Abstract
Diabetes associated injury healing and other tissue irregularities are viewed as a significant concern. The purpose of the study is to design the wound regeneration activity of Ficus carica extract (FFE) loaded amphiphilic polymeric scaffold of poly(xylitol-g-adipate-co-glutamide) (PXAG)–polyhydroxybutyrate (PHB) for potential diabetic affected wound regeneration. The PXAG copolymer was prepared by the condensation method, and the polymeric scaffolds of PXAG-PHB, PXAG-PHB/FFE were developed through the ultra-sonication process and magnetic stirrer processes. The chemical structure, crystalline nature, thermal stability, size, surface charge and surface morphology of PXAG-PHB and PXAG-PHB/FFE polymeric scaffolds were investigated. The PXAG-PHB/FFE exhibits 99.0% free radical scavenging activity which was determined by the DPPH method. The inhibition zones by the PXAG-PHB/FFE indicate it had a higher antibacterial activity with the Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive) pathogens. The PXAG, PXAG-PHB and PXAG-PHB/FFE polymeric scaffolds exhibited good viability against diabetic induced wound cells (WS1) in 100 μg/mL concentrations up to 72 h incubation. Since the synthesized PXAG-PHB/FFE polymeric scaffolds possess excellent thermal stability, bioactivity, biocompatibility and antioxidant activity along with potent antimicrobial activity, they play a potential role in diabetic wound tissue regenerations.
Introduction
Skin tissue is the essential organ in our human body, filling in as an obstruction against external infections, including microorganisms and other unfamiliar particles. An injury is a break or an interruption of the skin by heat, chemicals, mechanical or surgical damage [Citation1]. Healing of wounds is a specific and complicated process that includes a few curing stages, and the recovery of diabetic injuries is a worldwide clinical worry. A few variables are explicit to a person's age, obesity status, diabetes, medicine and the necessity for more viable yet additional low-cost dressing materials [Citation2]. The primary physiological role of wounds includes inflammation, coagulation and removal of affected cellular constituents, followed by angiogenesis, proliferation and migration of the cells. It is divided into haemostasis, inflammation, migration/proliferation, maturation or remodelling [Citation3,Citation4]. Since diabetic wound regeneration is difficult, and it takes 12 weeks for complete repairs.
Currently, wound dressings are traditionally used to protect the wound from contamination, be that as it may, they can be misused as stages to release bioactive particles to wound destinations [Citation5]. The utilization of bioactive compounds as in the formulation of liquids, creams and balms for bioactive agents release to the injury is not helpful as they quickly retain biological fluid and, all the while, lose their rheological qualities and become transferrable [Citation6]. Hence, the utilization of strong injury dressings is favoured on account of exudative injuries as they offer better exudates controlling and protracted residence at the wound area [Citation7]. Recently, the active wound healing dressing materials containing polymers, naturally occurring plant compounds, and nanoparticles were effectively used. Polymers are majorly applied to treat wound tissue engineering, and it can significantly affect natural derived bioactive polymers, naturally derived inert polymers and biodegradable synthetic polymers [Citation8–10].
Synthetic biodegradable polymers normally applied in wound regenerations include polyxylitol, polyethylene oxide (PEO), polyhydroxyl butyrate, polyvinylalcohol (PVA) and polyurethane [Citation11]. Their hydrophilic nature grants significant utilitarian injury repair qualities, for example, dampness ingestion limit and water absorption capacity, which permit support of a sodden injury condition while maintaining a strategic distance from the assortment of overabundance exudates [Citation12]. The engineered polymeric wound covering materials can be created utilizing different procedures, for example, electrospinning and hydrogel synthesis [Citation9]. The hydrophobic polymer of poly(3-hydroxybutyric acid) (PHB) has been used as biomaterials for tissue regeneration due to its biodegradable nature and excellent biocompatibility [Citation13]. Polyhydroxybutyrate (PHB) and gelatin (GEL), combined collagen (COL) nanofibres prepared as impersonates extracellular network and fills in as a successful biomaterial for extracellular matrix (ECM) designing uses, particularly for wound recuperating. The developed PHB-GEL-OSA-COL nanofibres are inspiring mechanical constancy, a vital obligation for wound repairing [Citation14].
A critical number of on-going exploration considers the significance of oxygen in the field of continuous injury repair, and the polymers polyxylitol, polyhydroxyl butyrate are abundant oxygen sourced polymers [Citation15]. Oxygen assumes an essential job on the side of cell development and contamination control. It is regularly acknowledged that insufficient cell oxygenation and perfusion prompts disabled injury repair, activating injury maceration and deferred healing. The polymers will supply oxygen and will reduce the hypoxia of the wound cells. Tang et al. fabricated the polydopamine (PDA) functionalized reduced graphene oxide (rGO)-loaded chitosan (CS) and silk fibroin (SF) composite materials for efficient wound dressing with good antioxidative properties [Citation16]. Previously the researcher used gamma radiation cross-connecting to get sterile hydrogels used in wound repair [Citation17,Citation18]. A single material cannot fulfil all the problems and the polymeric scaffold combined with natural bioactive compounds may reduce the issues of exciting treatment methods. The polymeric scaffold was involved in the curing of the diabetic affected wound repair. It can enhance cell proliferation, and it mimics the ECM. In the diabetic affected tissue regeneration, several natural materials can involve curing like turmeric, neem, aloe vera, eucalyptus and Ficus carica, etc. As of late, Ma et al., flavonoids with oligomeric proanthocyanidins contain extracts of grape-seed utilized as a natural photo bio-modulator compound for melanoma treatment and active natural compounds for wound repair [Citation19].
