?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
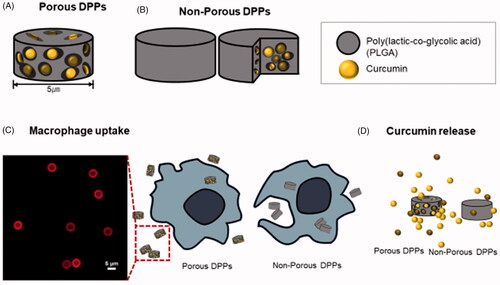
Curcumin has great potential in cancer treatment and prevention. However, free curcumin for anticancer effect is limited due to its low water solubility and instability. Delivery of free curcumin using biodegradable and biocompatible polymers, such as poly (lactic-co-glycolic acid) (PLGA), can improve these undesirable problems. In this study, a top-down fabrication method using PLGA was employed to deliver free curcumin, engineering size, shape, and surface properties. As a result, porous discoidal polymeric particles (DPPs) were produced in ammonium bicarbonate with a hydrodynamic diameter of 5 µm and a negatively charged surface. The loading amount of free curcumin in the porous DPPs was higher than non-porous DPPs. In vitro drug release study showed that curcumin release from porous DPPs was 1.4-fold higher than non-porous ones. The confocal microscopy and flow cytometry results demonstrated that porous DPPs decrease phagocytosis by macrophages than non-porous ones. This study suggests that porous DPPs have significant advantages for effective drug delivery of curcumin, minimizing phagocytosis.
Introduction
Curcumin, a hydrophobic polyphenol extracted from Curcuma longa plants, has been extensively studied for its pharmacological properties such as anti-inflammation, anticancer, and anti-angiogenesis [Citation1–3]. Anticancer effects of curcumin are due to the activation of the apoptotic pathway and inhibition of several cell signalling pathways involve in cancer pathogenesis [Citation4]. Regardless of its benefit in cancer therapy, limiting factors such as poor water solubility and inadequate bioavailability have limited its therapeutic application [Citation5–7]. Therefore, novel strategies for delivery are required for the successful implementation of curcumin.
Cancer is considered the most common disease in which cells expand and differentiate abnormally and out of control. For example, breast cancer is a life-threatening disease among women worldwide and is ranked as the most prevalent type of cancer. About 268,600 new cases of invasive breast cancer are estimated in 2019 in the USA [Citation8]. Therefore, new technologies that can distinguish normal and cancer cells and directly target the tumour are of great interest.
With that purpose, the inclusion of anticancer drugs in nanocarriers has emerged as a strategic approach to break traditional chemotherapy constraints. Some of the favourable consequences of drug delivery using nanocarriers include small size, biodegradability, high solubility, stability, enhanced drug permeability to the target site, and non-toxicity [Citation9–11]. The use of nanoparticulate carriers to transport active chemotherapeutic drugs is one strategy that has long been used to enhance the pharmacokinetics of anticancer drugs, including curcumin [Citation12]. Nanoparticulate carriers can be fabricated through a wide range of materials, but PLGA is extensively used due to its biocompatibility, biodegradability, and prolonged delivery of therapeutic drug levels for a sustained period [Citation13–15].
Nevertheless, non-specific uptake by the mononuclear phagocyte system and controlled release are the major obstacles that strongly affect therapeutic performance. Therefore, to improve it, there has been exponential growth in the use of porous particles [Citation16]. Compared to non-porous particles, porous particle possesses several distinctive characteristics like large surface area, high porosity, and low density. These features modulate to prevent rapid clearance and escape the phagocytic system [Citation17].
In this study, a novel development of discoidal porous polymeric particles encapsulated with curcumin was fabricated to avoid the uptake of macrophage cells and to deliver hydrophobic curcumin to the targeted site efficiently.
