Abstract
Oxidative stress has been proven to play a critical role in the pathogenesis of neuronal injury. As a novel adipocytokine, omentin is produced by visceral adipose with insulin sensitizing effects and has been revealed to possess anti-inflammatory effects. However, the possible effect of omentin on oxidative stress remains unknown. The present study aimed to detect the potential protective effect of omentin against hydrogen peroxide (H2O2)-induced cytotoxicity of PC12 cells. The results showed that no cytotoxic effect was shown in PC12 cells co-cultured with omentin alone at a concentration of 50–1000 ng/mL. The CCK8 and TUNEL assays suggested that omentin could remarkably attenuate apoptosis induced by 100 μM H2O2. The PCR and western blotting showed that the expression levels of Bax was significantly inhibited by omentin via the upregulation of miR-128-3p at its 3′-UTR. Taken together, these results indicated that omentin protects PC12 cells against H2O2-induced apoptosis, and further studies need to be conducted before utilization in the clinic for the treatment of neurodegenerative diseases.
Introduction
Parkinson’s disease (PD), a neurodegenerative disease, is characterized by cell damage or dysfunction of neurons in the striatum in an age-dependent manner [Citation1,Citation2]. The pharmacological treatment is preferred to relieve some symptoms. For example, dopamine agonists are used to prevent the motor complications arising from levodopa application [Citation3], yet it cannot cure PD completely. Although the specific cause of PD remains unknown, there is growing evidence suggesting that increased oxidative stress plays a role in the pathogenic factor of PD [Citation4,Citation5]. Therefore, antioxidants could be potential therapeutic management by inhibiting oxidative stress [Citation6].
Oxidative stress results from the imbalance between the antioxidant and the pro-oxidant activity in the body. Reactive oxygen species (ROS) are involved in oxidation-reduction processes. However, high concentration of ROS can be toxic and trigger oxidative stress, leading to cellular structural damage [Citation7] and alteration in components, such as proteins, nucleic acids, and lipids. These results in the activation of Bax and caspase-3 causing apoptosis [Citation8,Citation9]. ROS is a big family that includes hydrogen peroxide (H2O2), which could penetrate the cell membrane freely, thereby disabling nearby cells. Owing to this, H2O2 is the preferred agent to induce oxidative stress in numerous researches to ensure accuracy [Citation10–13].
MicroRNAs (miRNAs), a type of small non-coding RNA, are of great importance in many cellular activities, including mobility, proliferation, and apoptosis [Citation14,Citation15]. MiR-128-3p has been suggested to be related to many diseases. For instance, Chen et al. showed that it was involved in the repression of tumour growth in anaplastic thyroid cancer [Citation16]. In addition, miR-128-3p has been proven to modulate the injury of doxorubicin-treated liver cells by regulating sirtuin-1 [Citation17]. PD is associated with massive alteration in RNA metabolism, such as in patients’ leukocytes [Citation18,Citation19] and in brain neurons [Citation20]. Mor Hanan’s group found that miR-128 targets increased in PD patients and are involved in PD pathology [Citation21]. However, the potential contribution of miRNA-128 to oxidative stress is still unknown.
As a novel adipocytokine, omentin is produced by visceral adipose with insulin sensitizing effects. Normal concentration in human blood is from 100 to 800 ng/mL and is decreasing in the obese and overweight human subjects [Citation22,Citation23]. It is believed that omentin promotes vasodilation in blood vessels and has a protective effect against inflammation in endothelial cells [Citation24,Citation25]. Moreover, omentin has been demonstrated to attenuate acute ischaemic injury via the AMP-activated protein kinase (AMPK) and the Akt signalling pathways [Citation26]. Although substantial evidence has supported the effect of omentin on obesity and inflammation, the possible effect of omentin on oxidative stress remains unknown. Therefore, we hypothesize in the present study that omentin has potential protective effects against oxidative stress in H2O2-induced PC12 cells.
Materials and methods
Cell culture and treatments
Rat adrenal pheochromocytoma PC12 cells were obtained from American Type Culture Collection (ATCC). The cells were maintained in DMEM medium containing 10% FBS (AccuRef Scientific, Goshen, NY, China), 100 U/ml penicillin, and 100 U/ml streptomycin at 37 °C in a humidified incubator containing 5% CO2 for further examination, and the medium was changed every day. Human omentin was obtained from Sigma (Cat. No. 77037). The cells were pre-treated with different concentrations of omentin at different times. The cells were harvested for further examination after exposure with 100 μM H2O2 for 4 h.
