm. The decrease in the absorbance of DPPH solution indicates increase in the antioxidant activity of samples. Antioxidant activity by DPPH as percent radical scavenging activities (% RSA) was calculated as follows.
Antibacterial activity
The MLE and synthesised NPs were checked for their antibacterial activities against various bacterial strains, that is, Klebsiella pneumoniae (ATCC: 13882) (K. pneumoniae), Staphylococcus aureus (ATCC: 25923) (S. aureus), Bacillus subtilis (ATCC: 21332) (B. subtilis), using the standard protocol [Citation46]. K. pneumoniae, a Gram-negative, while S. aureus and B. subtilis as Gram-positive bacteria were subjected to trials. These organisms were stored in Muller-Hinton agar (MHA) in the refrigerator at 4 °C. Modified agar well diffusion procedure was followed to study antibacterial activity of MLE and synthesised NPs. (MHA) agar was used as a growth medium. The cultures were cultivated and then incubated at 37 °C for 24 to 72 h, and taken as triplicate. Sterilized Petri-dishes were used for broth culture, 0.6 ml of the broth culture was added and Petri-dishes were sterilized first and then 20 ml sterilized molten MHA was added to each petri-dish. Wells were bored in the medium and 0.2 ml volume of crude extract was added to each well with the help of a micro pipette, while 2 mg/mL of synthesized NPs were used to examine their antibacterial ability. Streptomycin (2 mg/mL) was used as a standard drug. Petri-dishes were kept in a laminar flow hood for 1 h for proper diffusion, later plates were incubated at 37 °C for 24 h. Next day the zone of inhibition was measured [Citation47].
Animals
BALB/c mice (20–28 g) were used in all experiments purchased from the National Institute of Health (NIH), Islamabad. Pakistan. They were fed with standard laboratory food and water ad libitum. Animals were kept under the standard condition of temperature and light. Before the start of the experiment, animals were acclimatized with laboratory conditions. The rulings of the institutes of Laboratory Animal Resources, Commission on Life Sciences, National Research Council were maintained during all the experiments. The experimental protocols were approved by the ethical committee (5/pharm) of the Pharmacy Department, University of Peshawar, Peshawar, Pakistan.
Acetic acid induced writhing test
The peripheral nociceptive activity of crude extracts and synthesized NPs was investigated by acetic acid-induced abdominal constriction test [Citation48–50]. The pre-screened animals were segregated into various groups (n = 6) and each group contain six animals of weighing 18–22 g. The pain was induced by intraperitoneal injection of 0.9% acetic acid (v/v, 0.1 ml/10 g body weight). One group of six animal received normal saline (10 ml/kg, i.p.) as control, second group received diclofenac (10 mg/kg i.p.) as a standard (a mostly commonly used standard drug for the antinociceptive test). The remaining groups received extracts and synthesized NPs at the dose of 50 and 100 mg/kg, i.p., respectively. The number of muscular contractions was counted over a period of 20 min after acetic acid injection. The number of writhes in each treated group was compared with control (Saline treated group).
Sedative activity
The apparatus design for sedative potential consists of an area of white wood (150 cm diameter) enclosed by stainless steel walls and divided in 19 squares by black lines. The open field was placed in a light and sound-attenuated room. Balb/c mice of either sex (n = 6) weighing 26 ± 4 g were used in this study. Animals were acclimatized under red light (40 Watt red bulb) one hour before the commencement of the experiment in laboratory with food and water available ad libitum. Animals were administered with distilled water as a blank (10 ml/kg) MLE (50 and 100 mg/kg), AgNPs and AuNPs (5 and 10 mg/kg, respectively) or diazepam (0.5 mg/kg, i.p). After 30 min each animal was placed in the centre of the box and the number of lines crossed was counted for each mouse [Citation48,Citation50].
Statistical analysis
Results were expressed as mean ± SEM. One-way ANOVA was used to compare test of significant differences among groups, followed by Dunnet’s multiple comparison post-test. A level of significance (p<.05 or .01) was considered for each test. The results of antibacterial and antioxidant activities are also presented as mean ± SEM of three different readings and their statistical analysis was carried out using the GraphPad program (GraphPad, San Diego, CA, USA).
Results and discussion
The results of phytochemical screenings of MLE are presented in . The phytochemical analysis showed the presence of bioactive secondary metabolites. Results of MLE indicated the presence of tannins, reducing sugar, saponins, terpenoids, steroids, cardiac glycosides, coumarin, anthocyanin, carbohydrates and soluble starch.