Method
Chemical and reagents
Commercially available acid-terminated poly-lactide-co-glycolide (PLGA) (lactide: glycolide lycolidegaw = 24,000–38,000 g/mol), acetone, dimethyl sulfoxide (DMSO), polyvinyl alcohol (PVA), curcumin, ammonium bicarbonate, fluorescein isothiocyanate (FITC), phosphate-buffered saline (PBS), tween 80, sylgard 184 elastomer and curing agent were purchased from Sigma Aldrich (St. Louis, MO) and used without further purification.
Fabrication process of porous and non-porous discoidal polymeric particles
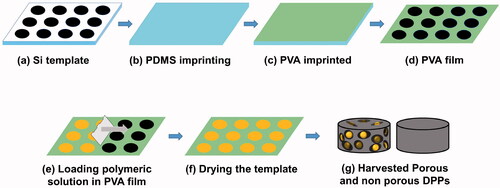
Curcumin loaded discoidal polymeric particles (DPPs) were fabricated by the top-down fabrication method using an electron beam lithography technique [Citation18–20]. Briefly, a silicon master template with an array of discoidal wells was purchased from the Korea Infrastructure Organization for Nanotechnology, South Korea. The fabricated silicon wafers were passivated with acetone and isopropanol and etched with a 4% hydrofluoric acid solution. After the silicon template was passivated, it was rinsed with deionized water and dried with nitrogen.
Polydimethylsiloxane (PDMS) mould was prepared using a silicon master template containing discoidal arrays. Briefly, the curing agent and sylgard 184 were mixed in the ratio of 1:10. The mixture was mixed homogeneously and was degassed in a vacuum chamber to remove air bubbles and then poured onto the master silicon template containing a well with a width of 5 and 2.4 µm to obtain a mould with the opposite shape of the silicon wafer. The mould was baked in an oven at 60 °C for 4 h and gently removed.
Polyvinyl alcohol solution (5.33%) was prepared to dissolve PVA in deionized (DI) water at 120 °C and cooled at room temperature. The PVA solution was poured onto the surface of the PDMS template and dried at 30 °C. The PVA template resembled the same discoidal wells as of the original silicon wafer.
About 70 mg PLGA was dissolved in 200 µl of acetone and vortexed. Thirty milligram of curcumin was dissolved in 50 µl of DMSO, and 4% of ammonium bicarbonate was dissolved in DI water. All three solutions were mixed together, and the mixture was sonicated and loaded on the PVA film. The film was dried at 37 °C for 5 min and dissolved in water. The solution was dissolved for 2 h and filtered and centrifuged. The particles were collected. For non-porous DPPs, ammonium bicarbonate was not used, and the method for fabrication was similar. For flow cytometry, FITC conjugated PLGA was used, as mentioned earlier [Citation21]. Briefly, 100 mg of PLGA was dissolved in 5 ml DMSO and activated using DCC and NHS. The reaction was carried out for 4 h. FITC was dissolved in activated PLGA, and the reaction was carried out for another 24 h. The sample was dialyzed against DI water to remove unreacted FITC and was lyophilized. The conjugation was confirmed by fluorescence microscopy.
Similarly, for Rhodamine B-loaded porous and non-porous DPPs, 70 mg PLGA and 10 µg Rhodamine B was dissolved in 100 µl acetone and 4% of ammonium bicarbonate. All three solutions were dissolved and sonicated. The solution was loaded on PVA, and further purification was performed, as mentioned earlier ).
Physicochemical characterization of curcumin DPPs
Scanning electron microscopy (SEM) images of porous and non-porous DPPs were taken by Incheon, South Korea, with an accelerating voltage of 5 kV, and coated with platinum under vacuum.
Particle size and zeta potential of the porous and non-porous DPPs were measured using dynamic light scattering, DLS (Zetasizer Nano ZS90; Malvern Instruments Ltd., Malvern, UK).
