?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Triamcinolone acetonide (TA) is widely indicated in the treatment of several ocular disorders, but the free drug suspension limits its clinical benefits and commercial compositions cause adverse ocular effects. In this study, TA was formulated in poly(d,l-lactide-co-glycolide) (PLGA)-chitosan (PLC) nanoparticles (NPs) for the treatment of ocular inflammatory diseases. TA-loaded PLC NPs exhibited excellent anti-inflammatory activity against human corneal epithelial (HCE) cells and significantly reduced the secretion of interleukin (IL)-6 in tumour necrosis factor (TNF)-α activated cells. In a rabbit model, TA-loaded PLC NPs did not show any typical clinical signs of eye inflammation and significantly alleviated inflammatory signs, compared with free TA suspension, at 24 h after a single dose. TA-loaded PLC NPs exhibited a greater aqueous humour transparency (%AHT), compared with that of normal saline (NS) or free TA suspension, indicating reduction in anterior chamber fogginess. Pharmacokinetic analysis of rabbit eyes revealed that TA-loaded PLC NPs peaked at 6 h. Substantial concentrations of TA were observed until 24 h, indicating the superiority of this PLC-based nanocarrier system. Overall, PLC-based NP formulations offer a new approach for the treatment of ocular inflammatory diseases.
Introduction
Ocular inflammation is the main cause of vision impairment and is considered a major public health concern. According to the World Health Organization, 250 million people are presumed to have vision impairment worldwide, especially in underdeveloped and developing nations [Citation1]. Cataracts inhibit the passage of light and cause clouding of the eye lens. Age-related cataracts are predominant and multifactorial in nature [Citation2,Citation3]. This multifactorial impairment results from multiple mechanisms that cause post-translational modifications and abnormal protein signalling in the eye lens, thereby contributing to the racemization of local cellular proteins [Citation4]. Furthermore, lenticular lesions are accelerated with the reduction of ocular protein solubility, due to the presence of oxidative stress-related metabolic products [Citation5]. To our knowledge, no single treatment strategy can effectively prevent ocular inflammation. Surgery is the primary treatment approach, despite its considerable expense for most developing countries. However, ocular surgery carries considerable risk and healthcare access is necessary after surgery [Citation6].
Corticosteroids such as dexamethasone and triamcinolone acetonide (TA) are often indicated in the treatment of eye disorders including cataracts, conjunctivitis, scleritis, uveitis, diabetic macular oedema and diabetic retinopathy [Citation7,Citation8]. Notably, the lipophilic nature of TA creates a non-specific distribution among ocular tissues that reduces bioavailability in the region of interest [Citation9]. Furthermore, TA is likely to reach sensitive tissues and cause adverse effects. Topical administration of free corticosteroids can damage the structure and function of local tissues such as Schlemm’s canal [Citation10,Citation11]. These adverse effects are observed during long-term administration of corticosteroids [Citation12]. Thus, an ophthalmic delivery system is needed to control the release of encapsulated drugs specifically in the region of interest and inhibit its accumulation in sensitive tissues [Citation13].
Nanomedicine has shown great potential to improve the therapeutic efficacy of poorly soluble drugs, such as TA. Multiple nanoscale carriers with polymer and lipid origins have shown considerable potential for controlling the release and biodistribution of encapsulated therapeutics [Citation14–18]. Due to their small size and adjustable surface characteristics, polymeric nanoparticles (NPs) may be suitable for ophthalmic applications [Citation19]. In contrast to a free drug solution that non-selectively distributes into sensitive cells, NPs can be optimized to facilitate cellular interactions and subsequent internalization into appropriate cells [Citation20]. We hypothesized that corticosteroid drugs loaded in polymeric NPs could preferentially accumulate in specific ocular tissues, thus minimizing the side effects associated with molecular dispersions that immediately demonstrate supra-therapeutic concentrations and subsequently exhibit sub-therapeutic concentrations. Due to their small size and adhesive properties, NPs could potentially prolong drug persistence in the precorneal region, thereby enhancing ocular bioavailability [Citation21]. In this study, we selected poly(d,l-lactide-co-glycolide) (PLGA) as a biopolymer to encapsulate the hydrophobic drug (i.e. TA) [Citation22]. PLGA exhibits excellent biodegradability, biocompatibility, low immunogenicity and low toxicity [Citation23]. PLGA NPs reportedly degrade by hydrolysis and release encapsulated therapeutics in a controlled manner. To control the release of drug from PLGA NPs, the NP surface was coated with cationic chitosan (CHT) polymer, which is highly biodegradable and biocompatible. A slight positive charge on CHT-coated NPs is presumed to enhance cellular uptake and targeting [Citation24–28].
