?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cervical cancer is the most important female genital cancer that develops from the cervix, a lower part of uterus. Houttuynia cordata is ubiquitously present in Asian countries, and traditionally prescribed to treat infections and oedema. Our study emphasizes on biological synthesis route for developing copper nanocomplex using Houttuynia cordata (Hc-CuONPs) plant extract. The UV–visible spectroscopy study of Hc-CuONPs revealed the maximum peak at 350 nm, which proved the formation of Hc-CuONPs and FT-IR absorption peaks revealed the existence of different functional groups. The results of high-resolution TEM and X-ray diffraction studies revealed that the Hc-CuONPs have face centred cubic structure along with 40–45 nm in size. The temperature conditions of the synthesized Hc-CuONPs were spherical and circular morphologies. Furthermore, the Hc-CuONPs (IC50=5 µg/ml) exhibited toxicity on cervical cancer cells (HeLa). The intracellular reactive oxygen species (ROS) level in the control and Hc-CuONPs-treated HeLa cells was monitored by DCFH-DA staining and the apoptotic cell death was detected by using the dual (AO/EtBr) staining, propidium iodide and DAPI staining assays. Our results from the fluorescent staining analysis evidenced that the Hc-CuONPs have inhibited the cell proliferation and promoted the apoptotic cell death in HeLa cells. The Hc-CuONPs promoted the apoptosis by targeting the PI3K/Akt signalling pathways in HeLa cells. Our results explored that the Hc-CuONPs are effective against in vitro HeLa cancer cells.
Introduction
Cervical cancer is the female genital cancer that forms underneath portion of the uterus that links to the vagina [Citation1], cancer that occurs in cervical cells. In year 2018, worldwide cancer incidence and mortality data estimates show nearly 570,000 cases with cervical cancer and 311,000 deaths at particular year. It is the fourth most occurring cancer among women. Cervical cancer considered as a serious health issue affected mid-aged women, peculiarly in low economic countries [Citation2]. The human papillomavirus types are the root cause for the infection which leads to the formation of cervical cancer. Currently, HPV16 and HPV18 types vaccines protect against the deadly disease cervical cancer in womens because these two strains are the causative agent of 75% of cervical cancer [Citation3]. Currently, radiotherapy and chemotherapy are applied in the interventions of cancers early stage. However, there are several side effects which are deleterious to the patients [Citation4] and cancer relapse was also prominent.
Hence, the current study is focussed on treatment with lesser side effect. Outcome of the patients with cervical cancer is a key concern along with finding a safer treatment for cervical cancer with lesser or no side effects is more important. Usage of nanoparticles in cancer therapy either as drug or drug delivery agent favours through their structure, dimension, distribution and various other property of nanoparticles [Citation5]. The extensive uses of inorganic metal in cancer therapy are attributed to their various biological properties which are not available in organic compounds [Citation6]. Among various other compounds, copper shows a greatest interest due to its stability and cost effectiveness [Citation7]. Metallic nanoparticles received great interests in numerous fields due to its minute size, huge surface area, optical and chemical properties and good conductivity [Citation8]. The copper nanoparticles possessed peculiar antimicrobial, antioxidant and anticancer activities [Citation9,Citation10].
Houttuynia cordata is a perennial herb belongs to the family of Saururaceae is an indigenous species of Asia, Japan and China. It is an inhabitant of moist environment and utilized as both edible food and also as a medicine for various ailments [Citation11]. H. cordata proven to possess various pharmacological properties such as antidiabetic, antioxidant, antiinflammatory, antihypertension, adjuvanticity and antiviral activities [Citation12]. It is also prescribed to respiratory diseases such as pneumonia, severe acute respiratory syndrome, etc. [Citation13–15]. Nanotechnology is a boon for various fields especially in the field of medicine; it plays a remarkable role. Hence, researchers were focussed on utilizing nanotechnology to formulate a drug with traditional herbal medicine since nanodrugs are more cite targeted and it also increases the efficacy of traditional medicine, Therefore in the current study, we formulated a copper nanodrug with the plant extract of H. cordata (Hc-CuONPs) and assessed its efficacy against cervical cancer cell line.
Materials and methods
Chemicals
The analytical grade of copper sulphate (CuSO4·5H2O), acridine orange (AO), ethidium bromide (EtBr), DAPI and MTT were procured from Sigma-Aldrich (St. Louis, MO, USA) and Hi-Media Labs (West Chester, PA, USA), respectively.