CCK8 assay
The viability of PC12 cells was detected by CCK8 assay (EXPrecision, Xi’an, China) as previously reported [Citation27]. Briefly, the PC12 cells were cultured in 96-well plates with 2 × 104 per well and pre-treated with different concentrations of omentin (50, 200, 500, and 1000 ng/mL) for 24 h, 1000 ng of omentin at different times (1, 2, 5, 12, and 24 h), or 100 μM H2O2 for 4 h to assess the cytotoxic effects of omentin and H2O2. After applying the different treatments, 2 mg/mL CCK8 solution was added for another 4 h followed by the examination of optical density by a microplate reader (TECAN, infinite F50).
Tunel assay
A terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay kit (AccuRef Scientific) was used to determine apoptosis according to the manufacturer’s protocol. Preincubated PC12 cells were mixed in PBS buffer containing 4% formaldehyde for 15 min, followed by permeabilization with 0.2% Triton X-100 in PBS for another 10 min under the same conditions. After washing with PBS twice, the cells were incubated with a fluorometric terminal deoxytransferase (TdT) mixture at 37 °C for 1 h and then wash twice with PBS buffer. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Fluorescent images were captured by a Nikon Eclipse Ti Microscope.
Flow cytometric analysis of apoptosis
Flow cytometry was used to determine the alteration of apoptosis. PC12 cells were pre-treated with different concentrations of omentin, followed by H2O2. The cells were harvested, washed with PBS twice, and fixed in ice cold 70% ethanol. After that, the cells were stained with FITC-Annexin V and propidium iodide using Annexin V-FITC Apoptosis Detection Kit (Cat. No. BMS500FI-300, Invitrogen), and detected using FACSCalibur (BD Biosciences). Data were analyzed using the FlowJo software v7.6.1 (FlowJo, LLC).
SOD and CAT activity determination, GSH-px, and MDA content determination
Superoxide dismutase (SOD) and catalase (CAT) activity determination and glutathione peroxidase (GSH-px) and malondialdehyde (MDA) content determination were carried out using commercial kits (Beyotime Biotech, Shanghai, China). Protein concentrations were determined using BCA protein quantification kit (Solarbio Sci & Tech, Beijing, China).
RT-qPCR
To clarify the potential effect of omentin against H2O2-induced PC12 cells on a molecular level, a Trizol reagent (EXPrecision) was used to extract the total RNA, followed by reverse-transcription with FastKing-RT SuperMix (Cat. No. KR118, TIANGEN BIOTECH) or miRcute Plus miRNA First-Strand cDNA Kit (Cat. No. KR211, TIANGEN BIOTECH) and amplification with SuperReal PreMix Plus (Cat. No. FP205, TIANGEN BIOTECH) or miRcute Plus miRNA qPCR kit (Cat. No. FP411, TIANGEN BIOTECH). The specific protocol is as follows: denaturation under 95 °C for 30 s repeated twice, annealing under 60 °C for 10 s, and elongation at 75 °C for 15 s. The whole process was repeated 40 times. The sequences for the primers are listed as follows: miR-214-3p primer: Forward 5′-GTCTGCCTGTCTACACTTGCT-3′; miR-216a-3p primer: Forward 5′-CTCAGCTGGCAACTGTGAGA-3′; miR-520a-5p primer: Forward 5′-TGTGACCCTCCAGAGGGAAGT-3′; miR-525-5p primer: Forward 5′-CTCTCCAGAGGGATGCACTT-3′; miR-128-3p primer: Forward 5′-TTGGATTCGGGGCCGTAG-3′; miR-3681-3p primer: Forward 5′-TTCCAGTAGTGGATG-3′; miR-1276 primer: Forward 5′-TAGGTAAAGAGCCCTGTGGAGA-3′; miR-766-5p primer: Forward 5′-GGGTGGTAGGAGGAATTGGTG-3′; miR-4640-3p primer: Forward 5′-GAGCAGCTGGTGGGTGG-3′; Bax primers: Forward 5′-TCATGGGCTGGACATTGGAC-3′, Reverse 5′-GCGTCCCAAAGTAGGAGAGG-3′.