The main aim of this study was to optimize the design of CHT-coated PLGA NPs to encapsulate TA, thereby improving the therapeutic efficacy of TA in cataract treatment. Detailed physicochemical characterizations were performed and therapeutic efficacy was assessed by interleukin (IL)-6 assays and clinical inflammation analyses in a rabbit model.
Materials and methods
Preparation of TA-loaded PLGA-CHT (PLC) NPs
TA-loaded PLC NPs were prepared by an emulsion-evaporation method. Briefly, 50 mg of PLGA and 10 mg of TA were dissolved in dichloromethane (3 ml) and stirred for 5 min. The organic phase was added slowly to an aqueous phase consisting of 5 ml of 1% polyvinyl alcohol and immediately homogenized at 13,000 rpm using an Ultra-Turrax T-25 homogenizer to form an oil-in-water emulsion, which was then sonicated at 100% W for 5 min employing a probe sonicator (Vibracell VCX130; Sonics, Newtown, CT). The organic solvent was gradually evaporated using a rotary evaporator at room temperature. TA-loaded PLGA NPs were dialysed briefly for 12 h to remove unencapsulated drugs. The PLGA NP concentrate was collected and CHT solution (pH 4.0) was added at a weight ratio of 10/1 w/w (PLGA/CHT), then incubated for 30 min at room temperature. NPs were centrifuged at 10,000 rpm for 10 min and the final PLC NPs were collected and stored until further use. The particle size distribution, polydispersity index and surface charge were evaluated using a ZetaSizer Nano ZS90 (Malvern Instruments Inc., Worcestershire, UK) at 25 °C. Particle morphology was evaluated using a Morgagni transmission electron microscope (FEI Co., Hillsboro, OR) at 100 kV. The entrapment and loading efficiency of TA were evaluated by high-performance liquid chromatography (HPLC). The stabilities of all NPs were evaluated at 4 °C for 45 days. Throughout the study period, change in particle size was used as an indicator of stability.
In vitro drug-release studies
TA-loaded PLGA NPs (PL NPs) and PLC NPs were freeze-dried using 1% trehalose and 2-mg equivalents of TA-containing NPs were used for release studies. Briefly, freeze-dried NPs were suspended in 1 ml of physiological buffer (pH 7.4) and packed in a dialysis tube (MWCO = 5000), then placed in a tube containing 25 ml of release buffer. The samples were shaken at 100 rpm at 37 °C and samples were collected periodically during incubation. The amount of drug released was calculated via HPLC using a Shimadzu Prominencei LC-2030C 3D liquid chromatograph with diode array detector (Kyoto, Japan). An Altima C18 RP18 HP column (250 × 4.6 mm2, with 5-µm particles) was used for drug separation. The mobile phase consisted of methanol/water at a ratio of 72/28, with a flow rate of 1 ml/min and detection at 254 nm. The mobile phase was filtered and degassed before use. The samples were prepared using a 0.45-µm filter and a 10-µl analysis volume.
Cell culture and cell viability assay
Human corneal epithelial (HCE) cells were used for in vitro cell viability assays. HCE cells were grown in DMEM/F-12 media with 15% foetal bovine serum and 1% antibiotic mixture. The media were also supplemented with 0.5% dimethyl sulphoxide, 10 ng/ml and 5 mg/ml insulin. Cells were maintained in ambient conditions in an incubator. For the cell-viability assay, HCE cells were seeded in 96-well plates (1 × 104 cells/well) and allowed to adhere overnight. The cells were incubated with one of three NP formulations for 24 h: empty PLC, TA-loaded PLC (0.1%) and TA-loaded PLC (1%). The media were replaced with fresh media containing MTT reagent and incubated for 4 h, then mixed with 100 µl of 0.1 N HCl in isopropanol anhydride. The resulting absorbance was read at 570 nm with a microplate reader.
In vitro corneal permeation assay
The in vitro corneal permeation study was performed on a freshly excised rabbit cornea using a Franz diffusion cell apparatus that consist of a donor compartment and receptor compartment with an 0.90 cm i.d., effective diffusion area of 0.70 cm2 and volume of 7.8 ml. The excised rabbit cornea was mounted between receptor and donor compartment in the modified Franz diffusion cell and the receptor chamber was filled with PBS (pH 7.4) buffer which is constantly stirred at 50 rpm and maintained at 37 °C. A 100 µl of sample was withdrawn at designated time and replaced with equal amount of fresh samples. The amount of TA released from free suspension as well as from PLC-TA was quantified using HPLC.