Sample collection, preparation of extract and synthesis of CuONPs using H. cordata extract
The complete plant sample of H. cordata was recognized and obtained from the region of China. The species of the plant was classified and approved by the botanist (specimen number: 43/2019). The complete plant materials were thoroughly cleaned with the running tap water to elicit the unwanted particles. The plant sample from the plant should be dried in shaded place for 2 weeks. The obtained dried plant content was grinded into a superfine course powder using electronic mixer. Weighed about 25 g of powdered sample from plant was combined with Millipore water of about 100 ml in a conical flask with rounded bottom. The sample contents were tended to boil at 60 °C using electric oven with temperature controller. Then, the samples were uniformly mixed by the magnetic stirrer in the electric oven for the 45 min. The samples were cooled at room temperature and the mixture of sample contents was centrifuged at 5000 rpm for 15 min. The Whatman filter paper was used to obtain the crude extract of Houttuynia cordata from the sample contents. Ten millilitres of crude extract was mixed with the 50 ml of 3 µM CuSO4·5H2O (Sigma-Aldrich, St. Louis, MO, USA) and placed in shaker incubator at 45 ± 2 °C. The blue colour copper salt in sample which tends to form dark green colour solution proves the synthesis of CuONPs. The CuONPs containing extract were centrifuged thrice at 10,000 rpm for 20 min. The clear supernatant was discarded repeatedly. The collected Hc-CuONPs were heated and dried in the oven at 50 ± 2 °C and the Hc-CuONPs was used to study the anticancer effects against the cervical cancer cell lines.
Characterization of synthesized Hc-CuONPs
The highest wavelength values of prepared Hc-CuONPs were analysed by Agilent Cary UV/Vis System (Agilent Technologies, Santa Clara, CA, USA) between the range of 300–700 nm. The Houttuynia cordata extract was light in colour. The deep green colour was developed after mixing with the copper sulphate solution. This confirms the formation of the Hc-CuONPs. The highest wavelength intensity of Hc-CuONPs was captured after completion of the reaction. This confirms the stability and integrity of Hc-CuONPs. FT-IR analysis was used to explore the chemical bonds and functional groups in the extract as well as Hc-CuONPs. The Hc-CuONPs size and shape were captured with the HR-TEM. The morphological structure and functional group of green synthesized Hc-CuONPs were validated by XRD and FT-IR.
Cell culture
HeLa cervical cancer cell line and normal fibroblast L929 cells were procured from ATCC (Manassas, VA, USA). Later, the cells were trypsinized and cultured in with DMEM medium with 10% of FBS at 37 C and 5% CO2. Upon obtaining 80% confluency, the cells were trypsinized and subcultured for further experiments.
MTT assay
Cell viability and proliferation of Hc-CuONPs treated normal murine fibroblast (L929) cells and human cervical cancer (HeLa) cells were analysed using MTT assay. The test was performed to measure cell viability in control and Hc-CuONPs treated cells. It is also used to observe the cancer cell proliferation and activation in culture medium. The transmittance value was measured at 595 nm utilizing UV-spectrophotometer. The viability of L929 and HeLa cancer cell line was investigated by applying the formula:
Apoptosis and structural alterations in the cells
The different staining techniques were followed to evaluate the apoptosis mechanism in the cancer cells. Some of the fundamental features of these cells may include shrinking of cell plasma, chromatin shortening and cytoplasm condensation, cell-cell detachment, nucleus fragmentation and membrane aberration. The HeLa cell line was treated with Hc-CuONPs at two different concentrations (5 and 7.5 µg). The cells were analysed utilizing inverted microscope with ×40 magnification (Nikon, Tokyo, Japan).
Reactive oxygen species
To measure reactive oxygen species (ROS) induction in HeLa cell line, the cells were treated with 5 and 7.5 µg of Hc-CuONPs for 24 h under controlled condition. After incubation, the cells were washed with newly prepared phosphate buffer saline and resuspended in plain culture medium (no serum) containing 10 µM DCFH-DA. Subsequently, ROS production was detected by fluorescence microscope.
Ethidium bromide and acridine orange staining
The combination of EtBr/AO staining helps to identify the difference between dormant and active cells in cell culture. Thus, the apoptotic cells having a vast portion of denatured DNA, exhibit extreme red fluorescence and diminished green emission in contrast with active cells.
DAPI staining
The apoptosis was also extensively studied by the ensuing DAPI staining technique in cancer cell lines. The dye gives rise to fluorescent colours having ds-DNA. In this case, the nuclei of the apoptotic cells are deeply stained and also fragmented with shortened chromatin.
Propidium iodide
The HeLa cells were treated with 5 and 7.5 µg of Hc-CuONPs for 24 h. After incubation, the cells were washed with newly prepared PBS, and resuspended in plain culture medium (no serum) containing propidium iodide (Pi). The stained cells were detected with fluorescence microscope.
Quantitative real-time PCR
The HeLa cancer cells treated with Hc-CuONPs were analysed for mRNA expression for apoptotic and anti-apoptotic genes and the PI3K/Akt/mTOR signalling proteins. The control cells and treated cells were used to isolate the RNA using commercially available RNAEasy kit (Qiagen, Hilden, Germany). The isolation steps were done as per the manufacturer’s protocol. The quality and concentration of RNA were measured and assessed by using Nanodrop spectrophotometer. For PCR reaction, the Sybr green PCR kit was used to analyse the apoptotic and anti-apoptotic genes and the PI3K/Akt/mTOR signalling proteins. The relative intensity of targeted genes data was obtained and analysed. The primer sequences for the targeted genes are obtained from PUBMED sequence with reference to previous literature (). The mRNA expressions of target markers were standardized to the housekeeping gene GAPDH, and the relative expressions were expressed using 2−ΔΔCT formula.