Western blotting assay
Following the different treatments, we detected the alternation of protein related to apoptosis. A RIPA lysis buffer (AccuRef Scientific) was recruited to extract the protein from PC12 cells. The protein concentration in the lysis buffer was determined using a BCA protein quantification kit (AccuRef Scientific). Equal amounts of proteins (10–15 mg) were separated by SDS-PAGE (14%) and transferred to a PVDF membrane (ATTO, Tokyo Japan). After being blocked with 0.5% skim milk, the membranes were incubated with primary Bcl-2 antibody (ABCam, ab182858), Bax antibody (ABCam, ab32503), Caspase-3 antibody (ABCam, ab32351), cleaved-Caspase-3 antibody (ABCam, ab2302), and β-actin antibody (ABCam, ab6276, 1:500 dilution) at 4 °C overnight, and the membrane-bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (1:10,000 dilution, 1 h) and the EZ-ECL system (Biological Industries, Kibbutz Beit-Haemek, Israel). Meanwhile, β-actin was used as the internal control. Then, the relative intensities were calculated as a densitometric ratio between the sample and β-actin by Image J (V1.50b). Each experiment was repeated for three times.
Dual-luciferase reporter assay
Dual-luciferase reporter assay was performed using commercial Kit (E1980, Promega). In brief, wild type (WT) or mutant type (MUT) Bax 3′-UTR was constructed into dual-luciferase reporter vector pGL4.10. Then, HEK 293 T cells were co-transfected with pGL4.10-WT or pGL4.10-MUT Bax 3′-UTR and miR-NC or miR-128-3p mimics. Luciferase reporter activity was measured using the DLRTM assay systems according to the manufacture’s instruction.
Statistical analysis
All data were shown as mean ± SD of three independent experiments. Statistical analysis was performed using one-way analysis of variance and Tukey’s post hoc test in IBM SPSS statistics version 22.0. A p-value of less than 0.05 (p<.05) was regarded as statistically significant.
Results
Omentin inhibited H2O2-induced PC12 cell death
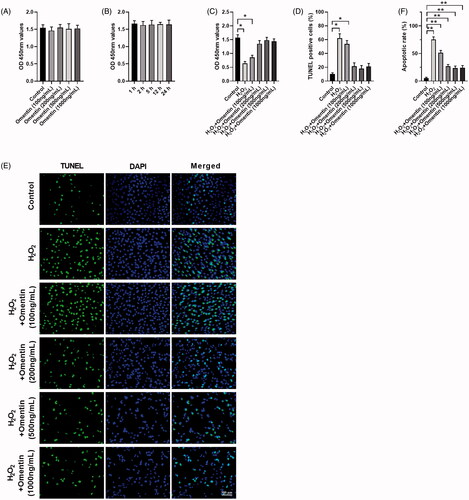
The dopamine-containing cell line, PC12, is a widely used cellular model for investigating oxidative stress in PD due to their similarities to the physiological properties of dopaminergic neurons [Citation28–30]. In the present study, we first investigated the effect of omentin on H2O2-induced PC12 cell death by CCK8 and TUNEL assays. We cultured PC12 cells with different doses of omentin (100, 200, 500, and 1000 ng/mL) for 24 h and 1000 ng/mL at different times (1, 2, 5, 12, and 24 h). As shown in , the effect of omentin on PC12 cell viability did not show a dose-manner or time-manner relationship, which means it did not have any cytotoxic effect. However, incubation with 100 μM H2O2 for 4 h resulted in a decrease in cell viability to 0.64. Pre-treated cells with omentin significantly inhibited H2O2-induced cell death in a dose-dependent manner (), 53.7% at 100 ng/mL and 0% at 200, 500, and 1000 ng/mL, which was confirmed by TUNEL (). In addition, apoptotic tendency was inhibited (), although omentin alone cannot affect cell viability. The potential protective effect of omentin against H2O2-induced cell death was demonstrated.
Figure 1. Omentin protected PC12 cell death against H2O2-induced apoptosis. (A,B) The effect of omentin on the viability of PC12 cells. The cells were incubated with different concentrations of omentin (100, 200, 500, and 1000 ng/mL) for 24 h, 1000 ng/mL omentin for different time (1, 2, 5, 12, and 24 h), respectively. (C–F) The protective effect of omentin on H2O2-induced injury. After preincubation with different dose of omentin for 24 h, PC12 cells were treated with 100 μM H2O2 for 4 h. Cell viability was assessed by CCK8 (C), and cell apoptosis rates were assessed by TUNEL assays (D–E) and flow cytometry (F). Data are expressed as mean ± SD (n = 3) (*p<.05; **p<.01).