TABLE 1. Effect of phytochemical tests of MLE.
Synthesis of NPs
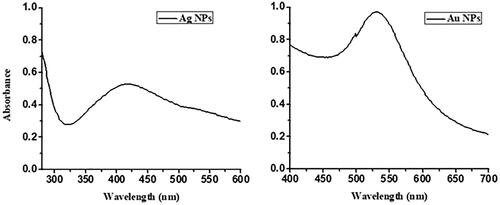
The colour change easily indicates the reduction process followed by synthesis of AgNPs and AuNPs for methanolic and aqueous extracts. The colour of MLE was changed from green to brown for AgNPs (), and green to pink while in case of AuNPs (), which indicates the formation of these MNPs [Citation51]. The UV-Visible spectra of AgNPs and AuNPs revealed their characteristic SPR at 435 nm and 550 nm, respectively as shown in [Citation51,Citation52]. The aqueous extract did not form any stable NPs at any ratio.
Figure 1. Image of (A) MLE have green colour due to the presence of UV active natural product, (B) Silver Solution are colourless due to the UV inactive ions (C) AgNPs having brown colour due to the surface plasmon resonance (D) Gold solution having no colour due the UV inactive ions of gold and (E) AuNPs having pink colour due to the surface plasmon resonance.

FT-IR analysis
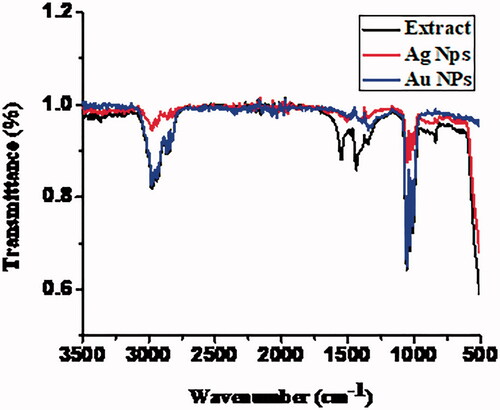
The plant contains a large number of chemical constituents such as flavonoids, tannins, alkaloids, monoterpenes and saponins, which plays important role in the synthesis of AgNPs and AuNPs. These compounds may actively involve in the reduction of Ag and Au ions to metallic silver and gold [Citation51]. The crude methanolic extract showed a broad peak at 3400 cm−1 that confirmed the presence of –OH and –NH2 group (flavonoids [Citation53], carbohydrates, terpenoids [Citation54], tannins [Citation53], saponins [Citation54,Citation55], reducing sugar [Citation56], cardiac glycosides [Citation56], steroids [Citation53], and coumarine) and a sharp peak at 2900 cm−1 corresponds to –NH2 stretching. A characteristic peak at 1700 cm−1 was observed by C = O stretching (carbonyl group of carbohydrates, glycosides, coumarine and tannins) [Citation53]. In case of AuNPs and AgNPs, the intensity of –OH peak further increased, while the peak at 3000 cm−1 completely disappeared, indicating that –OH and –NH2 were involved in the formation of NPs [Citation57]. The peak observed for C = O at 1700 cm−1 in the extract was also enhanced and red shift was noticed 1650 cm−1, which strongly supported the formation of Ag and Au NPs ). Phytochemical analysis indicated the presence of flavonoids, steroids, saponins, cardiac glycosides, tannins, alkaloids, terpenoids and anthraquinones. FT-IR analysis revealed the presence of phenolic flavonoids, saponins, tannins as major functional groups [Citation53]. Yassin et al. studied methanolic extract against cancer cells and confirmed the presence of above compound with certain functionality via GC-MS analyses [Citation58]. Therefore, our results are in close agreement with the findings of Yassin et al. FTIR spectral analysis of M. longifolia plant extract and silver and gold NPs are given in .
Table 2. FTIR spectral analysis of MLE, and AuNPs and AgNPs.
AFM imagining
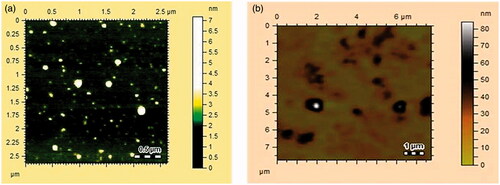
AFM used to examine the morphology, size and shape of the synthesised AgNPs and AuNPs [Citation51,Citation62]. AFM images of AgNPs and AuNPs are shown in . AgNPs and AuNPs were round oval in shape with the size ranges from 10–15 nm and 20–60 nm, respectively.