Table 1. The list of primers used in the study.
Statistical analysis
All the experiments were performed in triplicates and the results were assessed using statistical software GraphPad Prism (La Jolla, CA, USA). The data were subjected to one-way ANOVA followed by post hoc Dunnett’s test. p≥.05 was considered to be statistically significant and the values were expressed as mean ± SD.
Results
Assessment of Hc-CuONPs by UV method
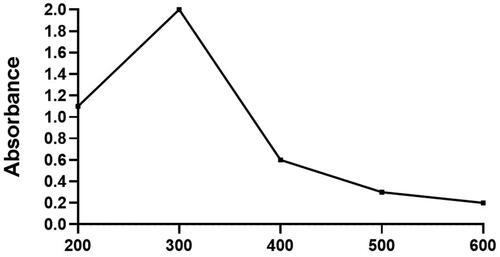
shows that the Houttuynia cordata plant extract was in light colour initially before it reacts with the copper nanoparticles. Then the extract colour was turned into deep green after the reaction with added copper nanoparticles. illustrates that the Hc-CuONPs synthesized with H. cordata extract which produced a sharp constant peak at 350 nm proves the production of Hc-CuONPs and its stability.
Analysis of Hc-CuONPs using FTIR
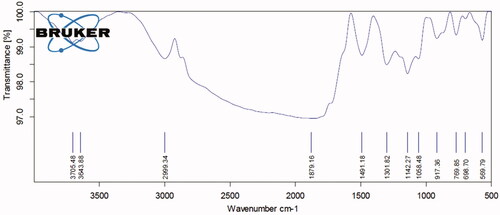
The results of Hc-CuONPs FTIR analysis are depicted in . The spectra at FTIR absorption peaks seen at 3705.48, 3643.88, 2999.34, 1879.16, 1491.18, 1301.82, 1142.27, 1058.48, 917.36, 769.85, 698.70 and 569.79 cm−1 represent chemical constituents such as alcohols, amines, aromatic amino acids and ethers. The binding capacity of the both functional groups with copper nanoparticles indicates the presence of alkaloids, terpenoids, alcohols, ketones, aldehyde and carboxylic acids.
Analysis of Houttuynia cordata extract by using FTIR
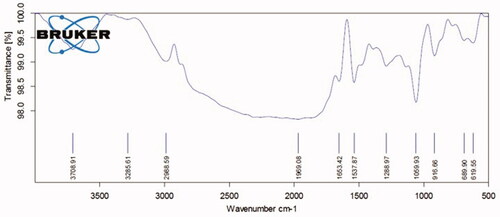
shows the results of FTIR analysis of crude extract of Houttuynia cordata. The spectrum at FTIR absorption peaks is noted at 3708.91, 3285.61, 2968.59, 1969.08, 1653.42, 1537.87, 1288.97, 1056.93, 916.66, 689.90 and 619.55 cm−1 that demonstrate the existence of different functional molecules like amines, amino acids and ethers. This outcome relates with the FTIR analysis results of synthesized Hc-CuONPs, which exhibit the presence of functional groups with copper, alkaloids, terpenoids, ketones and carboxylic acids ().
Analysis of Hc-CuONPs using HR-TEM
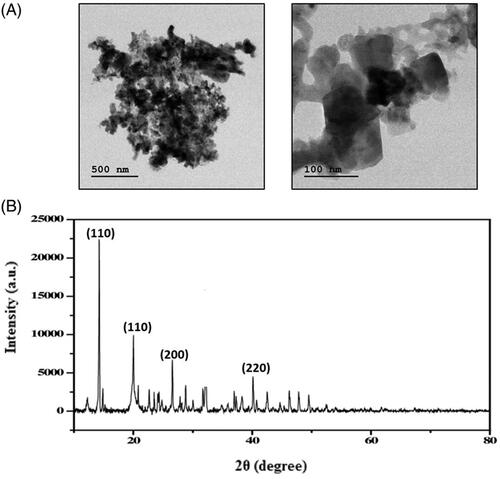
The size and morphology of the synthesized Hc-CuONPs were validated by the TEM. The image from TEM confirmed that the synthesized Hc-CuONPs diameter was about 40–45 nm (). The observed images proved that the synthesized Hc-CuONPs structure was in spherical in shape and crystalline in nature.
Analysis of Hc-CuONPs using XRD analysis
The XRD analysis was carried out to confirm the crystallinity and purity of the synthesized Hc-CuONPs. The outcome of XRD analysis supports and correlates to the TEM data. The synthesized Hc-CuONPs diffraction peaks located at 2θ angles: 41.69, 50.09 and 74.34 corresponded to (111), (200) and (220) which indicate face centred cubic structure (). The high and sharp intensity peaks confirmed the high purity of Hc-CuONPs. Based on the values of full width at half maximum (FWHM), the size of the CuONPs was calculated as 40–45 nm.