Effect of omentin on Bcl-2, Bax, pro-caspase3, and cleaved-caspase3 expression in H2O2-induced PC12 cells
The protective effects of omentin on the cells from oxidative stress were determined by the quantification of the reactivity of the antioxidant enzymes, namely SOD, CAT, GSH-Px, and MDA by ELISA, as shown in . The activity of SOD, CAT, and GSH-Px were significantly decreased in the H2O2 group, while MDA activity was increased compared to the control group. However, pre-treating with 500 ng/mL of omentin before H2O2 treatment greatly elevated the activity of SOD, CAT, and GSH-Px and inhibited the activity of MDA.
Figure 2. Omentin inhibited the apoptosis of PC12 cells induced by H2O2. (A) PC12 cells were pre-incubated with 500 ng/mL omentin for 24 h before exposure to H2O2 for 4 h. The antioxidant enzymes of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), and malondialdehyde (MDA) were analyzed. (B) The western blotting assay was used to detect the expression levels of proteins related to apoptosis, including Bcl-2, Bax, pro-Caspase3, and cleaved-Caspase3, while β-actin was utilized as an internal control. (C) PC12 cells were cultured with different doses of omentin for 24 h, and the western blotting assay was used to detect the expression levels of proteins related to apoptosis. Results are presented as mean ± SD (n = 3) (*p<.05; **p<.01).
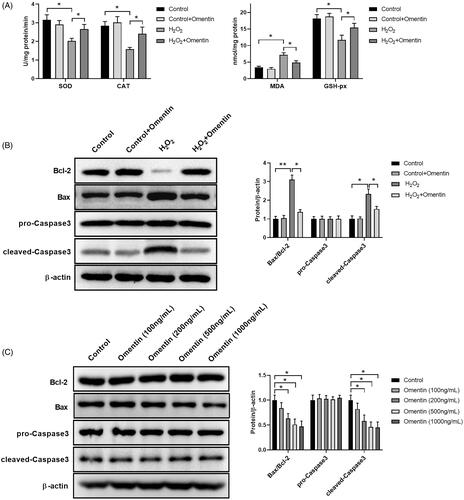
Many researches have proven that oxidative damages result in apoptosis. As a pro-apoptotic protein in the Bcl-2 family, Bax and Caspase-3 are involved in the activation of the death protease of the cysteine protease family, which is usually relevant to apoptosis [Citation31]. Overexpression of pro-apoptotic substances can significantly accelerate cell apoptosis [Citation32]. The expression of Bcl-2, Bax, pro-Caspase3, and cleaved-Caspase3 were determined by western blot analysis to investigate potential protective effect of omentin on H2O2-induced apoptosis. As shown in , H2O2 treatment induced the activation of Bax and cleaved-Caspase3, while inhibiting Bcl-2 activity. Conversely, pre-treatment with 500 ng/mL of omentin could significantly attenuate the abnormal expressions. It is noteworthy that preincubation with omentin inhibited Bax expression as opposed to other proteins. To further explore whether omentin could alter Bax expression, we treated PC12 with various concentrations of omentin for 24 h and detected Bcl-2, Bax, pro-Caspase3, and cleaved-Caspase3 expressions. As shown in , the Bax expression was markedly reduced by omentin in a concentration-dependent manner, while Bcl-2, pro-Caspase3, and cleaved-Caspase3 expressions had no effect. The results supported the idea that omentin can block H2O2-induced apoptosis via reducing Bax and cleaved-Caspase3 expressions, and Bax plays a role in the underlying mechanism of omentin on PC12 cells.
Mir-128-3p directly targets Bax
MiRNA can activate their biological functions by regulating target genes. In fact, each miRNA can target thousands of genes, and we involved those from three target gene prediction programs (StarBase, TargetScan and miRDB) for Venn diagram aggregation analysis. All three programs showed the same target genes in the centre (nine genes), which suggested that Bax is more likely bound to nine target miRNAs as shown in . The expression of related miRNA was detected after pre-incubation with omentin by qPCR to figure out the specific miRNA of Bax (). Pre-incubation with omentin before treating with H2O2 significantly increased the expression of hsa-miR-214-3p, hsa-miR-128-3p, hsa-miR-525-5p, hsa-miR-1276, and hsa-miR-766-5p, compared to treatment with H2O2 alone. And the changes of hsa-miR-128-3p expression level were the most (), suggesting that omentin decrease Bax expression maybe via mediating miR-128-3p expression.