Ficus is the most plentiful kind of angiosperms from the mulberry family. It has more than 800 types of trees, bushes, climbers, creepers and Hemi epiphytes in the tropical and subtropical districts everywhere throughout the world [Citation20]. Distinct plant parts like natural products, leaves, seeds, delicate, bark, latex and shoots have various restorative presentations, including hostile to diabetic specialists [Citation21–24]. The natural compounds of Ficus carica show a powerful wellspring of polyphenols and flavonoids and different mixes like β-carotines, glycosides, arabinose, β-sitosterols, β-amyrins and xanthotoxol [Citation25]. Saponins, coumarins, flavonoids, alkaloids and terpenes have likewise been accounted for in the fluid concentrate of the ready dry product of Ficus carica [Citation26].
This research work was planned to prepare a biodegradable scaffold of polyxylitol and polyhydroxyl butyrate amphiphilic polymer loaded with extracts of Ficus carica for the regeneration of diabetic wound tissues. The biocomposite scaffold established as a network with a biomimetic natural ECM is highly potentially appropriate for tissue formation to protect the external infections. The composite's novelty efficiently enhances the properties of antioxidant, anti-inflammatory activities, the proliferation of cells, angiogenesis and neovascularization, which are considered to be closely associated with its diabetic wound tissue regeneration by natural compounds. Besides, the materials reduce the hypoxia in the wound tissue to promote regenerations by the oxygenated polymers. The polymer biocomposite has satisfied the requirements; a perfect diabetic wound curing materials requirements to possess suitable biocompatibility, good pore size and good matching with the defect region to accelerate wound healing, studied in vitro cytocompatibility, and cell viability in normal diabetic wound cells models.
Materials and methods
Chemicals and reagents
Xylitol (MW 152.15, purity 99%), adipic acid (146.14, purity 99%), l-glutamic acid (MW 147.13, purity 98%), PHB (MW 2.3 × 105 g/mol), Span-80 (MW 428.6, purity 60%), ethyl acetate (MW 88.1, purity 99.8%), chloroform (CHCl3, MW 119.38, purity 99%), methanol (MW 32.04, purity 99.8%) and ethanol (46.07 g/mol, purity 99.8%) were purchased from Sigma Aldrich (Shanghai, China). Analytical grade chemicals were used without any further purification. Double distilled (DD) water was used in the whole experimentations.
Extraction of fig fruits (FFE)
Fresh fig fruit (Ficus carica) was collected from the fruit shop, Xi'an, China (34.3416° N, 108.9398° E). The fresh fig fruits were washed with DD water and cut into small pieces. The small pieces of fig fruits were further dehydrated in an oven at 40 °C for 24 h to obtain a fine particle and mashed well-using grinder. Furthermore, the 10 g of dried fig powders were boiled with 30 mL of ethyl acetate for 12 h [Citation25–27]. The boiled solution was filtered by using a Whatman 40 filter paper. Then, the ethyl acetate was evaporated from the filtrate. The extracts were dispersed in 20 mL of methanol and filtered once more to eliminate the remaining polymeric gum. A pale green colour fig fruit extract was collected and to be used for further reactions and experimental characterizations.
Synthesis of poly(xylitol-g-adipate-co-glutamide) (PXAG)
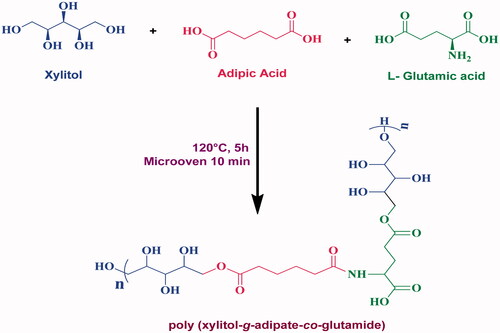
Polymerization of xylitol, adipic acid and l-glutamic acid was carried out by the polycondensation reactions with the previous report's slightly modified procedure [Citation28]. The 1:1:1 stoichiometric ratio of xylitol, adipic acid, l-glutamic acid monomers is accurately weighed 1 g and dissolved in a 1:1 ratio of DD water–ethanol solution, and these solutions were made up to 20 mL. The solutions were then taken in an RB flask to heat 120 °C with constant stirring (150 rpm) for 5 h to get a transparent solution of the PXAG prepolymer. The transparent solution was heated in a micro oven at 150 W for about 10 min to form the pale yellow solution of PXAG polymers. The polymer viscosity was elevated slowly with the cumulative reaction period. Finally, the PXAG polymeric solution was kept a room temperature (25 °C) for 24 h to get a solid palm leaves structure of PXAG copolymer. The formation of palm leaves structure polymer of PXAG polymer, which has an ester bond between the monomers of xylitol and adipic acid and the amide bonds between adipic acid and l-glutamic acid monomers (Scheme 1), was confirmed by FT-IR, 1H and 13C NMR spectroscopy. The synthetic procedure of PXAG synthesis is shown in Supplementary Figure 1.