In vitro cytotoxic effects of Hc-CuONPs
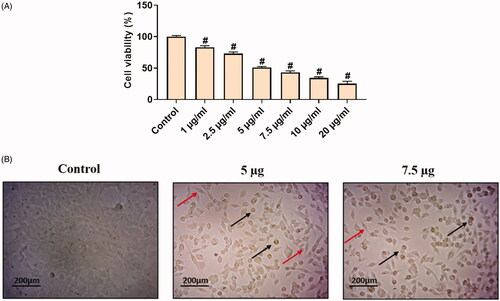
The in vitro cytotoxicity assays are best tools used so far to predict the toxicity effects of sample anticancer drugs on live cells (cell lines). The MTT assay was performed to detect the cytotoxic effects of synthesized Hc-CuONPs against the normal L929 on cervical cancer HeLa cell lines. As such, the results of the Hc-CuONPs were tested at various concentrations ranging from 1, 2.5, 5, 7.5, 10 and 20 µg/ml. The CuONPs treatment did not show any toxicity to the normal fibroblast L929 cells (). However, Hc-CuONPs showed 50% activity at 5 µg/ml concentration against HeLa cell line (). The occurrence of cell toxicity might be due to incitement ROS by copper nanoparticles and by their action on cellular components which prompts apoptosis. The IC50 value of Hc-CuONPs on HeLa cells was 5 µg/ml.
Figure 5. In vitro cytotoxic effects of Hc-CuONPs in normal L929 cells. L929 cells were treated with different concentrations of Hc-CuONPs (1–20 µg/ml) for 24 h and viability was analysed using MTT assay. Viability was calculated as a percentage of decrease or increase over the control. Data are expressed as mean ± SD triplicate measurement.
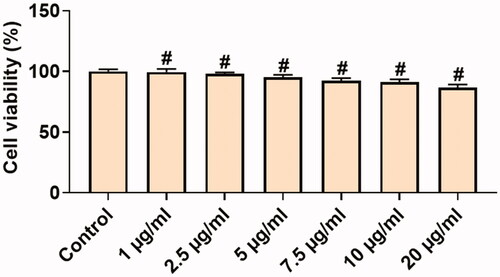
Figure 6. In vitro cytotoxic effects of Hc-CuONPs in cervical cancer HeLa cells. HeLa cells were treated with different concentrations of Hc-CuONPs (1–20 µg/ml) for 24 h and viability was analysed using MTT assay. Viability was calculated as a percentage of decrease or increase over the control. Data are expressed as mean ± SD triplicate measurement. The black arrows indicate the dead cells and the red arrows indicate the viable cells.
Morphological and nuclear changes of apoptosis
Cell morphology
Morphological changes of HeLa cells are visualized in . The cell was treated with Hc-CuONPs at different concentrations and observed under microscope. The results obtained were the majority of the Hc-CuONPs-treated HeLa cells changed from spindle to star-shaped, some of which become damaged and shrink morphology (). These morphological changes are typical of apoptosis and to confirm apoptosis further the study was carried to following methods.
Analysis of ROS by DCFH-DA staining method
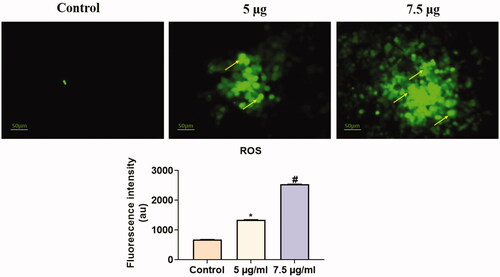
The Hc-CuONPs induced apoptosis mediated by ROS generation. HeLa cells were treated with Hc-CuONPs at 5 and 7.5 µg dramatically decreased the cell viability (). Collectively, these results indicated that Hc-CuONPs exerted anti-cervical cancer properties through ROS-mediated apoptosis.
Figure 7. Analysis of ROS by DCFH-DA staining method. HeLa cells were treated with different concentrations of Hc-CuONPs (5 and 7.5 µg) and then stained with DCFH-DA solution. ROS production was evaluated by fluorescence microscopy. Control cells possessed lower green fluorescence, Hc-CuONPs (5 and 7.5 µg) possessed bright and visible green fluorescence in HeLa cells (yellow arrows).
Detection of live and dead cell population by AO/EtBr double staining
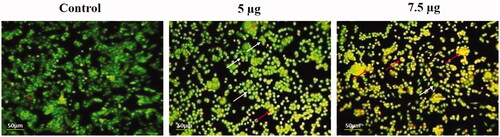
The dyes counterstained the ds-DNA located in the nucleus of the dormant cells. Acridine orange was utilized by active and dormant cells while EtBr was incorporated only in non-viable cells which discharges red fluorescence due to their interaction with DNA. As such, the results of AO/EtBr staining declared that the Hc-CuONPs induced more cell death (apoptosis) in HeLa cells (). The active cells emit green and dead cells emit red colour. Thus, the cell death induced by Hc-CuONPs was mediated via apoptosis. The nuclear alterations such as chromatin condensation and nuclear disintegration were seen extensively in the Hc-CuONPs treated cells. These findings were also confirmed by various other staining techniques which discriminate normal and apoptotic cells.