Figure 3. MiR-128-3p suppresses Bax expression. (A) Bioinformatics analysis of microRNA upstream of Bax. (B) The expression levels of different miRNAs in PC12 cells co-cultured with 500 ng/mL omentin. (C) The expression levels of different miRNAs in PC12 cells pre-incubated with 500 ng/mL omentin before exposed to H2O2. (D–G) The effect of miR-128-3p mimics (D & F) and inhibitor (E & G) on the Bax expression was detected by RT-qPCR (D & E) and Western blotting assay (F & G), respectively. (H) Luciferase assay of HEK 293 T co-transfected with wild-type (WT) or mutated (MUT) Bax 3′-UTR and miR-128-3p mimics. Results are presented as mean ± SD (n = 3) (*p<.05; **p<.01).
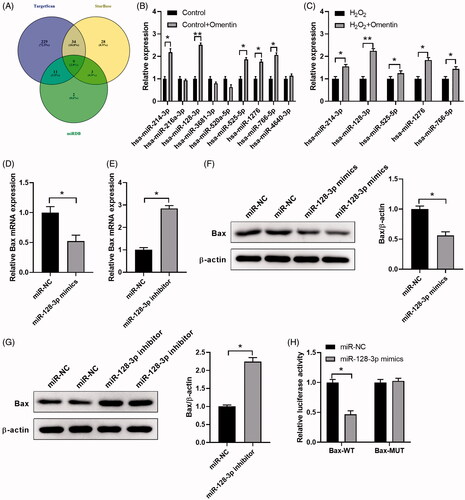
To verify the regulatory interaction between miR-128-3p and Bax, Bax mRNA expression level was determined after transfection with miR-128-3p mimics or miR-128-3p inhibitor. We found that miR-128-3p mimics remarkably reduced Bax mRNA expression, while miR-128-3p inhibitor promoted them (), which was also confirmed by western blot (). The luciferase activity was notably inhibited by co-transfection with miR-128-3p mimics and wild-type Bax 3′-UTR, while co-transfection with miR-128-3p mimics and mutant-type Bax 3′-UTR did not change the luciferase activity, which confirmed the direct binding of miR-128-3p to the 3′-UTR of Bax as shown in . These data provided concrete evidence that miR-128-3p targets Bax and represses its expression.
Protective effects of miR-128-3p on H2O2-induced apoptosis in PC12 cells
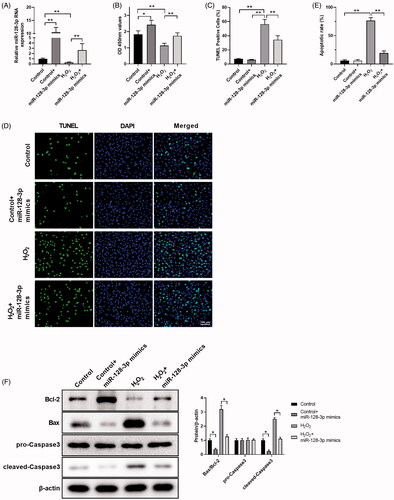
To analyze the function of miR-128-3p during H2O2-induced apoptosis, we transfected miR-128-3p into PC12 cells. The miR-128-3p expression was promoted by miR-128-3p mimics transfection, while it was significantly inhibited by H2O2 treatment as shown in . CCK8 data revealed that the overexpression of miR-128-3p showed a protective effect on cell viability (), which was confirmed by TUNEL assay () and flow cytometry (), when compared to treatment with H2O2 alone. We next determined the protective effect of miR-128-3p against H2O2 at the molecular level. MiR-128-3p mimics markedly downregulated Bax and cleaved-Caspase3 expression, when compared to the control and H2O2 group, respectively, as evaluated by western blotting shown in . Conversely, Bcl-2 was elevated by miR-128-3p mimics, which suggested that the protective effect of miR-128-3p against oxidative stress to the cells may result in the inhibition of Bax and cleaved-Caspase-3 expressions. These results are consistent with the effect of omentin against H2O2-induced apoptosis in PC12 cells.