Synthesis of PXAG-PHB polymeric scaffold
The hydrophilic nature of PXAG polymer and hydrophobic in nature of the PHB polymer was subjected to emulsion through the oil in water (O/W) emulsion technique with Span 80 surfactant [Citation29,Citation30]. The synthesized 0.5 g of PXAG copolymer was dissolved with 20 mL of ethanol to get a transparent solution. 0.5 g of PHB was dissolved in 20 mL of CHCl3 with slightly heating at 40 °C to get a milky white-colour solution. The span 80 surfactants were added in PHB solution with 10:1 ratio respectively to continuous stirring at room temperature for 30 min. The mixture of PXAG and PHB/span solutions was subjected to the probe ultra-sonication method and the normal magnetic stirring method.
Synthesis of PXAG-PHB with the assistance of ultrasonication
In brief, 20 mL of PHB/span 80 solutions were added dropwise into the PXAG (20 mL) solution and then directly exposed to sonication under ultra-probe sonicator (SONICS Vibra-cell, Warmensteinach, Germany with 20 Hz and 130 W) with 6-mm diameter probe for 1 h in a pulsed cycle of 3 s on and 2 s off. After 1 h, a white precipitate of the PXAG-PHB scaffold was formed [Citation31]. The polymeric scaffold was filtered with Whatman 40 filter paper and dried at room temperature. The formation of PXAG-PHB polymer was established by Fourier transform spectroscopy, SEM, XRD and DTA methods. The schematic method of preparation is presented in Supplementary Figure 2.
Synthesis of PXAG-PHB in the magnetic stirring method
The synthesis of PXAG-PHB polymer was also carried out through the magnetic stirring method. A 20 mL of the PXAG stock solution was taken in a beaker, and the PXAG solution was treated with a magnetic stirrer with 20 mL of PHB/span 80 solutions in a dropwise manner for 1 h with constant stirring (500 rpm) at 25 °C. After transferring the whole 20 mL of PHB/span solution into the PXAG solution, the stirring was stretched for an additional 2 h. Finally, the homogenous PXAG-PHB polymer was collected, and the solvent was evaporated to get a white precipitate of the PXAG-PHB polymer [Citation31]. The scaffold was filtered by using a Whatman 40 filter paper and dried a room temperature. The formed PXAG-PHB polymer was investigated by the physicochemical characterizations. The procedure for the preparation of the PXAG-PHB scaffold through the magnetic stirring method is demonstrated in Supplementary Figure 3.
Preparation of PXAG-PHB/FFE scaffold
The loading of FFE in the PXAG-PHB/FFE scaffold was followed by the above said emulsion method under probe ultra-sonication and magnetic stirrer assistance. The schematic representation of the preparation method was presented in Supplementary Figure 4. Briefly, 0.1 g of the FFE was taken and added to the PXAG polymer. The reaming protocol was followed by preparing the PXAG-PHB scaffold via ultra-sonication and magnetic stirring method.
Cell culture
The human wound cells (WS1) were cultured in Dulbecco's Modified Eagle Medium (DMEM, GIBCO, Carlsbad, CA), which consisted of a minimum essential medium (Hi-Media Laboratories, Mumbai, India), supplemented with 10% foetal bovine serum (FBS) and 24-well tissue culture plates used. Cells cultured at 37 °C under the 100% relative humidified atmosphere of 5% CO2 and 95% air. The maintained cultures passaged weekly, and the culture medium was changed twice a week. A diabetic cell model was attained in WS1 cells by constantly growing cells in supplemented MEM with an additional 17 mM/L d-glucose. Moreover, the MEM already contains a glucose concentration of 5.6 mM/L; thus, cells were grown for several passages in 22.6 mM/L d-glucose before experiments. For the study, wound cells were seeded at a density of 6 × 105 in 3.4 cm diameter tissue culture dishes. Wounded models are described in the respective sub-section below. Untreated cells were used as controls.
In vitro cell viability
The monolayer cells were detached using trypsin–ethylene diamine tetraacetic acid (EDTA) to make single-cell suspensions, and a haemocytometer totalled viable cells. It was diluted with a medium containing 5% FBS to give a final density of 1 × 106 cells/mL. One hundred microlitres per well of cell suspension were seeded into 96-well plates at a plating thickness of 10,000 cells/well were incubated to allow for cell attachment at 37 °C, 5% CO2 and 95% air. Cells were treated after 24 h with a serial concentration of the test samples. It was initially dispersed in phosphate-buffered saline (PBS). An aliquot of the sample solution was diluted twice for the desired final maximum test concentration with a serum-free medium. Aliquots of 100 μL of these different sample dilutions were added to the appropriate wells already containing 100 μL of medium with or without cells (blank), resulting in the required final sample concentration. The medium containing without samples served as a positive control, and triplicate was maintained for all masses. The plates were incubated for an extra 48 h at 37 °C, 5% CO2 and 95% air with samples' addition.
Cell proliferation by EZBlueTM cell assay
The 100 μL of 1 × 106 cells/mL cells seeded in 96-well microtiter plates in triplicate. The dish was incubated at 37 °C in a 5% CO2 environment. The cells were allowed to grow for up to 24 h or till confluence is reached. The medium was removed from the microtiter plate, added 100 µL RPMI 1640 medium, which contains a different concentration of samples; the plate was incubated at 37 °C for 24, 48 and 72 h in a 5% CO2 environment. After incubation, the plates were removed from the incubator and added EZBlueTM reagent to a final concentration of 10% of total volume. This volume should be the same as the volume used while determining optimum cell density. The plate was then covered with aluminium foil to avoid exposure to light; the plate was kept in the incubator for 1–8 h. After incubation, the dish was removed from the incubator and read the absorbance at 570 nm, keeping 600 nm as a reference wavelength.