In vitro anti-inflammatory assay
The anti-inflammatory activities of free TA and TA-loaded PLC NPs were evaluated using the method established by Enríquez-de-Salamanca et al. [Citation20]. HCE cells were seeded in 24-well plates (4 × 104 cells/well) and incubated for 36–48 h in regular media, as described above. Before the experiment, cells were incubated in serum-free media for 4 h, then with media containing 20 ng/ml tumour necrosis factor (TNF)-α for an additional 48 h. HCE cells were then treated with free TA and TA-loaded PLC NPs for 1 h, and the supernatant media were collected for the quantification of IL-6 secretion using an enzyme-linked immunosorbent assay kit. Cells treated with TNF-α alone were used as a positive control and the experiment was performed in triplicate.
In vivo therapeutic efficacy in an animal model
An endotoxin-induced uveitis rabbit model was used for this analysis. Briefly, male albino rabbits underwent topical anaesthesia induction using 0.5% proparacaine hydrochloride eye solution. Each rabbit was administered 50 µl of lipopolysaccharide (1 ng/ml) via intravitreal injection. The rabbits were divided into three groups with four rabbits per group, and treatment was initiated at 1 h after lipopolysaccharide injection. The animals underwent subconjunctival injection of 100 µl of TA (1%) suspension, TA-loaded PLC NPs (equivalent to 250 µg/ml) or normal saline (NS). The clinical signs after the respective treatment were observed through slit-lamp and binocular indirect ophthalmoscopy (Heine small pupil) using a 20D lens (VOLK) at 24 h, 48 h and 72 h, respectively, after the treatment. The NS groups were used for baseline response. The severity of treatment was observed in terms of inflammation score determined by corneal damage, anterior chamber cell flares, vitreous haze and iris vessels. The mean total score (MTS) was calculated using the following formula:
wherein, X1, X2, X3 and X4 are inflammation score of cornea, anterior chamber cell flares, vitreous haze and iris, respectively, and (n) is the number of animals (n = 3). The inflammation scores are graded as 0 = none; 1 = minimal; 2 = mild; 3 = moderate; 4 = marked; 5 = severe, respectively.
In a separate study, aqueous humour fogginess was photographed and the images were processed using ImageJ software (Bethesda, MD) to determine the differences in corneal reflections among animal groups. The change in fogginess among animal groups was quantified in terms of aqueous humour transparency (%AHT), calculated as %AHT=D/E × 100%, where D represents aqueous humour fogginess in the endotoxin-induced uveitis rabbit eye and E represents fogginess in the normal rabbit eye. All animal study protocols were approved by our institutional care and use committee.
Pharmacokinetic analysis
Pharmacokinetic analysis was performed using New Zealand Albino rabbits (n = 5, 2.5–3 kg). A single dose of free TA or TA-loaded PLC NPs (1 mg/ml equivalent) was administered to the lower conjunctival sac of one eye of each rabbit. The NPs were freshly prepared on the day of the experiment. Samples were then collected from the aqueous humour at predetermined time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 12 and 24 h). Each 20-µl sample was diluted with 80 µl of mobile phase (as mentioned above) and filtered through a 26-gauge needle, then centrifuged. A 50-µl volume of supernatant sample was used for HPLC drug analysis as mentioned above.
Statistical analysis
Statistical analysis was performed using SPSS 14.0 (Chicago, IL). Statistical significance was set at p<.05 and data are presented as means ± standard deviations.
Results
Characterization of TA-loaded PLC NPs
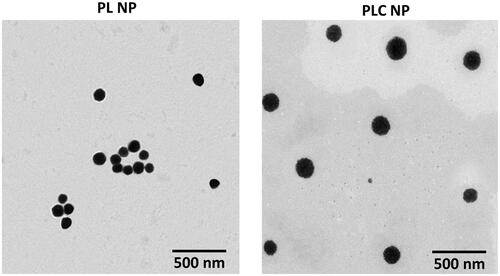
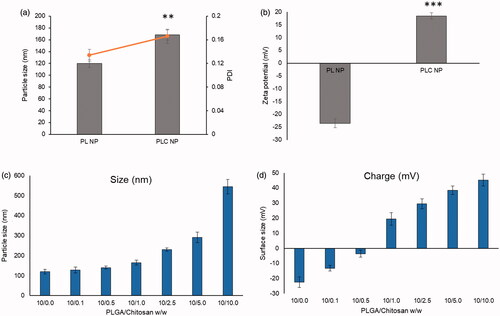
In this study, we synthesized PLC NPs as a stable delivery system for ophthalmic applications (). PL NPs exhibited average particle-size distributions of 120 nm with a narrow polydispersity index (0.124) and a negative surface charge of −22.5 mV. To further stabilize the PL NPs, their surfaces were coated with CHT. The negatively charged PL NPs exhibited electrostatic interactions with the positively charged CHT particles (). Several concentrations of CHT were investigated from 0.1 to 10 parts w/w of PL NPs. Notably, slight increases in particle size were observed until a 10/1 ratio was reached, but a steep increase in particle size was observed after the 10/1 ratio was reached and larger particles were observed (). The addition of CHT was further confirmed by zeta potential analysis, which showed that the zeta potential was 22.5 mV for PL NPs and began decreasing with increasing CHT concentration. At the 10/1 ratio, the charge was 20 mV, and increased above 40 mV for greater concentrations of CHT. We therefore selected the 10/1 ratio of PLGA to CHT as an ideal concentration for stable NPs with a final size of 165 nm and net positive charge of 20 mV. CLSM analysis showed an enhanced cellular uptake for PLC NP compared to that of PLGA NP in HCE cells (Figure S1).