Figure 4. MiR-128-3p mimics possess the potential effect against H2O2-induced apoptosis. (A) The changes in miR-128-3p expression levels transfected with miR-128-3p mimics followed by incubating with H2O2. (B–D) The cell viability of PC12 cells treated with H2O2 and miR-128-3p mimics was analyzed by CCK8 (B), and the cell apoptosis rates were detected by TUNEL assay (C,D). (E) The cell apoptosis rates were detected by flow cytometry. (F) The effect of H2O2 and miR-128-3p mimics on apoptosis-related proteins expression were detected by western blotting assay. Results were presented as mean ± SD (n = 3) (*p<.05, **p<.01).
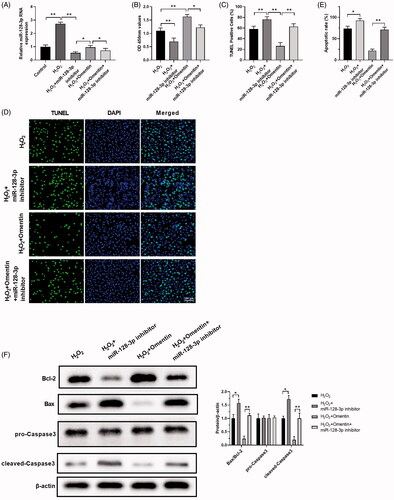
Mir-128-3p inhibitor attenuated omentin’s protective effect against H2O2-induced apoptosis
To further clarify the underlying mechanism of omentin’s protective effect on H2O2-induced PC12 cells, we involved in the miR-128-3p inhibitor. MiR-128-3p expression levels were detected using RT-qPCR when treating with/without H2O2, omentin, and the miR-128-3p inhibitor, as shown in . Treatment with omentin showed higher miR-128-3p expression level as compared to the miR-128-3p inhibitor in the H2O2 group, which was restrained by the miR-128-3p inhibitor. presents the changes of PC12 cell viability with different treatments. Omentin notably protected PC12 cells from H2O2 damage, while miR-128-3p inhibitor promoted cell death and attenuated omentin’s protection effect against H2O2-induced cell damage. These findings were consistent with TUNEL assay () and flow cytometry (). Moreover, we examined the effect of miR-128-3p inhibitor on the apoptotic proteins of PC12 cells, including Bcl-2, Bax, pro-caspase3, and cleaved-caspase3. As shown in , Bax and cleaved-Caspase3 were significantly up-regulated by the miR-128-3p inhibitor, while Bcl-2 was greatly down-regulated. However, omentin greatly reversed these effects, and co-treatment with miR-128-3p inhibitor reduced the omentin’s inhibitory effect on the expression of Bax and cleaved-Caspase3 in PC12 after exposure to H2O2. Thus, combining the miR-128-3p inhibitor with omentin restrains omentin’s protective effect against H2O2-induced apoptosis in PC12 cells.
Figure 5. MiR-128-3p inhibitors alleviate the protective effect of omentin against H2O2-induced apoptosis. (A) The changes of miR-128-3p expression level after treated with H2O2, H2O2 + miR-128-3p inhibitor, H2O2 + omentin, H2O2 + omentin + miR-128-3p inhibitor. (B–D) The cell viability of PC12 cells in different groups were detected by CCK8 assay (B), and the cell apoptosis rates were detected by TUNEL assay (C,D). (E) The cell apoptosis rates were detected by flow cytometry. (F) The effect of H2O2, miR-128-3p inhibitor, and omentin on the expression of proteins related to apoptosis were detected by Western blotting assay. Results were presented as mean ± SD (n = 3) (*p<.05; **p<.01).
Discussion
ROS, such as the superoxide radical (O–2), hydroxyl radical (.OH), and hydrogen peroxide (H2O2) are of great importance in apoptosis [Citation33]. ROS in high concentrations can be toxic and can trigger oxidative stress. This leads to cellular structure damage and alteration in its components, including proteins, nucleic acids, and lipids, resulting in apoptosis [Citation8]. Although the specific cause of PD remains unknown, an increasing number of studies have suggested that oxidative stress is closely related to its pathogenesis [Citation34,Citation35]. An adipocytokine named omentin has been reported to be inhibited in obesity [Citation36]. Substantial evidence shows that omentin is protective against cardiac diseases, where omentin-1 is negatively corelated with blood IL-6 level in patients with acute coronary syndrome and stable angina pectoris [Citation37]. Moreover, omentin was noted to restrain TNF-α-induced VCAM-1 expression and the production of superoxide through the inactivation of p38 and JNK [Citation38]. However, the potential effects of omentin against oxidative stress in PC12 cells remains unknown.