Figure 8. Detection of live and dead cell population by AO/EtBr double staining. HeLa cells were treated with different concentrations of Hc-CuONPs (5 and 7.5 µg) and then stained with AO/EtBr solution. Apoptotic cell death was evaluated by fluorescence microscopy. Viable cells show uniformly green fluorescence (white arrows). Hc-CuONPs treated cells shows with chromatin condensation, indicating early and late apoptosis with uniformly in bright yellow (red arrows).
Detection of nuclear changes by DAPI staining
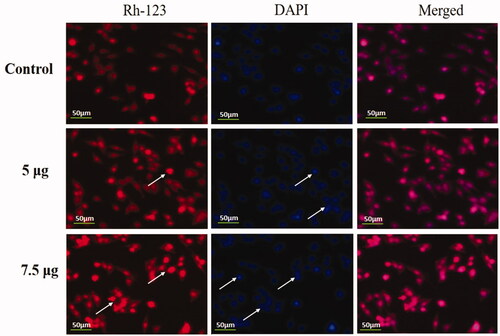
The apoptotic role of Hc-CuONPs at the concentration 5 and 7.5 µg was studied using DAPI along with Rh-123, which is used extensively in fluorescence microscopy to study apoptosis. Our findings proved that the copper nanoparticles treatment increased the count of apoptotic cells in HeLa cell lines compared with untreated control cells ().
Figure 9. Detection of nuclear changes by Rh-123 and DAPI staining. HeLa cells were treated with different concentrations of Hc-CuONPs (5 and 7.5 µg) and then stained with Rh-123 and DAPI stains, respectively. Control cells show normal mitochondrial matrix and viable cells. Hc-CuONPs treated cells possess the decreased mitochondrial membrane potential and apoptotic cell deaths in the HeLa cells (white arrows).
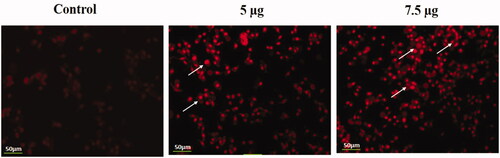
Identification of apoptotic cells by propidium iodide staining
The fluorescence staining of the cervical cancer cells by using Pi demonstrated the condensation of chromatin, blebbing of the membrane and apoptotic cells which clearly exhibited the apoptotic properties of the synthesized Hc-CuONPs at the dose of 5 and 7.5 μg/ml ().
Figure 10. Identification of apoptotic cells by propidium iodide staining. HeLa cells were treated with different concentrations of Hc-CuONPs (5 and 7.5 µg) and then stained with propidium iodide solution. Apoptotic cell death was evaluated by fluorescence microscopy. Viable cells show very mild red fluorescence. Hc-CuONPs treated cells show apoptotic cell death with bright red fluorescence (white arrows).
Effect of Hc-CuONPs on the mRNA expressions of apoptotic and anti-apoptotic genes and PI3K/AKT/mTOR signalling proteins in the HeLa cells by RT-PCR
The apoptotic and anti-apoptotic genes (Bax, Bad, Bcl-2 and Bcl-XL) were assessed in the HeLa cells using the real time PCR method. illustrates that the apoptotic genes (Bax, Bad) were significantly (*p≤.05) increased in the HeLa cells treated with 5 and 7.5 µg of Hc-CuONPs when compared with HeLa cells alone. The anti-apoptotic genes (Bcl, Bcl-XL) were significantly (*p≤.05) decreased in the HeLa cells treated with 5 and 7.5 µg of Hc-CuONPs when compared with untreated control cells.
Figure 11. Effect of Hc-CuONPs in HeLa cells apoptotic and anti-apoptotic gene expressions and PI3K/AKT/mTOR signalling proteins using real time PCR. Each value is mean ± SD of triplicate measurements. *p<.05, #p<.001 comparisons between the control and experimental groups.
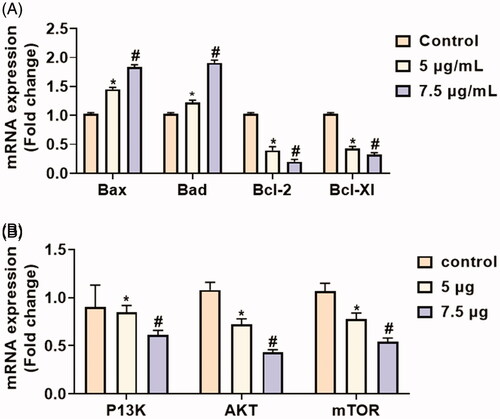
The expression pattern of cell signalling (PI3K, AKT, p-AKT, mTOR, and p-mTOR) proteins was analysed by RT-PCR (). The cell regulators and apoptotic proteins were evaluated in the HeLa cells. The two different concentrations (5 and 7.5 µg/ml of CuONPs) were tested. The highest concentration of Hc-CuONPs halts the cell proliferation and makes the cell leading to apoptosis. The 7.5 µg/ml concentration was used to intensify the apoptotic proteins and suppress cell regulatory protein expressions of PI3K/AKT/m-TOR in HeLa cells. The Hc-CuONPs treatment potentially activated the apoptotic pathway in HeLa cells.