Hoechst 33258 staining
The nuclear damage of normal diabetic wound cells on PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold at 100 μg/mL was assessed by Hoechst 33258 (bisbenzimide trihydrochloride) (Invitrogen, H21491, Thermo Fisher Scientific, Waltham, MA) staining. After 48 h incubation, the cells were washed with PBS thrice and incubated with Hoechst 33258 (10 μg/mL) at 37 °C in an atmosphere of 5% CO2 for 7 min. Cells were again washed thrice with PBS to remove the excess unwanted dye and viewed under a Carl Zeiss Axio Observer Z1 with Axio Vision software (352Ex/461Em).
In vitro central scratch wound assay
In vitro wounded cell models were performed through the central scratch assay, and the wounds were created in diabetic induced WS1 cells. Briefly, WS1 cells (6 × 105 cells/dish) were seeded into six-well plates and allowed to grow to 80% confluence. A central scratch was created by scraping the cell monolayer using a sterile p200 pipette tip. After scraping, the cells were incubated in a CO2 incubator for 30 min, and the wells were washed twice with PBS. About 2 mL of fresh MEM medium (high glucose (17 mM/L) medium for diabetic groups) and PXAG, PXAG-PHB and PXAG-PHB/FFE Scaffold at 100 μg/mL were added, and cells were grown for 72 h or until wound closure. Cellular migration towards and into the central scratch was observed, and images were taken every 24–72 h using an inverted light microscope coupled to a camera (Olympus CX41, Tokyo, Japan).
Statistical analysis
Statistical reports are given as standard error of the mean (SEM) (n = 3). ANOVA (one-way analysis of variance) and Student's t-test were used to determine significant differences between controls and experiments and between groups. Significant probability is shown as *p ≤ .05.
Results and discussion
PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold characterization
The characterization of the synthesized PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold was characterized by various techniques for the confirmations of the structure of the polymer and extract loaded scaffold formations. The synthesized PXAG copolymer structure was described using FT-IR, XRD, DLS, TGA, SEM, DPPH assay, antimicrobial activity and Cell proliferation EZBlueTM Cell Assay.
FT-IR investigation
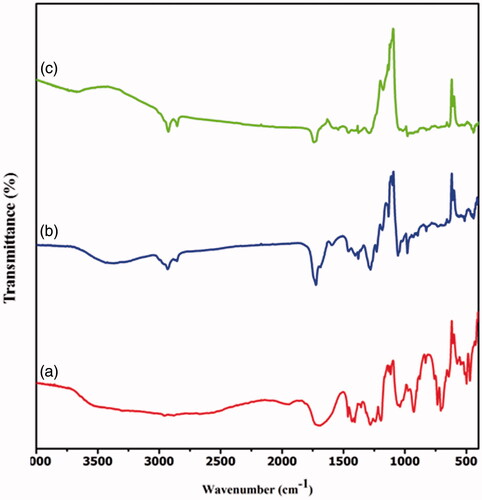
FT-IR analysis was achieved to authorize the development and interaction between the polymers of PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold (). The PXAG copolymer, which exists in the band at 1720 cm−1 and 1645 cm−1, corresponds to the ester and amide carbonyl group's (C=O) stretching vibration, and the peak at 2962 cm−1 corresponds to the C–H stretching vibrations. The broadband around 3200 cm−1 and a sharp peak at 1429 cm−1 correspond to the O–H stretching vibrations. The band at 1118 cm−1 confirms the presence of C–O–C functionality in PXAG polymer [Citation32]. In comparison, the bends at 1047 and 1312 cm−1 were exhibiting C–O bond in the structure (). In general, PXAG-PHB polymer shows the same bends as PHB, with few band peaks shifted to lower frequencies due to the physical interactions with PXAG copolymer. The characteristic peak from 1720 cm−1 was moved to 1727 cm−1 due to the formation of the PXAG-PHB scaffold. The band at 1284 cm−1 relates to the –CH gathering, though groups somewhere in the range of 1000 and 1300 cm−1 show extending the ester group's C–O obligation. The groups at 2926 cm−1 show the nearness of a C–H gathering, and the band situated at 1377 cm−1 relates to the symmetric bowing –CH3 gathering.
The peak at 1452 cm−1 is identified as the –CH2 or –CH3 group's asymmetric twisting. Likewise, curves of minor pertinence at 3434 cm−1 are determined with a terminal –OH gathering of the PHB portion. These bends confirm PHB's presence along with the PXAG to the formation of the PXAG-PHB scaffold () [Citation33]. In the fruit extract of Ficus carica loaded PXAG-PHB scaffold, FTIR spectrum shows that strong absorption peaks at 3590, 2936, 1721, 1455, 1337, 1274, 1085, 886 and 780 cm−1, which corresponds to the compounds present in the extracts of their functional groups like alcohols, C=O, aromatics, esters, ethers, alkyl halide and alkyne groups, respectively. These bands confirm the presence of FFE in the PXAG-PHB scaffold () [Citation34].