Loading amount
Drug-loading amounts of curcumin in porous and non-porous discoidal polymeric particles were determined by a plate reader. Briefly, 1 mg of porous and non-porous DPPs was dissolved in 1 ml DMSO, and the absorbance value was measured at 420 nm. The concentration was calculated using a standard curve. The experiment was repeated three times. The calculation equation is as follows:
In vitro drug release
In vitro curcumin release from porous and non-porous DPPs was examined using the dialysis method. Briefly, 1 mg of porous and non-porous DPPs were suspended in 1 ml mixture of phosphate-buffered saline and 20% tween 80 (pH 7.40) and was transferred into a dialysis cup. About 1 ml of PBS saline and tween 80 solutions was put in the Eppendorf tube, and above that, the dialysis cup containing curcumin loaded porous and non-porous DPPs solution was kept. The Eppendorf tubes were then placed in a water bath stirring at 80 RPM at 37 °C. The release test was measured using a microplate reader at every 24-h interval.
Cytotoxicity assay and cell inhibition
Human breast cancer cell (MCF 7) was cultured in DMEM supplemented with 10% FBS and 1% penicillin at 37 °C in a humidified atmosphere of 5% CO2. MCF 7 cells were seeded in 96 well plates at a density of 5 × 103 cells/well and incubated for 24 h and subsequently treated with different concentrations of blank porous and non-porous DPPs for 24 h and free curcumin, and curcumin loaded porous and non-porous DPPs for another 48 and 72 h, respectively. The cells were washed three times with PBS and reacted with CCK-8, and incubated for 4 h. The cell viability was measured through a microplate reader at 450 nm. Cells without treatment with particles were used as a negative control. The experiment was performed three times.
Cell uptake studies
MCF 7 breast cancer cells and Raw 264.7 macrophage cells were grown under DMEM supplemented with 10% FBS and 1% penicillin. Cellular uptake of curcumin loaded porous and non-porous DPPs was determined using a fluorescence microscope. For this experiment, 1 × 105 cells were seeded on the confocal dish and incubated at 37 °C until they reached about 60–70% confluency. The cells were then exposed to 100 µg/ml concentration of curcumin-loaded porous and non-porous DPPs for 12 h. The cells were washed several times using PBS, fixed in 10% formalin, and stained with DAPI. Cells were viewed under the microscope to determine maximum uptake.
Flow cytometry measurement
For flow cytometry measurements, 2 × 105 of Raw 264.7 cells were seeded in 6 well plates. After the cell reached about 60–70% confluency, 100 µg/ml FITC conjugated porous and non-porous DPPs were exposed for 12 h. After exposure, cells were washed with DPBS (Dulbecco’s Phosphate Buffered Saline), trypsinized, centrifuged at 3000 RPM for 1 min, and resuspended in DPBS. Then, these cells were analyzed using a flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). FACS express software was used for further analysis [Citation22].
Statistical analysis
All data were processed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Results are expressed as mean ± standard deviation. Comparison of porous and non-porous data was performed using a t-test.
Results and discussion
Synthesis and characterization of porous and non-porous curcumin-loaded DPPs
Nanoparticle’s shape, size distribution, and zeta potential play an essential role in regulating their release, cellular uptake, and in vivo bio distribution [Citation23]. Therefore, designing the nanoparticles in terms of shape, size, and surface chemistry can maximize therapeutic efficacy. The morphology of synthesized porous and non-porous DPPs was found to be discoidal from SEM micrographs with an average diameter of 5 µm. Representative SEM image shows that non-porous DPPs have a very smooth surface, while porous DPPs show a highly porous surface ().
Figure 2. Morphology of porous and non-porous DPPs. (a) Scanning electron microscope (SEM) image of the porous and non-porous DPPs. Microscope image of the (b) rhodamine B-loaded DPPs and (c) curcumin-loaded DPPs. The scale bar equals 1 µm.