Figure 1. Schematic presentations of preparation of triamcinolone acetonide-loaded PLGA-Chitosan polymer nanoparticles. The PLGA was prepared by solvent-evaporated method and chitosan was coated by electrostatic interactions.
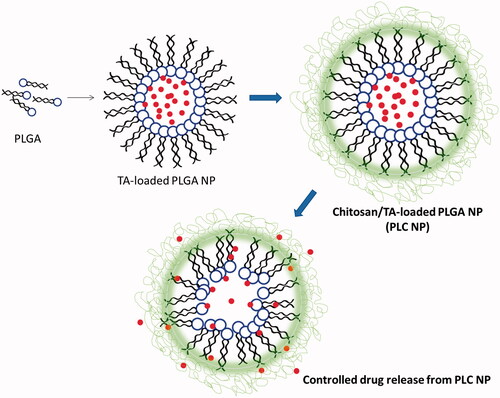
Figure 2. (a) Change in particle size and polydispersity index; (b) change in zeta potential; (c) optimization of PLGA-chitosan NP in terms of particle size; (d) optimization in terms of zeta potential. **p < 0.01 and ***p < 0.0001.
Particle morphology investigation by transmission electron microscopy revealed that PL NPs were smaller than PLC NPs (as indicated by dynamic light scattering analysis). Importantly, the CHT coating on PLGA NPs was clearly visible as a thin greyish layer surrounding the black PLGA core. Nevertheless, this small particle size was presumed to allow suitable distribution in the ocular chamber ().
Stability analysis and in vitro drug-release study
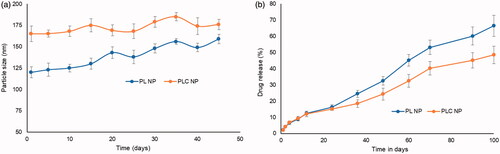
The results showed that PL NPs varied in size from 120 to 160 nm over a period of 45 days. In contrast, PLC NPs varied in size from 165 to 176 nm, indicating excellent stability (). Simulated physiological media (pH 7.4) were used to evaluate the release kinetics of PL NPs and PLC NPs. After 12 h of incubation, approximately, 10% of TA was released from both NPs. Drug-release patterns noticeably differed between PL NPs and PLC NPs by 48 h, and a significant difference in drug release was observed by 60 h (). For example, 45% of the drug was released from PL NPs, compared with 32% from PLC NPs.
Figure 4. (a) Stability analysis of PL NP and PLC NP over 45 days at 4 °C. The stability was performed in terms of particle size; (b) in vitro release characteristics of triamcinolone acetonide from PL NP and PLC NP at 37 °C. The release was quantified using HPLC technique.
Analysis of release kinetics was performed by fitting the data to Higuchi’s model. The release profiles plotted against the square root of time resulted in linear plots for both NPs, indicating controlled release of the encapsulated drugs. The data were also fitted to the Korsmeyer–Peppas equation, wherein the exponent “n” is indicative of the release mechanism. Our results indicate that the n values for PL NPs and TA-loaded PLC NPs were 0.65 and 0.68, respectively (Figure S2).