NMR analysis
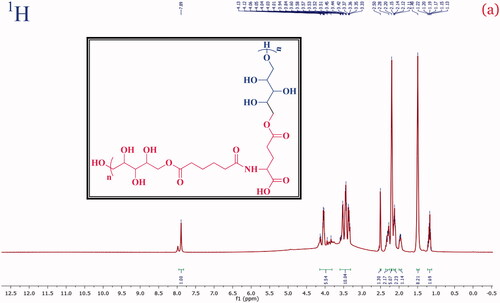
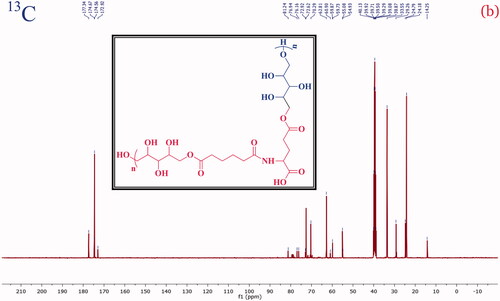
A structural confirmation of the poly-(xylitol-g-adipate-co-glutamide) co-polymer formation with the characteristic signal of the 1H and 13C was investigated and presented in and , respectively. The NMR 1H (400 MHz, DMSO) values are 7.89 (s, NH), 4.17–3.77 (m, CH), 3.61–3.29 (m, CH2), 2.36–2.25 (m, CH2), 2.20 (s, OH), 2.16–2.08 (m, CH2), 2.00–1.93 (m, CH), 1.49 (s, CH) and 1.23–1.11 (m, CH). Besides, the 13C NMR values: (101 MHz, DMSO) δ 177.34, 174.67, 174.56, 172.92, 81.24, 76.94, 76.16, 72.92, 72.62, 70.29, 62.81, 60.90, 59.87, 59.73, 55.08, 54.93, 33.55, 29.26, 24.79, 24.18 and 14.25 were observed. In the typical spectrum, a strong signal detected 177.34, 174.67, 174.56 and 172.92 ppm was accountable to the C=O (ester, amide and acid carbonyl) signals. These NMR spectral data confirm the formation of PXAG polymer through simple polycondensation reaction.
X-ray diffraction studies
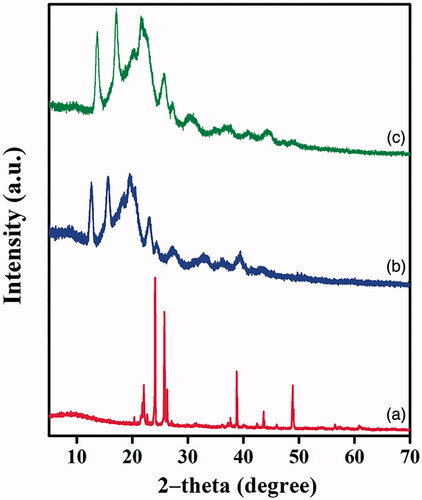
The physical phase of free scaffold and extract loaded scaffold was investigated using X-ray diffraction analysis, which helped provide useful information for the interaction of the biological fluid with tissue engineering materials [Citation35,Citation36]. The diffraction patterns at 22.66°, 24.02°, 26.22°, 37.7°, 38.8° and 48.93° observed correspond to xylitol molecules present in the PXAG [Citation21] (). The peaks at 20.45°, 25.6° and 26.22° reveal l-glutamic acid compounds in PXAG copolymer [Citation37]. The presence of adipic acid in PXAG is authenticated from the diffraction peak at 22.09° [Citation38]. The XRD pattern from reveals the high crystalline nature of the prepared PXAG copolymer. shows that the pattern intensities of PXAG copolymer decreased due to the combination of hydrophobic nature PHB polymer and the slight shifting in the diffraction peaks confirms the existence of physical interaction between PXAG and PHB polymers. Finally, FFE loaded PXAG-PHP scaffold intensity of the patterns further decreased due to the extract's covering in the PXAG-PHP scaffold (). Wound tissue materials crystalline phase made of synthetic polymers, for example, amphibolic polymers, polyesters likewise have the mechanical resistance that obliges development of the influenced body part and can retain more exudate than thin-film dressing [Citation39].
SEM analysis
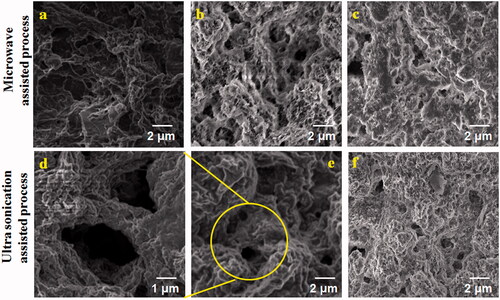
The surface morphology of PXAG-PHB and PXAG-PHB/FFE was evaluated by SEM analysis, as shown in . The non-linear structure morphology of the PXAG polymer is observed in . The macroporous morphology of PXAG-PHB was formed, which is shown in ,e). The larger porous was observed in ultra-sonication processed PXAG-PHB scaffold, and it will give a better permeable nature of the wound to higher proliferation (). Porous scaffold materials could be promising injury improving dressings that keep up a damp domain in the injury site and quicken wound repair [Citation40]. After FFE was added to the PXAG-PHB scaffold, the overall porous carrier was filled with FFE, as shown in . It is due to the reaction of the kinetic energy of molecules, which is high, and thus, the molecules in the mixture vibrate more, and the interaction is also high. As shown in , PXAG-PHB has been a micro and nanoporous structure, and added FFE into PXAG-PHB polymer has changed porous and filled with FFE magnetic stirrer method. The formation of micro and nanoporous structures due to the mixture of the molecule vibrates very low and does not continue the reaction. Hence, the molecules' interaction is low, so it is more porous than the ultrasonication method (). However, it is well-known that the PXAG-PHB/FFC scaffold will help prepare bandages to wound regeneration. The surface of the microspheres was hydrophobic and hydrophilic due to the three blocks copolymer. The crude surface may also absorb more cells to adhere to the microspheres. Thus, the porous microspheres with a rough surface can attract cell adhesion proteins, such as vitronectin and fibronectin, at significantly higher levels than the solid microspheres with a smooth surface [Citation41].