Discussion
Traditional medicines are potent alternatives for allopathy drugs which render hilarious side effects. Chinese and Indian traditional medicinal practices used various plant sources to treat numerous ailments without causing side effects. Houttuynia cordata is one such herb present throughout South East Asia, China and Japan proven to possess various pharmacological properties such as antiviral, antiinflammatory, antioxidant, etc. [Citation16–24]. The major drawback of phytochemicals to prescribe as drug is its bioavailability. Therefore, in the present we utilized nanotechnology to formulate a phyto based nanodrug to treat cervical cancer. Copper oxide nanoparticles are proven to effectively target various types of cancer cells and induce apoptosis [Citation25]. Copper nanoparticles are cost effective and it is a potent alternative for other metal based nanoparticles such as gold, silver, platinum and lead [Citation26].
Copper nanoparticles are used in various fields such as electronics, cosmetics, textiles, biosensing, food processing, pharmacological and medical diagnosis [Citation27,Citation28] . The synthesis of nanoparticles is carried out by three different mechanism physical, chemical and biological processes. Compared to physical and chemical, biological process is more potent and it is biocompatible to the environment [Citation29–32]. Plant extracts possess numerous phytochemicals like alkaloids, flavonoids, ascorbic acid, glycosides, terpenoids, etc. which act as reducing agent for nanoparticle synthesis [Citation33]. In the present study, we synthesized green copper oxide nanoparticle using the plant extract of H. cordata. Our UV spectroscopic analysis confirmed the synthesis of Hc-CuONPs which show sharp intense band at 350 nm. The intense peak at 350 nm may be due to the reduction of copper ions by the phytochemicals of H. cordata extract which correlates with the previous studies where the plant extracts reduced copper ions to copper oxide nanoparticles [Citation10]. The reduction of copper ions into copper nanoparticles is measured with the Debye–Scherrer formula D=kλ/βcosθ, where D is the average crystalline size (Å), k is a constant 1, λ is the wavelength of X-ray source (0.1541 nm), β is the angular line FWHM intensity in radians and θ is Bragg’s angle. Therefore, the crystalline nature of synthesized Hc-CuONPs assessed with XRD analysis characteristic peaks was observed at (111), (200) and (220) located at 2θ angle of 41, 50 and 74 which is also observed by prior reports utilized with plant extracts [Citation34]; and it also matches with XRD pattern of copper oxide nanoparticles (JCPDS card no. 05-0667). FTIR analysis was performed to analyse the bioactive compounds present in the nanoparticles sharp intense peaks which were observed between 500 and 3700 cm−1. Peaks at 3423 cm−1 and 3412 cm−1 represent the presence of OH group form polyphenol, 2925–2920 cm−1 which may be due to the C–H bond stretch. The peaks at 1629 and 1440 cm−1 are due to the COOH and the peak at 1070 cm−1 alkoxy C–O stretches. These results confirm the bioactive compounds present in H. cordata extract which was effectively synthesized CuO nanoparticles. This outcome was supported by the previous report by Mali et al. [Citation35] and Das et al. [Citation36]. The size and shape of the Hc-CuONPs were further confirmed with TEM analysis. Our TEM results confirm the size of the Hc-CuONPs as 40–45 nm and it is spherical agglomerate cluster which correlates with the results of XRD analysis. The outcomes from the UV spectroscopic analysis, FTIR, XRD and TEM analysis authentically confirmed the bioreduction of Hc-CuONPs. After that, we further assessed the anticancer property of Hc-CuONPs against HeLa, human cervical cancer cell line. The cytotoxicity of Hc-CuONPs was estimated with MTT assay. Hc-CuONPs effectively inhibited the viability of HeLa cell line in a dose dependent manner. The 50% of cell apoptosis is observed at a concentration of 20 µg/ml Hc-CuONPs treatment. It was reported that copper nanoparticles were effectively internalized into the breast and lung cancer cells thereby inducing apoptosis [Citation37,Citation38].
Apoptosis plays a major role in identifying potent anticancer drug in in vitro anticancer studies. In cancer research, the toxicity of compound on cancer cells are in turn mentioned as cell death due to apoptosis. Various techniques were adopted to confirm apoptosis in anticancer study. The common techniques which are used rapidly are presented in this study. DCFH-DA staining method is used to confirm ROS mediated apoptosis, to differentiate live and dead cells in a single image to confirm the apoptosis AO/EtBr double staining method. Chromatin condensation and nuclear fragmentation were the hall mark of apoptosis, in our study, the nuclear staining DAPI and PI were used to confirm the apoptosis in HeLa cell line. The results obtained were Hc-CuONPs significantly induced apoptosis in HeLa cell line. There, it supports that Hc-CuONPs is potent inducer of apoptosis mediated cell death in cancer.