In vitro cell viability assay
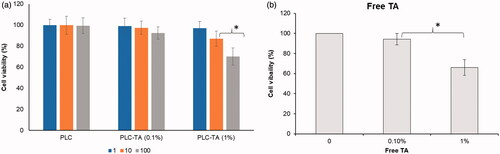
The in vitro toxicities of blank and drug-loaded NPs were investigated by cell viability assay (). The cells were treated with blank PLC NPs and TA (0.1 and 1%)-loaded PLC NPs at concentrations of 1 µg/ml, 10 µg/ml and 100 µg/ml. Cell viability was not significantly affected by any concentration of blank PLC NPs. However, a notable reduction in cell viability was observed for TA-loaded PLC NPs (1%), compared with TA-loaded PLC NPs (0.1%), possibly because of dose-dependent toxicity in HCE cells. In the present study, we have performed additional study on the effect of free TA suspension on the cell viability. As shown, significant decrease in cell viability was observed at a concentration of TA 1% compared to that at 0.1%. It is worth noting that no significant difference in cell viability was observed between PLC-TA (1%) and TA (1%) suspension after 24 h incubation, respectively.
In vitro corneal permeation study
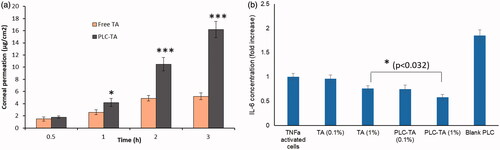
The in vitro corneal permeation study clearly demonstrated the linear relationship between amounts of drug penetrated and time indicating that the permeation rate of the respective groups was steady. PLC-TA exhibited a significantly higher corneal permeation compared to that of free TA suspension after 0.5 h of incubation period until 3 h indicating the enhanced permeation potential of the CHT-based formulations.
In vitro anti-inflammatory assay
The therapeutic efficacies of the NPs were first evaluated using an in vitro model to assess the release of IL-6, a cytokine that causes ocular inflammation (). Cells were treated with free TA suspension and two concentrations of TA-loaded PLC NPs. Blank PLC NPs did not reduce the IL-6 secretion, compared with that of TNF-α activated control cells, indicating a lack of therapeutic efficacy. Furthermore, TA (0.1%) did not show any notable effect, but TA (1%) caused a significant reduction in IL-6 secretion. Importantly, the maximum anti-inflammatory effect was observed using a TA-loaded PLC NP (1%) formulation, which caused a significant reduction in IL-6 secretion compared with all other groups.
Figure 6. (a) In vitro corneal permeation analysis of free TA and PLC-TA for a time period ranging from 0.5 to 3 h, respectively; (b) ELISA based quantification of IL-6 secretion by TNFa activated HCE cells after treatment with free TA suspensions and PLC-TA formulations. *p <0.05, **p < 0.01 and ***p < 0.0001.
In vivo therapeutic efficacy assessment in a rabbit model
Rabbits administered NS showed extensive conjunctival redness and congestion (). These inflammatory signs were significantly reduced at 24 h after administration of TA-loaded PLC NPs, compared with free TA (1%) suspension at an equivalent concentration. Ophthalmic administration of free TA suspension at an equivalent concentration did not alleviate clinical signs of inflammation, compared with NS-treated controls. Additionally, TA-loaded PLC NPs did not cause any adverse effects after ophthalmic administration, further confirming their safety.
Figure 7. (a) Clinical inflammatory score of rabbit treated with normal saline, TA suspension and PLC-TA formulations at 0.1 and 1% concentrations; (b) percentage of aqueous humour transparency (%AHT) in the EIU model after 24–72 h. **p < 0.01 and ***p < 0.0001.
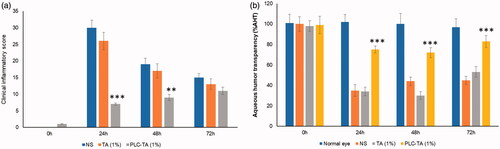
The %AHT effects of NS, free TA and TA-loaded PLC NPs were compared with the presentation in normal eyes. Administration of TA-loaded PLC NPs resulted in greater %AHT, compared with that of either NS or free TA suspension, indicating substantial reduction in ocular fogginess. In contrast, administration of free TA suspension did not show any significant difference in %AHT, compared with NS.
Pharmacokinetic analysis
TA concentrations in rabbit aqueous humour were measured at multiple intervals (). The plasma concentrations of TA following the administration of free TA and TA-loaded PLC NPs are shown in , and pharmacokinetic parameters were established using WinNonlin software (Pharsight Corporation, Mountain View, CA). The free TA concentration in the aqueous humour peaked at 1 h after ophthalmic administration and subsided gradually, such that it did not significantly differ from baseline at 6 h after administration. Conversely, the TA-loaded PLC NP concentration in the aqueous humour peaked at 6 h and significantly differed from baseline until 24 h after administration. The pharmacokinetic parameters are presented in .
Figure 8. Pharmacokinetic analysis of plasma concentration of TA after ophthalmic administration of free TA suspension and PLC-TA. The study was conducted until 24 h and analysed using HPLC technique. ***p < 0.0001.