In vitro cell viability of PXAG, PXAG-PHB and PXAG-PHB/FFE polymers
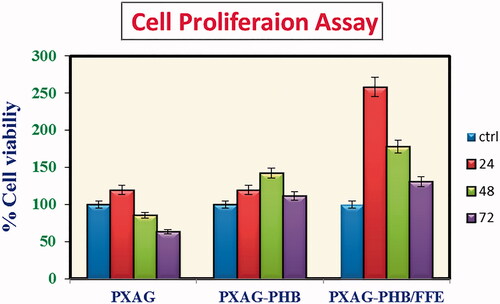
The biocompatibility of the 100 μg/mL concentration of the prepared PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold was treated with diabetic induced wound cells (WS1). The cell proliferation/cytotoxic effect of PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold polymers was observed 24, 48 and 72 h using EZBlueTM cell assay. As shown in , with the addition of PXAG copolymers in the WS1 cell line, the cells could proliferate within 24 h. Formerly, with the addition of PHB in PXAG, the cells proliferated more than PXAG. PXAG-PHB/FFE scaffold could induce more proliferation within 24 h when compared to control cells. As seen in the plot, the PXAG-PHB/FFE scaffold showed more cell proliferation than other polymers. It indicates the polymeric scaffold's non-toxic nature, and these results conclude that the biocompatible and cell growth behaviour of PXAG and PHB polymer. Evidence of the PXAG-PHB/FFE scaffold polymers can serve as a potential composite for wound healing [Citation5,Citation37]. Tissue frameworks have been shown to be helpful in wound dressing or skin tissue designing in an assortment of wound conditions. Tissue frameworks cannot exclusively cover the injury and give an actual hindrance, yet can likewise offer a cell skin with excretive natural segments to invigorate re-epithelialization and arrangement of granulation tissue [Citation42].
Hoechst 33258 staining
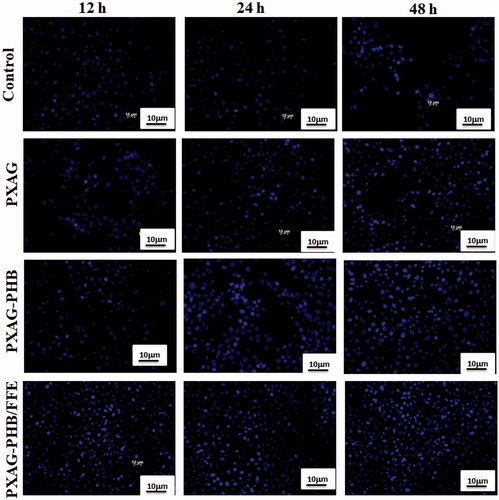
The Hoechst 33258 nuclear stain was used to detect the viable nature of PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold, inducing proliferation and cell growth of live cells. Hoechst 33258 is a blue fluorescent DNA labelling stain generally used to visualize nuclei and mitochondria [Citation43]. As experiential in , control cell nuclei appeared to be intact and oval, with few cells. After 48 h incubation, PXAG-PHB/FFE scaffold treated diabetic wounded cells showed maximum clear spherical nuclei that reflect the cell growth and proliferation in a time-dependent manner, and composite treated cells showed uniformly fluorescent nuclei [Citation44]. The main factor of the cell adhesion and cell growth is the PXAG-PHB surface and porous nature. The surface morphology of the biomaterial has substantial impacts on cell attachment and cell development [Citation45,Citation46]. Higher microroughness the anchorage of dressing material on natural tissues and does not limit their adhesion and growth [Citation47].
In vitro scratch assay
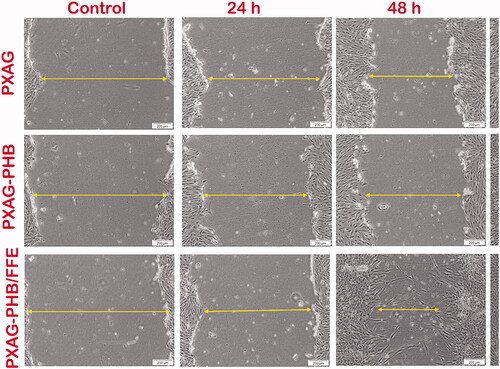
Cell migration and proliferation play a vital role in wound healing processes. The influence of the non-adhering dressing on wound healing was determined using the in vitro scratch wound assay [Citation5] in diabetic wounded cell models (). In vitro wound closure was investigated at control, 24, 48 and 72 h post-injury and treatment with 100 μg/mL of PXAG, PXAG-PHB and PXAG-PHB/FFE scaffold. The PXAG-PHB/FFE scaffold treated diabetic wounded cells demonstrate complete closure throughout 72 h compared with control cells. Likewise, the migration of diabetic injured cells treated with the PXAG-PHB/FFE scaffold significantly increased at 48 and 72 h (as indicated by arrows). During wound regeneration, cell movement is significant for injury reconstruction and the later restoring stages. In this way, studying wound cell migration influenced by PXAG-PHB/FFE scaffold may assist with focussing on treatments for improved wound regeneration [Citation24]. Goorani et al. studied the Anethum graveolens aqueous extract ointment could significantly reduce the levels of the lymphocyte, total cell, wound area and enhance the levels of hexosamine, hydroxyproline, wound contracture and fibrocyte as compared with the basal ointment and control groups [Citation48]. Ponrasu and Suguna reported Annona squamosa L extract to COL and glycosaminoglycans formation during wound healing [Citation49].