Mitochondrial mediated apoptotic pathway was regulated by the Bcl2 group of proteins. These proteins have common BH domain and they are classified into antiapoptotic proteins (Bcl2 and Bcl-xl) and proapoptotic proteins (Bid, Bax, Bad). Most of the anticancer drugs were designed thereby targeting the Bcl2 group of proteins and the drugs which are successful entered clinical trials [Citation39]. It is reported that Houttuynia cordata Thunb. (HCT) inhibits cell growth and induces apoptosis in human primary colorectal cancer cells [Citation13], lung cancer [Citation40], gastric cancer [Citation41], breast cancer [Citation42] and colon adenocarcinoma [Citation43]. In the present study, Hc-CuONPs effectively decreased the expression of antiapoptotic proteins Bcl2 and Bcl-xl and increased the expression of proapoptotic proteins Bax and Bad which correlates with the results of MTT assay.
PI3K/Akt/mTOR signalling pathway plays a key role in both physiological and pathological processes [Citation44]. In most of the human cancers such as ovarian, gastric and breast cancer, activation of PI3K/Akt/mTOR signalling pathway and overexpression of mTOR [Citation45] were reported. Hence in the present study, we assessed the impact of Hc-CuONPs on PI3K/Akt/mTOR signalling pathway in HeLa cell line. Hc-CuONPs effectively decreased the expression of total and phosphorylated proteins PI3K, AKT and mTOR which clearly signify Hc-CuONPs effectively inhibits the growth of cervical cancer cells and induces apoptosis in in vitro condition.
Conclusions
Green synthesized Hc-CuONPs showed potent cytotoxicity against human cervical cancer HeLa cells by inducing apoptosis and altering PI3k/AKT/mTOR signalling. The apoptotic changes were well documented and validated through the increased expression of pro-apoptotic proteins Bax, Bad and decreased expression of anti-apoptotic protein Bcl2 and Bcl-xl. The study provided the valuable information to understand the cytotoxic aetiology and apoptosis pathway using Hc-CuONPs against HeLa cells. Thus, green synthesized Hc-CuONPs can be considered as potent anticancer agent against cervical cancer in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Khazaei Z, Sohrabivafa M, Mansori K, et al. Incidence and mortality of cervix cancer and their relationship with the human development index in 185 countries in the world: an ecology study in 2018. Adv Hum Biol. 2019;9(3):222–227.
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide. Lancet Glob Health. 2020;8(2):e191–e202.
- Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069.
- Alexandra Hofsjö, Nina Bohm-Starke, Karin Bergmark, Britt Masironi, Lena Sahlin. (2019) Sex steroid hormone receptor expression in the vaginal wall in cervical cancer survivors after radiotherapy. Acta Oncologica 58:8, pages 1107–1115.
- Rizvi SAA, Saleh AM. Application of nanoparticle system in drug delivery technology. Saudi Pharm J. 2018;26:64–70.
- Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, Dou QP. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des. 2010;16(16):1813–1825.
- Nasrollahzadeh M, Maham M, Sajadi SM. Green synthesis of CuO nanoparticles by aqueous extract of Gundelia tournefortii and evaluation of their catalytic activity for the synthesis of N-monosubstituted ureas and reduction of 4-nitrophenol. J Colloid Interface Sci. 2015;455:245–253.
- Priya DD, Elango G, Roopan SM, et al. Abutilon indicum mediated CuO nanoparticles: eco-approach, optimum process of congo red dye degradation, and mathematical model for multistage operation. Chem Select. 2020;5(28):8572–8576.
- Devipriya D, Roopan SM. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater Sci Eng C Mater Biol Appl. 2017;80:38–44.
- Gnanavel V, Palanichamy V, Roopan SM. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J Photochem Photobiol B. 2017;171:133–138.
- Wang JH, Bose S, Shin NR, Chin YW, Choi YH, Kim H. Pharmaceutical Impact of Houttuynia Cordata and Metformin Combination on High-Fat-Diet-Induced Metabolic Disorders: Link to Intestinal Microbiota and Metabolic Endotoxemia. Front Endocrinol (Lausanne). 2018(24);9:620.
- Kumar M, Prasad SK, Krishnamurthy S, Hemalatha S. Antihyperglycemic Activity of Houttuynia cordata Thunb. in Streptozotocin-Induced Diabetic Rats. Adv Pharmacol Sci. 2014;2014:809438.
- Lai KC, Chiu YJ, Tang YJ, et al. Houttuynia cordata Thunb extract inhibits cell growth and induces apoptosis inhuman primary colorectal cancer cells. Anticancer Res. 2010;30(9):3549–3556.
- Lau KM, Lee KM, Koon CM, et al. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008;118(1):79–85.
- Nuengchamnong N, Krittasilp K, Ingkaninan K. Rapid screening and identification of antioxidants in aqueous extracts of Houttuynia cordata using LC–ESI–MS coupled with DPPH assay. Food Chem. 2009;117(4):750–756.
- Chang JS, Chiang LC, Chen CC, et al. Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am J Chin Med. 2001;29(2):303–312.