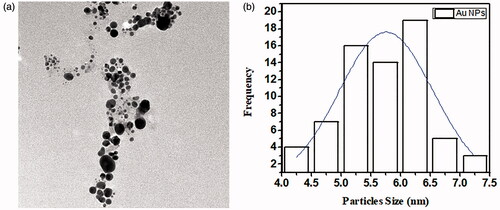
TEM analysis
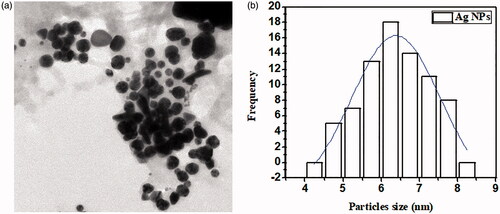
The size of AuNPs and AgNPs of M. longifolia was studied through transmission electron microscopy (TEM, TEM-1400 Electron Microscope, JEOL, Japan) [Citation11]. Average particles sizes of AgNPs and AuNPs were calculated using Image J software. As depicted in and , TEM showed that AuNPs and AgNPs were round oval in shape with an average particles size of 10.23 ± 2 and 13.45 ± 2 nm, respectively, which is in close agreement with AFM analysis.
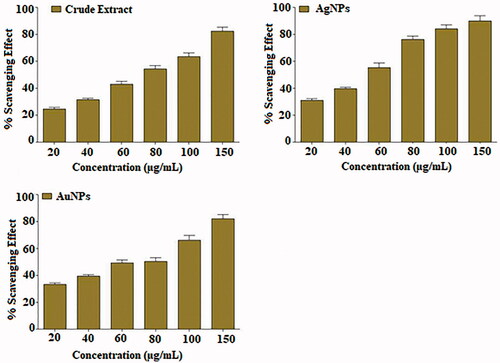
DPPH radical scavenging assay
Plant source antioxidants have the potential to reduce different diseases. Most of the antioxidant compounds in a typical diet are derived from plant sources caring wide range of physical and chemical properties [Citation63,Citation64]. DPPH is a stable free radical compound with ability to trap free radicals, and use to study the scavenging effect of plants with a characteristic absorption at 517 nm. The antioxidant profile of MLE and AuNPs and AgNPs are presented in which display the maximum antioxidant potential of crude extract and NPs at different concentration. The methanolic extract of the plant along with NPs exhibited varying degrees of scavenging abilities at dose depended manners (). Result showed that highest RSA was observed in AgNPs as compared to AuNPs at 80 µg/mL. The degree of discolouration indicates the scavenging potentials of the antioxidant present in MLE and both NPs.
Antibacterial assay
Antibacterial activity of MLE and their NPs are presented in in comparison with the standard drug, Streptomycin [Citation52]. MLE did not show any activity; however, their synthesized NPs exhibited some degree of antibacterial activity against human pathogenic microorganisms such as K. pneumoniae, S. aureus and B. subtilis bacterial strains. The zone of inhibition for NPs of MLE ranged from 10 to 12 mm. The MLE and their respective AuNP’s and AgNP’s did not show any potential activity against K. pn. While both AuNPs and AgNPs of the MLE showed good inhibition of S. aureus with zone of inhibition = 10 ± 0.01 and 12 ± 0.03 mm, respectively. Whereas, only AgNPs showed good inhibitory activity for B. subtilis with zone of inhibition 10 ± 0.01 mm.
Table 3. Antibacterial activity of MLE and AuNPs and AgNPs.
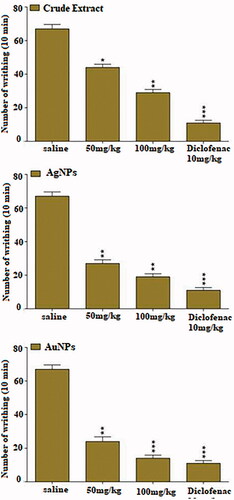
Effect in acetic acid-induced writhing test
The acetic acid-induced writhing test is one of the primary tools used for characterization of analgesic potential of test compounds. Injection of acetic acid caused the release of various endogenous pain producing mediators such as bradykinin, serotonin, histamine, substance P. The local peritoneal receptor cause abdominal pain (writhing) which is symbolised by contraction of the abdominal muscle accompanied by an extension of the forelimbs and body elongation. The antinociceptive effect of MLE and both NPs are presented in . Pre-treatment of MLE (*p<.05, **p<.01) and AgNPs (**p<.01) and AuNPs (**p<.01, ***p<.001) evoked dose-dependent antinociceptive effect in acetic acid-induced writhing test. The overall effect of both NPs was better than the corresponding extract, while less than standard drug, diclofenac. However, the antinociceptive potential of AuNPs (100 mg/kg i.p.) was comparable with standard drug (10 mg/kg i.p). The unwanted effects of synthetic analgesic drugs are already been reported [Citation49,Citation50]. The current antinociceptive findings, therefore, suggested the AuNPs as an effective alternative to synthetic drugs. The pain-relieving property of a compound or extract is related to lot of receptors/enzymes including cyclo-oxygenase (COX-I and COX-II), opioids receptors and GABA ionic channel receptors. These receptors and enzymes are widely distributed in the body including central nervous system.