In the present study, H2O2 was used to induce oxidative stress in PC12 cells and maintain the accuracy in all the experiments. Firstly, we examined the toxicity of omentin on PC12 cell viability by con-culturing with varying concentrations and durations using the CCK8 assay. The data showed that the effect of omentin on PC12 cell viability was not dose-dependent nor time- dependent. In addition, after exposure to 100 μM H2O2 for 4 h, the preincubation of omentin remarkably augmented cell viability. These results indicate a potential protective effect of omentin against H2O2-induced apoptosis. To clarify the underlying mechanism of omentin’s potential protective effect, the protein expression levels of those related to apoptosis were detected. The data suggested that omentin altered Bax expression directly, which was mediated by miR-128-3p through the targeting of its 3′UTR ( and ). To further confirm the potential mechanism, we also involved the miR-128-3p inhibitor and its mimics, since omentin is known as a miR-128-3p activator, in order to show its protective effect against H2O2-induced PC12 cells, and was found to be consistent with our previous findings in and .
Extensive studies have reported the regulatory role of miR-128-3p during the pathogenesis of a variety of human diseases, such as Parkinson’s disease, myocardial inflammation, multiple sclerosis, and liver ischaemia-reperfusion injury [Citation39–42] etc. Mao et al. found that increased miR-128-3p level could reduce the protein level of p38α thus inhibit the activation of pro-apoptotic pathway and prevent neuronal death during cerebral ischaemia in mice [Citation43]. In a recent study by Liu et al., the protective role of miR-128-3p was confirmed by a Lipopolysaccharide (LPS)-induced myocardial inflammation chicken model, which showed that LPS administration inhibited miR-128-3p and increased the expression of p38 MAPK, resulting the occurrence of myocardial inflammation [Citation40]. Besides, the increased expression of miR-128-3p treatment alleviate the ROS production and oxidative injury in cardiomyocytes. In consistent with their findings, we found omentin treatment notably protects PC12 cells from H2O2 damage, while miR-128-3p inhibitor attenuated omentin’s protection effect against H2O2-induced cell damage ().
Taken together, our present research is the first to show that omentin can attenuate the cytotoxicity and apoptosis of PC12 cells through the inhibition of Bax expression via the targeting of miR-128-3p at 3′UTR after exposure to H2O2. The findings of the present study may shed light on the molecular basis for the clinical application of omentin for the treatment of PD.
Supplemental Material
Download TIFF Image (307.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and/or analyzed during the present study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Jenner P, Olanow CW. Understanding cell death in Parkinson's disease. Ann Neurol. 1998;44(3 Suppl 1):S72–S84.
- Zhang S, Tang M-B, Luo H-Y, et al. Necroptosis in neurodegenerative diseases: a potential therapeutic target. Cell Death Dis. 2017;8(6):e2905.
- Schaeffer E, Pilotto A, Berg D. Pharmacological strategies for the management of levodopa-induced dyskinesia in patients with Parkinson's disease. CNS Drugs. 2014;28(12):1155–1184.
- Niedzielska E, Smaga I, Gawlik M, et al. Oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2016;53(6):4094–4125.
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: evidence supporting it. Ann Neurol. 1992;32(6):804–812.
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–975.
- Klaunig JE, Wang Z, Pu X, et al. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254(2):86–99.
- Fico A, Paglialunga F, Cigliano L, et al. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11(8):823–831.
- Radi E, Formichi P, Battisti C, et al. Apoptosis and oxidative stress in neurodegenerative diseases. JAD. 2014;42(s3):S125–S152.
- Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006;20(9):1501–1503.
- Han Q, Wang H, Xiao C, et al. Oroxylin A inhibits H2O2-induced oxidative stress in PC12 cells. Nat Prod Res. 2017;31(11):1339–1342.
- Chen L, Wu X, Shen T, et al. Protective effects of ethyl gallate on H2O2-induced mitochondrial dysfunction in PC12 cells. Metab Brain Dis. 2019;34(2):545–555.
- Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619.
- Christensen LL, Holm A, Rantala J, et al. Functional screening identifies miRNAs influencing apoptosis and proliferation in colorectal cancer. PLOS One. 2014;9(6):e96767.
- Luu HN, Lin H-Y, Sørensen KD, et al. miRNAs associated with prostate cancer risk and progression. BMC Urol. 2017;17(1):18.