Conclusions
The PXAG-PHB/FFE polymeric scaffold was successfully synthesized with the loading of natural diabetic inhibiting compounds from Ficus carica fruit by a simple, eco-friendly and cost-effective method. Various analytical techniques investigated the bonding, crystallinity, thermal stability, size, surface charge, surface morphology and in vitro analysis of PXAG-PHB and PXAG-PHB/FFE polymeric scaffolds. The prepared PXAG, PXAG-PHB and PXAG-PHB/FFE polymeric scaffolds exhibited good viability in diabetic induced wound cells (WS1) in 100 μg/mL concentrations for 72 h. Among them, PXAG-PHB/FFE reveals excellent cell viability in 72 h. The synthesized PXAG-PHB/FFE scaffolds possess excellent thermal stability, bioactivity, biocompatibility and antioxidant activity along with strong antimicrobial activity, which plays a potential role in diabetic wound tissue engineering applications.
Supplemental Material
Download MS Word (2.8 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting this study's findings are available within the article and its supplementary materials.
Additional information
Funding
References
- Sabale P, Bhimani B, Prajapati C, et al. An overview of medicinal plants as wound healers. J Appl Pharm Sci. 2012;2:143–150.
- Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci. 2015;104(11):3653–3680.
- Sharifi S, Javad Hajipour M, Gould L, et al. Nanomedicine in healing chronic wounds: opportunities and challenges. Mol Pharm. 2020; 18 (2) 550–575.
- Si H, Xing T, Ding Y, et al. 3D bioprinting of the sustained drug release wound dressing with double-crosslinked hyaluronic-acid-based hydrogels. Polymers. 2019;11(10):1584.
- Anishiya Chella Daisya ER, Rajendran NK, Houreld NN, et al. Curcumin and Gymnema sylvestre extract loaded graphene oxide–polyhydroxybutyrate–sodium alginate composite for diabetic wound regeneration. React Funct Polym. 2020;154:104671.
- Boateng JS, Matthews KH, Stevens HN, et al. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923.
- Thomas D, Nath MS, Mathew N, et al. Alginate film modified with aloevera gel and cellulose nanocrystals for wound dressing application: preparation, characterization and in vitro evaluation. J Drug Deliv Sci Technol. 2020;59:101894.
- Sumathra M, Rajan M, Amarnath Praphakar R, et al. In vivo assessment of a hydroxyapatite/κ-carrageenan–maleic anhydride–casein/doxorubicin composite-coated titanium bone implant. ACS Biomater Sci Eng. 2020;6(3):1650–1662.
- Rajwade JM, Paknikar KM, Kumbhar JV. Applications of bacterial cellulose and its composites in biomedicine. Appl Microbiol Biotechnol. 2015;99(6):2491–2511.
- Pan J, Prabakaran S, Rajan M. In-vivo assessment of minerals substituted hydroxyapatite/poly sorbitol sebacate glutamate (PSSG) composite coating on titanium metal implant for orthopedic implantation . Biomed Pharmacother. 2019;119:109404.
- Rahimi M, Noruzi EB, Sheykhsaran E, et al. Carbohydrate polymer-based silver nanocomposites: recent progress in the antimicrobial wound dressings. Carbohydr Polym. 2020;231:115696.
- Mickelson MA, Mans C, Colopy SA. Principles of wound management and wound healing in the exotic pets. Vet Clin North Am Exot Anim Pract. 2016;19(1):33–53.
- Piras AM, Chiellini F, Chiellini E, et al. New multicomponent bioerodible electrospun nanofibers for dual-controlled drug release. J Bioact Compat Polym. 2008;23(5):423–443.
- Kandhasamy S, Perumal S, Madhan B, et al. Synthesis and fabrication of collagen-coated ostholamide electrospun nanofiber scaffold for wound healing. ACS Appl Mater Interfaces. 2017;9(10):8556–8568.
- Rubnov S, Kashman Y, Rabinowitz R, et al. Suppressors of cancer cell proliferation from Fig (Ficus carica) resin: isolation and structure elucidation. J Nat Prod. 2001;64(7):993–996.
- Tang P, Han L, Li P, et al. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl Mater Interfaces. 2019;11(8):7703–7714.
- Bennett NT, Schultz GS. Growth factors and wound healing: part II. Role in normal and chronic wound healing. Am J Surg. 1993;166(1):74–81.
- Fonder MA, Lazarus GS, Cowan DA, et al. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58(2):185–206.
- Ma H, Zhou Q, Chang J, et al. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano. 2019;13(4):4302–4311.
- Badgujar SB, Patel VV, Bandivdekar AH, et al. Traditional uses, phytochemistry and pharmacology of Ficus carica: a review. Pharm Biol. 2014;52(11):1487–1503.
- Jones V, Grey JE, Harding KG. Wound dressings. BMJ. 2006;332(7544):777–780.