Figure 8. Effect of intraperitoneal administration of AuNPs and AgNPs in acetic acid induced test. Values are expressed as mean ± SEM *p<.05, **p<.01, ***p<.001 as compared to control. n = 6.
The MLE and synthesized NPs were also evaluated for their sedative potential. The results of open field, that is, sedative effect is presented in . Diazepam, a reference drug, exhibited maximum sedation, which was significantly (p<.001) different from saline control group. The methanolic crude extract exhibited mild sedative effect (p < .05) at the doses of 50 and 100 mg/kg, as compared to diazepam. AgNPs and AuNPs also exhibited significant (p<.01) sedative effect in doses of 5 and 10 mg/kg as compared to MLE and standard drug (diazepam). Diazepam showed superior sedative effects then NPs and MLE (). The sedative effect may be due to increase action of GABA binding with GABA channels. GABA channels stimulates the cell by keeping them polarised and decrease the release of neurotransmitter including (substance P), therefore, acts as potential pain desensitisers [Citation65]. It is hypothesized that MLE and NPs may follow similar interaction, thus act as painkiller and sedative.
Table 4. Sedative effect of MLE and NPs in open field test (Locomotive activity).
Conclusion
Current study present synthesis and characterization of AuNPs and AgNPs of MLE by using advance techniques, such as UV, FT-IR, AFM and TEM. The phytochemical analysis reflects the presence of several bioactive secondary metabolites. The synthesis of AgNPs and AuNPs was confirmed via UV-Vis spectroscopy with SPR at 300–500 and 500–700 nm, respectively. The FT-IR spectra showed the presence of chemical constituents like flavonoids, tannins, alkaloids, monoterpenes and saponins, which play a key role in the synthesis of AgNPs and AuNPs. The size and shapes of both NPs were confirmed by AFM and TEM techniques, which showed poly dispersed and spherical shapes of AgNPs and AuNPs of 10.23 ± 2 nm and 13.45 ± 2 nm, respectively. As compared to MLE, the NPs significantly increase the antibacterial, antioxidant, antinociceptive, analgesic and sedative activities.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research group Project under grant number [R.G.P. 2/26/42].
Disclosure statement
No potential conflict of interest was reported by the author(s).s
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Rajeshkumar S, Kannan C, Annadurai G. Synthesis and characterization of antimicrobial silver nanoparticles using marine brown seaweed Padina tetrastromatica. Drug Invent. 2012;4(10):511–513.
- Ahmed S, Ahmad M, Swami BL, et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1)17-28.
- Bar H, Bhui DK, Sahoo GP, et al. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colliod Surf. A. 2009;339(1–3):134–139.
- Bachheti RK, Fikadu A, Bachheti A, et al. Biogenic fabrication of nanomaterials from flower-based chemical compounds, characterization and their various applications: a review. Saudi J Biol Sci. 2020;27(10):2551–2562.
- Siddiqi KS, Husen A. Current status of plant metabolite-based fabrication of copper/copper oxide nanoparticles and their applications: a review. Biomater Res. 2020;24:11.
- Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13(10):2638–2650.
- Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotechnol. 2009;29(4):279–306.
- Das J, Das MP, Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta A Mol Biomol Spectrosc. 2013;104:265–270.
- Mallikarjuna K, Narasimha G, Dillip GR, et al. Green synthesis of silver nanoparticles using Ocimum leaf extract and their characterization. Digest J Nano and Biostruct. 2011;6(1):181–186.
- Isaac RS, Sakthivel G, Murthy CH. Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J Nanotech. 2013;2013:1–6.
- Islam NU, Jalil K, Shahid M, et al. Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arabian J Chem. 2019;12(8):2310–2319.