- Chen J, Zhao D, Meng Q. Knockdown of HCP5 exerts tumor-suppressive functions by up-regulating tumor suppressor miR-128-3p in anaplastic thyroid cancer. Biomed Pharmacother. 2019;116:108966.
- Zhao X, Jin Y, Li L, et al. MicroRNA-128-3p aggravates doxorubicin-induced liver injury by promoting oxidative stress via targeting Sirtuin-1. Pharmacol Res. 2019;146:104276.
- Soreq L, Israel Z, Bergman H, et al. Advanced microarray analysis highlights modified neuro-immune signaling in nucleated blood cells from Parkinson's disease patients. J Neuroimmunol. 2008;201–202:227–236.
- Soreq L, Guffanti A, Salomonis N, et al. Long non-coding RNA and alternative splicing modulations in Parkinson's leukocytes identified by RNA sequencing. PLOS Comput Biol. 2014;10(3):e1003517.
- Liu EY, Cali CP, Lee EB. RNA metabolism in neurodegenerative disease. Dis Model Mech. 2017;10(5):509–517.
- Hanan M, Simchovitz A, Yayon N, et al. A Parkinson's disease CircRNAs Resource reveals a link between circSLC8A1 and oxidative stress. EMBO Mol Med. 2020;12(9):e11942.
- Batista CMdS, Yang R-Z, Lee M-J, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–1661.
- Yan P, Li L, Yang M, et al. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on plasma omentin-1 levels in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;92(3):368–374.
- Yamawaki H, Tsubaki N, Mukohda M, et al. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem Biophys Res Commun. 2010;393(4):668–672.
- Yamawaki H, Kuramoto J, Kameshima S, et al. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun. 2011;408(2):339–343.
- Kataoka Y, Shibata R, Ohashi K, et al. Omentin prevents myocardial ischemic injury through AMP-activated protein kinase- and Akt-dependent mechanisms. J Am Coll Cardiol. 2014;63(24):2722–2733.
- Li H, Li C, Shen T, et al. R-eriodictyol and S-eriodictyol exhibited comparable effect against H2O2-induced oxidative stress in EA.hy926 cells. Drug Discov Ther. 2014;8(5):218–224.
- Greene L, Tischler A. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73(7):2424–2428.
- Offen D, Panet H, Melamed E. Catechol-O-methyltransferase reduced levodopa toxicity in PC12 cells: implications for treatment of Parkinson's disease. Ann Neurol. 1999;46(3):459–459.
- Sun H-J, Wang Y, Hao T, et al. Efficient GSH delivery using PAMAM-GSH into MPP-induced PC12 cellular model for Parkinson's disease. Regen Biomater. 2016;3(5):299–307.
- Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309(5733):481–484.
- Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166(1):29–43.
- Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21(3):83–86.
- Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(S7):S18–S25.
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59(5):1609–1623.
- Moreno-Navarrete JM, Catalán V, Ortega F, et al. Circulating omentin concentration increases after weight loss. Nutr Metab. 2010;7(1):27.
- Zhong X, Zhang H-y, Tan H, et al. Association of serum omentin-1 levels with coronary artery disease. Acta Pharmacol Sin. 2011;32(7):873–878.
- Kazama K, Usui T, Okada M, et al. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol. 2012;686(1–3):116–123.
- Zhang G, Chen L, Liu J, et al. HIF-1α/microRNA-128-3p axis protects hippocampal neurons from apoptosis via the Axin1-mediated Wnt/β-catenin signaling pathway in Parkinson's disease models. Aging. 2020;12(5):4067–4081.
- Liu J, Wang S, Zhang Q, et al. Selenomethionine alleviates LPS-induced chicken myocardial inflammation by regulating the miR-128-3p-p38 MAPK axis and oxidative stress. Metallomics. 2020;12(1):54–64.
- Zanoni M, Orlandi E, Rossetti G, et al. Upregulated serum miR-128-3p in progressive and relapse-free multiple sclerosis patients. Acta Neurol Scand. 2020;142(5):511–516.
- Mou T, Luo Y, Huang Z, et al. Inhibition of microRNA-128-3p alleviates liver ischaemia-reperfusion injury in mice through repressing the Rnd3/NF-κB axis. Innate Immun. 2020;26(6):528–536.
- Mao G, Ren P, Wang G, et al. MicroRNA-128-3p protects mouse against cerebral ischemia through reducing p38α mitogen-activated protein kinase activity. J Mol Neurosci. 2017;61(2):152–158.