- Chen YY, Liu JF, Chen CM, et al. A study of the antioxidative and antimutagenic effects of Houttuynia cordata Thunb. using an oxidized frying oil-fed model. J Nutr Sci Vitaminol. 2003;49(5):327–333.
- Chiang LC, Chang JS, Chen CC, et al. Anti-Herpes simplex virus activity of Bidens pilosa and Houttuynia cordata. Am J Chin Med. 2003;31(3):355–362.
- Lu H, Liang Y, Yi L, et al. Anti-inflammatory effect of Houttuynia cordata injection. J Ethnopharmacol. 2006;104(1–2):245–249.
- Kim IS, Kim JH, Kim JS, et al. The inhibitory effect of Houttuynia cordata extract on stem cell factor-induced HMC-1 cell migration. J Ethnopharmacol. 2007;112(1):90–95.
- Kim SK, Ryu SY, No J, et al. Cytotoxic alkaloids from Houttuynia cordata. Arch Pharm Res. 2001;24(6):518–521.
- Li GZ, Chai OH, Lee MS, et al. Inhibitory effects of Houttuynia cordata water extracts on anaphylactic reaction and mast cell activation. Biol Pharm Bull. 2005;28(10):1864–1868.
- Ng LT, Yen FL, Liao CW, et al. Protective effect of Houttuynia cordata extract on bleomycin-induced pulmonary fibrosis in rats. Am J Chin Med. 2007;35(3):465–475.
- Park E, Kum S, Wang C, et al. Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am J Chin Med. 2005;33(3):415–424.
- Karlsson HL, Toprak MS, Fadeel B. Chapter 4 – toxicity of metal and metal oxide nanoparticles. In: Nordberg GF, Fowler BA, Nordberg M, editors. Handbook on the toxicology of metals. 4th ed. Burlington: Academic Press; 2015. p. 75–112.
- Abbas S, Nasreen S, Haroon A, Ashraf MA. Synhesis of Silver and Copper Nanoparticles from Plants and Application as Adsorbents for Naphthalene decontamination. Saudi J Biol Sci. 2020;27(4):1016–1023.
- da Silva PB, Machado RTA, Pironi AM, Alves RC, de Araújo PR, Dragalzew AC, Dalberto I, Chorilli M. Recent Advances in the Use of Metallic Nanoparticles with Antitumoral Action - Review. Curr Med Chem. 2019;26(12):2108–2146.
- Sarkar J, Chakraborty N, Chatterjee A, Bhattacharjee A, Dasgupta D, Acharya K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials (Basel). 2020(12);10(2):312.
- Khandel P, Yadaw RK, Soni DK, et al. Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J Nanostruct Chem. 2018;8(3):217–254.
- Peralta-Videa JR, Huang Y, Parsons JG, et al. Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol Environ Eng. 2016;1(1):4.
- Shah M, Fawcett D, Sharma S, et al. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8(11):7278–7308.
- Singh J, Dutta T, Kim KH, et al. 'Green' synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol. 2018;16(1):84.
- Della Pelle F, Scroccarello A, Scarano S, Compagnone D (2019) Silver nanoparticles-based plasmonic assay for the determination of sugar content in food matrices. Anal Chim Acta 1051:129–137.
- Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, Yawar W, ul Hasan MM. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Annals of microbiology. 2010;60(1):75–80.
- Mali SC, Dhaka A, Githala CK, et al. Green synthesis of copper nanoparticles using Celastrus paniculatus Willd. leaf extract and their photocatalytic and antifungal properties. Biotechnol Rep. 2020;27:e00518.
- Das PE, Abu-Yousef IA, Majdalawieh AF, et al. Green synthesis of encapsulated copper nanoparticles using a hydroalcoholic extract of Moring oleifera leaves and assessment of their antioxidant and antimicrobial activities. Molecules. 2020;25(3):555.
- Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, et al. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int J Biol Macromol. 2020;164:2074–2084.
- Saravanakumar K, Shanmugam S, Varukattu NB, et al. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J Photochem Photobiol B. 2019;190:103–109.
- Abou-Nassar K, Brown JR. Novel agents for the treatment of chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2010;8(12):886–895.
- Chen Y-F, Yang J-S, Chang W-S, et al. Houttuynia cordata Thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J Biomed Sci. 2013;20:18.
- Jeong JB, Hong S, Eo H, et al. Houttuynia cordata Thunberg exhibits anti-tumorigenic activity in human gastric cancer cells. Korean J Herbol. 2013;28(6):155–160.
- Subhawa S, Chewonarin T, Banjerdpongchai R. The effects of Houttuynia cordata Thunb and Piper ribesioides Wall extracts on breast carcinoma cell proliferation, migration, invasion and apoptosis. Molecules. 2020;25(5):1196.
- Tang Y, Yang J, Lin C, et al. Houttuynia cordata Thunb extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncol Rep. 2009;22(5):1051–1056.
- Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64.
- Tapia O, Riquelme I, Leal P, et al. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465(1):25–33.