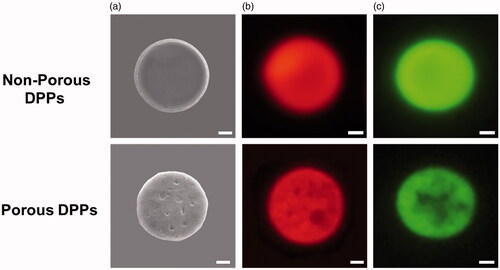
The drug-loading amount was found to be 18.9 and 16.7% for curcumin-loaded porous and non-porous DPPs, respectively. Porous DPPs showed a high drug-loading amount compared to non-porous, which can be beneficial for an efficient drug delivery system (). The higher loading amount can be explained because of decomposition products of ammonium bicarbonate, which lowers the vapour pressure of organic solvents and accelerate the solidification of the polymeric matrix [Citation23].
Table 1. Characterization of porous and non-porous curcumin-loaded DPPs.
In vitro release of curcumin
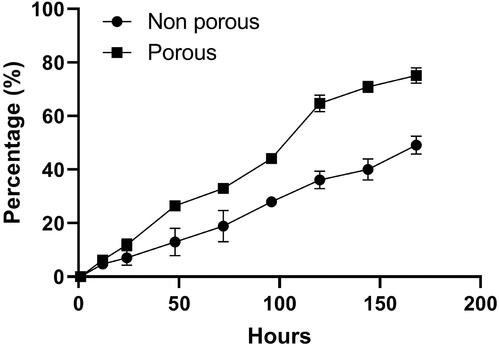
In vitro release behaviour of curcumin encapsulated porous and non-porous DPPs was studied for 7 days. shows the cumulative release percent of curcumin from non-porous and porous DPPs at 7.4 pH. Over a period of 7 days, 73% of curcumin was released from porous DPPs, while only 52% was released from non-porous DPPs. In both cases, the release was gradual, with no burst release. The higher cumulative release from porous DPPs is because they are able to diffuse more easily through the leaky structures that are existed in the porous DPPs.
In vitro cytotoxicity
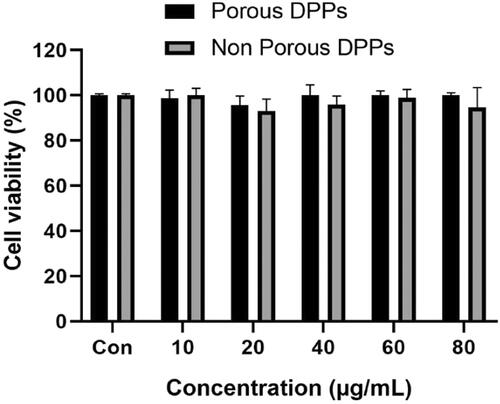
The in vitro cytotoxic effect of blank porous and non-porous DPPs was tested using CCK-8 on MCF 7 cell line. shows the viability of MCF 7 cells with porous and non-porous DPPs at a concentration ranging from 10 to 80 µg/ml. The result indicates that both porous and non-porous DPPs were nearly non-toxic to MCF 7 cells within the designated interval.
Cell inhibition
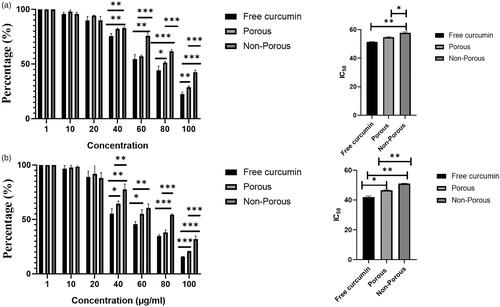
The anti-proliferation effect on MCF 7 cells induced by free curcumin and curcumin loaded porous and non-porous DPPs were evaluated through CCK-8 assay. As shown in , concentration ranging from 10 to 100 µg/ml inhibits the proliferation of MCF 7 cells. With increasing concentration, the cell viability exhibited a decreasing tendency with only 22.5% for free curcumin, while 28.83% for porous DPPs and 42% for non-porous DPPs at the interval of 48 h and only 15% for free curcumin, 20% for porous DPPs and 32% for non-porous DPPs at 72 h interval. The half maximal inhibitory concentration (IC50) values of free curcumin, curcumin-loaded porous DPPs and curcumin-loaded non-porous DPPs were calculated, which were 51.5 µg/ml, 54.7 µg/ml and 57.7 µg/ml, respectively, at 48 h, and 42 µg/ml, 46.7 µg/ml and 51.04 µg/ml at 72 h, respectively. As expected, free curcumin showed a higher cytotoxic effect than curcumin-loaded porous and non-porous DPPs via direct exposure. The curcumin-loaded porous DPPs showed a higher cytotoxic effect than non-porous DPPs due to faster drug release.