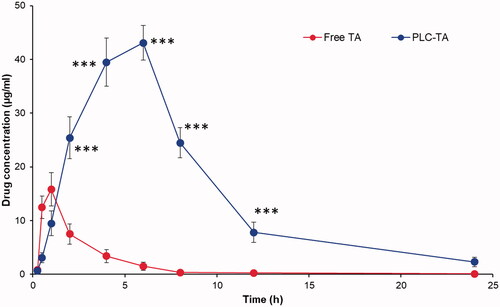
Table 1. Pharmacokinetic parameters after administration of free TA suspension and PLC-TA in rabbit model.
Discussion
TA has been shown to possess excellent anti-inflammatory and immunomodulatory effects in eye disorders such as uveitis. Regardless of its excellent pharmacological actions, the administration of TA suspensions often has clinical limitations. Furthermore, the lipophilic nature of TA creates non-specific distribution across ocular tissues, resulting in reduced bioavailability in the region of interest. TA is also likely to reach sensitive tissues and cause adverse effects. Nanomedicine-based TA administration presents advantages including the sustained release of encapsulated therapeutics in the eye, as well as targeting of the region of interest. PL NPs exhibited average particle-size distributions of 120 nm with a narrow polydispersity index (0.124) and a negative surface charge of −22.5 mV. Importantly, particle size gradually increased with an increasing concentration of CHT. However, greater concentrations of CHT resulted in larger particle sizes and were unstable. During the initial addition of CHT, polymers may have been non-continuous on the PLGA surface and formed a stable consistent layer at a 10/1 ratio. Subsequently, the bulky mass of CHT increased the particle size, thus forming aggregates and leading to unstable particles. The positive surface charge of PLC-TA resulted in enhanced cellular uptake compared to that of PLGA NP in HCE cells [Citation29,Citation30].
Stability was compared between PL NPs and PLC NPs. The results showed that PL NPs varied in size from 120 to 160 nm over a period of 45 days. This substantial change in particle size might have been caused by particle aggregation. In contrast, PLC NPs varied in size from 165 to 176 nm, indicating excellent stability (). The strong surface charge on PLC NPs might have been responsible for this excellent stability. After 24 h of incubation, >20% of TA was released from both NPs, indicating that the carriers were stable in physiological media and could consistently control drug release. Drug-release patterns noticeably differed between PL NPs and PLC NPs by 48 h, and a significant difference in drug release was observed by 60 h. This considerable difference in drug release was observed from 36 h through 100 h. Slower drug release by PLC NPs was presumably due to the stable presence of the outer CHT layer and increased path length. Overall, controlled release of drug from the NP core was considered beneficial for long-term ophthalmic treatment with few side effects. Analysis of release kinetics was performed by fitting the data to Higuchi’s model. Our results indicate that the n values for PL NPs and TA-loaded PLC NPs were 0.65 and 0.68, respectively, which exhibited non-Fickian kinetics and suggested that multiple mechanisms (e.g. diffusion and erosion/degradation) might be involved in TA release.
Although cells behave differently in vitro and in vivo, cell-based experiments offer first-hand evidence concerning cytotoxicity and biocompatibility. Cell viability was not significantly affected by any concentration of blank PLC NPs, indicating the excellent biocompatibility of the designed nanocarriers. However, a notable reduction in cell viability was observed for TA-loaded PLC NPs (1%), compared with TA-loaded PLC NPs (0.1%), possibly because of dose-dependent toxicity in HCE cells. Importantly, free TA reportedly exhibits considerable toxicity, compared with that of TA-loaded PLC NPs. The toxicity of free TA suspension was attributed to the presence of several excipients, but TA-loaded PLC NPs are considered safe for ophthalmic applications [Citation31]. Equivalent concentrations of commercial formulations of Fortcinolona® 40 have shown greater toxicity and low cell viability. The in vitro corneal permeation study clearly demonstrated the linear relationship between amounts of drug penetrated and time indicating that the permeation rate of the respective groups was steady. The presence of nanocarriers facilitated the easy entry of TA through cornea resulting in appreciable permeation rate, while lack of any carrier in free TA suspension limited the penetration of free small molecules through the tightly bound corneal epithelia. Furthermore, positive surface charge of PLC-TA might allow favourable interaction with negatively charged corneal membrane and might enhance the corneal permeation [Citation32,Citation33].