- Kumar PPNV, Pammi SVN, Kollu P, et al. Green synthesis and characterization of silver nanoparticles using Boerhaavia diffusa plant extract and their anti bacterial activity. Indus Crops Prod. 2014;52:562–566.
- Ali K, Dwivedi S, Azam A, et al. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J Colloid Interface Sci. 2016;472:145–156.
- Beshah F, Hunde Y, Getachew M, et al. Ethnopharmacological, phytochemistry and other potential applications of Dodonaea genus: A comprehensive review. Curr Biotechnol. 2020;2:103–119.
- Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18(10):105104.
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583.
- Ankamwar B, Damle C, Ahmad A, et al. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 2005;5(10):1665–1671.
- Song JY, Jang H-K, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009;44(10):1133–1138.
- Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces. 2009;68(1):55–60.
- Vijayan R, Joseph S, Mathew B. Anticancer, antimicrobial, antioxidant, and catalytic activities of green-synthesized silver and gold nanoparticles using Bauhinia purpurea leaf extract. Bioprocess Biosyst Eng. 2019;42(2):305–319.
- Ali K, Saquib Q, Ahmed B, et al. Bio-functionalized CuO nanoparticles induced apoptotic activities in human breast carcinoma cells and toxicity against Aspergillus flavus: an in vitro approach. J Photochem Photobiol Biol. 2020;91:387–397.
- Ghramh HA, Khan KA, Ibrahim EH. Biological activities of Euphorbia peplus leaves ethanolic extract and the extract fabricated gold nanoparticles (AuNPs). Molecules. 2019;24(7):1431.
- Valsalam S, Agastian P, Esmail GA, et al. Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J Photochem Photobiol B Biol. 2019;201:111670.
- Ali K, Ahmed B, Ansari SM, Saquib Q, et al. Comparative in situ ROS mediated killing of bacteria with bulk analogue, Eucalyptus leaf extract (ELE)-capped and bare surface copper oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;100:747–758.
- Siddiqi KS, Husen A, Rao RAK. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnology. 2018;16(1):14.
- Bunsawat J, Elliott NE, Hertweck KL, et al. Phylogenetics of Mentha (Lamiaceae): evidence from chloroplast DNA sequences. Syst Bot. 2004;29(4):959–964.
- Harley RM, Atkins S, Budantsev AL, et al. Labiatae. The families and genera of vascular plants, VII, flowering plants, dicotyledons, lamiales, except acanthaceae including avicenniaceae. Berlin Heidelberg: Springer; 2004. pp 167–275.
- Codd LEW: Lamiaceae: Flora of Southern Africa.Botanical Research Institute. Pretoria. 1985;28(4):1–247.
- Brickell C, Cole T, Cathey HM. The American Horticultural Society encyclopedia of plants and flowers (American Horticultural Society Practical Guides). New York, NY, USA: DK Publishing; 2002.
- Van Wyk B-E, Oudtshoorn B, Gericke N. Medicinal plants of South Africa. Pretoria, South Africa: Briza; 1997.
- Foster S, Duke JA. A field guide to medicinal plants and herbs of eastern and central North America. Vol. 2. New York: Houghton Mifflin Harcourt; 2000.
- Oyedeji OA, Afolayan AJ, Eloff JN. Comparative study of the essential oil composition and antimicrobial activity of Leonotis leonurus and L. ocymifolia in the Eastern Cape. South Africa S Afr J Bot. 2005;71(1):114–116.
- Amin GR. Popular medicinal plants of Iran. Vol. 1. Tehran: Iranian Research Institute of Medicinal Plants; 1991.
- Gulluce M, Sahin F, Sokmen M, et al. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103(4):1449–1456.
- Shah AJ, Bhulani NN, Khan SH, et al. Calcium channel blocking activity of Mentha longifolia L. explains its medicinal use in diarrhoea and gut spasm. Phytother Res. 2010;24(9):1392–1397.
- Mimica-Dukic N, Popovic M, Jakovljevic V, et al. Pharmacological studies of Mentha longifolia phenolic extracts. II. Hepatoprotective activity. Pharm. Bio. 1999;37(3):221–224.
- Odeyemi OO, Masika P, Afolayan AJ. Insecticidal activities of essential oil from the leaves of Mentha longifolia L. subsp. capensis against Sitophilus zeamais (Motschulsky)(Coleoptera: Curculionidae). Afr Entomol. 2008;16(2):220–225.