- Soni N, Mehta S, Satpathy G, et al. Estimation of nutritional, phytochemical, antioxidant and antibacterial activity of dried fig (Ficus carica). J Pharmacogn Phytochem. 2014;3:158–165.
- Moradi R, Hajialiani M, Salmani S, et al. Effect of aqueous extract of Allium saralicum R.M. Fritsch on fatty liver induced by high-fat diet in Wistar rats. Comp Clin Pathol. 2019;28(5):1205–1211.
- Goorani S, Shariatifar N, Seydi N, et al. The aqueous extract of Allium saralicum R.M. Fritsch effectively treat induced anemia: experimental study on Wistar rats. Orient Pharm Exp Med. 2019;19(4):403–413.
- Slatnar A, Klancar U, Stampar F, et al. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J Agric Food Chem. 2011;59(21):11696–11702.
- Jeong MR, Kim HY, Cha JD. Antimicrobial activity of methanol extract from Ficus carica leaves against oral bacteria. J Bacteriol Virol. 2009;39(2):97–102.
- Meziant L, Boutiche M, Bachir Bey M, et al. Standardization of monomeric anthocyanins extraction from fig fruit peels (Ficus carica L.) using single factor methodology. Food Meas. 2018;12(4):2865–2873.
- Dong W, Li T, Xiang S, et al. Influence of glutamic acid on the properties of poly(xylitol glutamate sebacate) bioelastomer. Polymers. 2013;5(4):1339–1351.
- Praphakar RA, Shakila H, Azger Dusthackeer VN, et al. Mannose conjugated multi-layered polymeric nano carrier system for controlled and targeted release on alveolar macrophages. Polym Chem. 2018;9(5):656–667.
- Li J, Ni X, Li X, et al. Micellization phenomena of biodegradable amphiphilic triblock copolymers consisting of poly(beta-hydroxyalkanoic acid) and poly(ethylene oxide)). Langmuir. 2005;21(19):8681–8685.
- Pich A, Schiemenz N, Corten C, et al. Preparation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) particles in O/W emulsion. Polymer. 2006;47(6):1912–1920.
- Ramezani M, Amoozegar MA, Ventosa A. Screening and comparative assay of poly-hydroxyalkanoates produced by bacteria isolated from the Gavkhooni Wetland in Iran and evaluation of poly-β-hydroxybutyrate production by halotolerant bacterium Oceanimonas sp. GK1. Ann Microbiol. 2015;65(1):517–526.
- Tanasea EE, Popaa ME, Rapab M, et al. PHB/cellulose fibers based materials: physical, mechanical and barrier properties. Agric Agric Sci Proc. 2015;6:608–615.
- George B, Shanmugam S. Phytochemical screening and antimicrobial activity of fruit extract of Sapindus mukorossi. Int J Curr Microbiol Appl Sci. 2014;3:604–611.
- Sumathra M, Rajan M, Alyahya SA, et al. Development of self-repair nano-rod scaffold materials for implantation of osteosarcoma affected bone tissue. New J Chem. 2018;42(1):725–734.
- Li X, Vinothini K, Ramesh T, et al. Combined photodynamic–chemotherapy investigation of cancer cells using carbon quantum dot-based drug carrier system. Drug Deliv. 2020;27(1):791–804.
- Cruz LCd, Miranda CSd, Santos WJd, et al. Development of starch biofilms using different carboxylic acids as plasticizers. Mat Res. 2015;18(Suppl. 2):297–301.
- Moshe H, Levi G, Mastai Y. Polymorphism stabilization by crystal adsorption on a self-assembled monolayer. Cryst Eng Comm. 2013;15(44):9203.
- Vermeulen H, Ubbink DT, Goossens A, et al. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92(6):665–672.
- Xue H, Hu L, Xiong Y, et al. Quaternized chitosan–matrigel–polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr Polym. 2019;226:115302.
- Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10(5):398–406.
- Schneider GB, Perinpanayagam H, Clegg M, et al. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003;82(5):372–376.
- Rajan M, Raj V. Formation and characterization of chitosan–polylactic acid–polyethylene glycol–gelatin nanoparticles: a novel biosystem for controlled drug delivery. Carbohydr Polym. 2013;98(1):951–958.
- Bucevicius J, Lukinavicius G, Gerasimaite R. The use of Hoechst dyes for DNA staining and beyond. Chemosensors. 2018;6:18.
- Bacáková L, Starý V, Kofronová O, et al. Polishing and coating carbon fiber‐reinforced carbon composites with a carbon‐titanium layer enhances adhesion and growth of osteoblast‐like MG63 cells and vascular smooth muscle cells in vitro. J Biomed Mater Res. 2001;54(4):567–578.
- Tan J, Saltzman WM. Biomaterials with hierarchically defined micro- and nanoscale structure. Biomaterials. 2004;25(17):3593–3601.
- Vagaska B, Bacakova L, Filova E, et al. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol Res. 2010;59:309–322.
- Goorani S, Koohi MK, Zangeneh MM, et al. Healing and cytotoxicity potentials of ointment containing aqueous extract of Anethum graveolens on cutaneous wounds in male rats. Comp Clin Pathol. 2019;28(5):1471–1481.
- Ponrasu T, Suguna L. Efficacy of Annona squamosa L in the synthesis of glycosaminoglycans and collagen during wound repair in streptozotocin induced diabetic rats. BioMed Res Int. 2014;2014:1–10.