The therapeutic efficacies of the NPs were first evaluated using an in vitro model to assess the release of IL-6, a cytokine that causes ocular inflammation (). This in vitro inflammation model was selected because of its suitability for evaluating the anti-inflammatory efficacies of NPs. A maximum anti-inflammatory effect was observed using a TA-loaded PLC NP (1%) formulation, which caused a significant reduction in IL-6 secretion compared with all other groups. This enhanced anti-inflammatory response associated with TA-loaded PLC NPs (1%) was presumably related to the controlled release of encapsulated therapeutics from nanosized particles and better presentation of TA to TNF-α activated cell. The correlation between drug release at 1 h and anti-inflammatory response after 1 h incubation is interesting. We anticipated that the drug release pattern might be different in the cellular compartments than at in vitro conditions. These results emphasize that TA loading in NPs did not affect its biological activity.
An endotoxin-induced uveitis rabbit model was used to investigate the therapeutic efficacy of TA-loaded PLC NPs, based on clinical signs of intraocular inflammation at 24 h after intravitreal lipopolysaccharide administration. Rabbits administered NS showed extensive conjunctival redness and congestion. Typical fibrin deposits were noted in the cornea and crystalline lens, with reduced intraocular pressure relative to baseline [Citation34]. Furthermore, inflammation of the iris and ciliary body were attributed to the Tyndall effect, which results in anterior chamber fogginess and indicates typical ocular inflammation. These inflammatory signs were significantly reduced at 24 h after administration of TA-loaded PLC NPs, compared with free TA (1%) suspension at an equivalent concentration. The mean grey difference in light reflection between the cornea and anterior chamber was quantified in terms of fogginess (calculated as %AHT). Administration of TA-loaded PLC NPs resulted in greater %AHT, compared with that of either NS or free TA suspension, indicating substantial reduction in ocular fogginess. Consistent with the reduced clinical inflammation findings, the administration of TA-loaded PLC NPs led to greater ocular transparency, signifying superior therapeutic efficacy in the rabbit model. The free TA concentration in the aqueous humour peaked at 1 h after ophthalmic administration and subsided gradually, such that it did not significantly differ from baseline at 6 h after administration, which indicated rapid clearance of the free drug. The TA-loaded PLC NP concentration in the aqueous humour peaked at 6 h and significantly differed from baseline until 24 h after administration, indicating the superiority of the PLC-based nanocarrier system. TA-loaded PLC NPs exhibited a six-fold greater area under the curve value and a nearly threefold greater mediator release test value, compared with those of free TA suspension, indicating the superior pharmacokinetic dynamics of the nanocarrier system. The encapsulation of drug in the NP core allowed gradual sustained release of TA in the rabbit aqueous humour.
Conclusions
TA was successfully formulated in PLC NPs for the treatment of ocular inflammatory diseases. PLC NPs were subjected to detailed characterization with regard to size, shape and stability. PLC NPs demonstrated controlled drug release for 100 h, compared with PLGA NPs, indicating the importance of the CHT coating for prolonged drug release. Blank PLC NPs did not influence cell viability, indicating that the designed nanocarriers had excellent biocompatibility. TA-loaded PLC NPs exhibited excellent anti-inflammatory activity in studies of HCE cells, and significantly reduced the secretion of IL-6 by TNF-α activated HCE cells. In a rabbit model, TA-loaded PLC NPs did not show any clinical signs of typical ocular inflammation and significantly alleviated signs of inflammation, compared with free TA suspension, at 24 h after a single dose. Moreover, TA-loaded PLC NPs exhibited greater %AHT, compared with that of either NS or free TA suspension, indicating considerable reduction in anterior chamber fogginess. Pharmacokinetic analysis in rabbit eyes revealed that TA-loaded PLC NP concentrations peaked at 6 h and remained high for 24 h, indicating the superiority of a PLC-based nanocarrier system. Overall, PLC-based NP formulations offer a new therapeutic approach for ocular inflammatory diseases.
Supplemental Material
Download MS Word (772.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- WHO. Priority eye diseases; 2010; [cited 2019 Jul 15]. Available from: http://www.who.int/blindness/causes/priority/en/index1.html
- WHO. Visual impairment and blindness; 2014; [cited 2019 Jul 10]. Available from: http://www.who.int/mediacentre/factsheets/fs282/en/
- Khairallah M, Kahloun R, Bourne R, et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Invest Ophthalmol Vis Sci. 2015;56(11):6762–6910.
- Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002;1(3):465–479.
- Pescosolido N, Barbato A, Giannotti R, et al. Age-related changes in the kinetics of human lenses: prevention of the cataract. Int J Ophthalmol. 2016;9:1506–1710.
- Prokofyeva E, Wegener A, Zrenner E. Cataract prevalence and prevention in Europe: a literature review. Acta Ophthalmol. 2013;91(5):395–405.
- Abraldes MJ, Fernandez M, Gomez-Ulla F. Intravitreal triamcinolone in diabetic retinopathy. Curr Diabetes Rev. 2009;5(1):18–25.