- Nagell A, Hefendehl FW, Hoyer J. Two Stereoisomeric 1, 2-Epoxymenthylacetates from an Oil of Mentha rotundifolia x Mentha longifolia. Zeitschrift Fur Naturforschung Section C Biosci. 1974;29(5-6):294–295.
- Ali MS, Saleem M, Ahmad W, et al. A chlorinated monoterpene ketone, acylated Î2-sitosterol glycosides and a flavanone glycoside from Mentha longifolia (Lamiaceae). Phytochemistry. 2002;59(8):889–895.
- Idrissi AI, Fkih-Tetouani S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia. 2001;72(5):596–598.
- Al-Bayati FA. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Ann Clin Microbiol Antimicrob. 2009;8(1):20.
- Javed B, Nadhman A, Mashwani ZUR. Optimization, characterization and antimicrobial activity of silver nanoparticles against plant bacterial pathogens phyto-synthesized by Mentha longifolia. Mater Res Express. 2020;7(8):085406.
- Uddin G, Rauf A, Siddiqui BS, et al. Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Mid J Sci Res. 2011;10(1):78–81.
- Uddin G, Rauf A, Qaisar M, et al. Preliminary phytochemical screening and antimicrobial activity of Hedera helix. Mid J Sci Res. 2011;8(1):198–202.
- Uddin G, Rauf A, Arfan M, et al. Preliminary phytochemical screening and antioxidant activity of Bergenia caliata. Mid J Sci Res. 2012;11(8):1140–1142.
- Heatley N. A method for the assay of penicillin. Biochem J. 1944;38(1):61–65.
- Owuama CI. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr J Microbiol Res. 2017;11(23):977–980.
- Rauf A, Khan R, Khan H, et al. Antipyretic and antinociceptive potential of extract/fractions of Potentilla and its isolated compound, acacetin. BMC Compl Alternative Med. 2014;14(1):448.
- Rauf A, Uddin G, Siddiqui BS, et al. In-vivo antinociceptive, anti-inflammatory and antipyretic activity of pistagremic acid isolated from Pistacia integerrima. Phytomedicine. 2014;21(12):1509–1515.
- Koster R. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–417.
- Islam NU, Jalil K, Shahid M, Rauf A, et al. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab J Chem. 2019;12(8):2914–2925.
- Nisar M, Khan SA, Shah MR, et al. Moxifloxacin-capped noble metal nanoparticles as potential urease inhibitors. New J Chem. 2015;39(10):8080–8086.
- Zahoor MK, Zahoor MA, Mubarik MS, et al. Insecticidal, biological and biochemical response of Musca domestica (Diptera: Muscidae) to some indigenous weed plant extracts. Saudi J Biol Sci. 2020;27(1):106–116.
- Kareru P, Keriko J, Gachanja A, et al. Direct detection of triterpenoid saponins in medicinal plants. Afr J Tradit Complement Altern Med. 2008;5(1):56–60.
- Almutairi MS, Ali M. Direct detection of saponins in crude extracts of soapnuts by FTIR. Nat Prod Res. 2015;29(13):1271–1275.
- Kumaravel S, Muthukumaran P, Phytochemical TN, et al. Analysis of Papaver somniferum L. J Pharm Biol Sci. 2019;7(1):1–8.
- Ali K, Ahmed B, Khan MS, et al. Differential surface contact killing of pristine and low EPS Pseudomonas aeruginosa with Aloevera capped hematite (α-Fe2O3) nanoparticles. J Photochem Photobiol Biol. 2018;188:146–158
- Yassin MT, Mostafa AA, Al-Askar AA. Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J King Saud Univ Sci. 2020;32(3):2046–2052.
- Salavati-Niasari M, Davar F, Mahmoudi TJP. A simple route to synthesize nanocrystalline nickel ferrite (NiFe2O4) in the presence of octanoic acid as a surfactant. Polyhedron. 2009;28(8):1455–1458
- Dovbeshko GI, Gridina NY, Kruglova EB, et al. FTIR spectroscopy studies of nucleic acid damage. Talanta. 2000;53(1):233–246.
- Depciuch J, Kaznowska E, Szmuc K, et al. Comparing paraffined and deparaffinized breast cancer tissue samples and an analysis of Raman spectroscopy and infrared methods. Infrared Phys Technol. 2016;76:217–226.
- Ateeq M, Shah MR, Ali H, et al. Hepatoprotective and urease inhibitory activities of garlic conjugated gold nanoparticles. New J Chem. 2015;39(6):5003–5007.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270–278.
- Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–126.
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6(7):565–575.