- Degenring R, Jonas J. Intravitreal injection of triamcinolone acetonide as treatment for chronic uveitis. Br J Ophthalmol. 2003;87(3):361.
- Chen H, Sun S, Li J, et al. Different intravitreal properties of three triamcinolone formulations and their possible impact on retina practice. Invest Ophthalmol Vis Sci. 2013;54(3):2178–2185.
- Smithen LM, Ober MD, Maranan L, et al. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138(5):740–743.
- Choy YB, Prausnitz MR. The rule of five for non-oral routes of drug delivery: ophthalmic, inhalation and transdermal. Pharm Res. 2011;28(5):943–948.
- Cekic O, Chang S, Tseng JJ, et al. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol. 2005;139(6):993–998.
- Quiram PA, Gonzales CR, Schwartz SD. Severe steroid-induced glaucoma following intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 2006;141(3):580–582.
- Araújo J, Gonzalez E, Egea MA, et al. Nanomedicines for ocular NSAIDs: safety on drug delivery. Nanomedicine. 2009;5(4):394–401.
- Wong CW, Metselaar JM, Storm G, et al. A review of the clinical applications of drug delivery systems for the treatment of ocular anterior segment inflammation. Br J Ophthalmol. 2020.
- Ramasamy T, Ruttala HB, Gupta B, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J Control Release. 2017;258:226–253.
- Jian HJ, Wu RS, Lin TY, et al. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano. 2017;11(7):6703–6716.
- Luo LJ, Lin TY, Yao CH, et al. Dual-functional gelatin-capped silver nanoparticles for antibacterial and antiangiogenic treatment of bacterial keratitis. J Colloid Interface Sci. 2019;536:112–126.
- Li YJ, Luo LJ, Harroun SG, et al. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale. 2019;11(12):5580–5594.
- Manzoor AA, Lindner LH, Landon CD, Park JY, Simnick AJ, Dreher MR, Das S, Hanna G, Park W, Chilkoti A, Koning GA, ten Hagen TL, Needham D, Dewhirst MW. Overcoming limitations in nanoparticle drug delivery: triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012 Nov 1;72(21):5566–75. doi: https://doi.org/10.1158/0008-5472.CAN-12-1683.
- Luo LJ, Nguyen DD, Lai JY. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: a push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials. 2020;243:119961.
- Danhier F, Ansorena E, Silva JM, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522.
- Stevanovic M, Uskokovic D. Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci. 2009;5(1):1–14.
- Nguyen DD, Luo LJ, Lai JY. Effects of shell thickness of hollow poly(lactic acid) nanoparticles on sustained drug delivery for pharmacological treatment of glaucoma. Acta Biomater. 2020;111:302–315.
- Lu B, Lv X, Le Y. Chitosan-modified PLGA nanoparticles for control-released drug delivery. Polymers (Basel). 2019;11(2):304.
- Zou P, Yang X, Wang J, et al. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016;190:1174–1181.
- Huang WF, Tsui CP, Tang CY, et al. Surface charge switchable and pH-responsive chitosan/polymer core–shell composite nanoparticles for drug delivery application. Compos Part B Eng. 2017;121:83–91.
- Ramasamy T, Haidar ZS, Tran TH, et al. Layer-by-layer assembly of liposomal nanoparticles with PEGylated polyelectrolytes enhances systemic delivery of multiple anticancer drugs. Acta Biomater. 2014;10(12):5116–5127.
- Ramasamy T, Munusamy S, Ruttala HB, et al. Smart nanocarriers for the delivery of nucleic acid-based therapeutics: a comprehensive review. Biotechnol J. 2021;16(2):e1900408.
- Ramasamy T, Ruttala HB, Kaliraj K, et al. Polypeptide derivative of metformin with the combined advantage of a gene carrier and anticancer activity. ACS Biomater Sci Eng. 2019;5(10):5159–5168.
- Narayanan R, Mungcal JK, Kenney MC, et al. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47(2):722–728.
- Gallarate M, Chirio D, Bussano R, et al. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int J Pharm. 2013;440(2):126–134.
- Abdullah TA, Ibrahim NJ, Warsi MH. Chondroitin sulfate-chitosan nanoparticles for ocular delivery of bromfenac sodium: improved permeation, retention, and penetration. Int J Pharm Investig. 2016;6(2):96–105.
- Calles JA, Lopez-Garcia A, Valles EM, et al. Preliminary characterization of dexamethasone-loaded cross-linked hyaluronic acid films for topical ocular therapy. Int J Pharm. 2016;509(1–2):237–243.