Figure 5. Effect of curcumin loaded porous and non-porous DPPs on the growth of MCF 7 cells. Cell inhibition of porous and non-porous DPPs at a given concentration and IC50 value. MCF 7 cells were treated with different concentrations (10–100 µg/ml) of free curcumin, curcumin-loaded porous, and non-porous DPPs for (a) 48 h and (b) 72 h. The data were expressed as mean value ± SD, n = 3, (*p<.05; **p<.01; ***p<.001).
Intracellular uptake
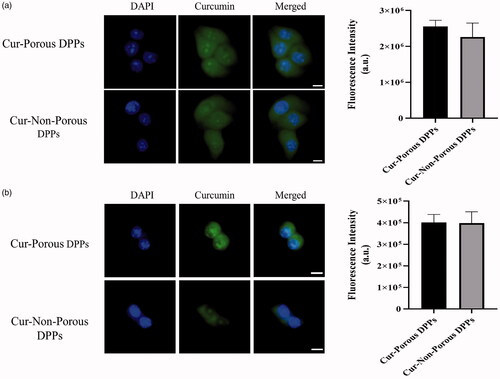
Cellular uptake was carried out to evaluate the internalization of the curcumin loaded DPPs in MCF 7 breast cancer cells and Raw 264.7 macrophage cells. The inherent green fluorescence property of curcumin was used to observe the cellular entry of curcumin-loaded porous and non-porous DPPs. A confocal microscope was used to confirm the cellular uptake at the incubation time point of 12 h. Curcumin accumulation was detected from cells treated with both porous and non-porous DPPs. It was also noted that fluorescence intensity was higher on cells treated with curcumin loaded porous DPPs () in comparison to non-porous DPPs. However, there was no statistical significant difference between the fluorescence intensity of porous and non-porous DPPs as verified by image J.
Figure 6. Cell internalization was observed by confocal fluorescence microscopy. (a) MCF 7 cells incubated with curcumin-loaded porous and non-porous DPPs. (b) Raw 264.7 cells incubated with curcumin-loaded porous and non-porous DPPs. Curcumin was released from the DPPs and diffused into the MCF 7 and Raw 264.7 cells. The nucleus was stained using DAPI. The scale bar equals 10 micrometres.
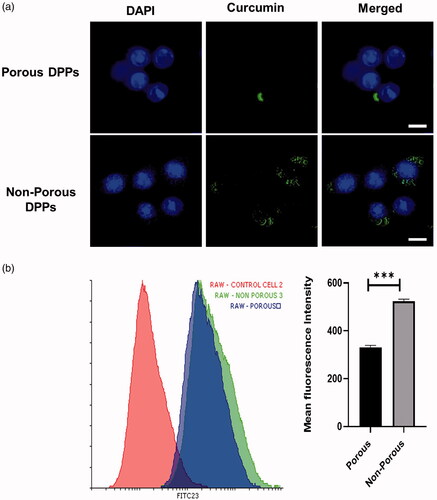
To determine that curcumin was internalized on the Raw 264.7 cells, FITC-conjugated porous and non-porous DPPs were used and measured by using flow cytometry and confocal imaging (). To calculate the cellular uptake efficiency between porous and non-porous DPPs, we treated Raw 264.7 macrophage cells for 12 h with FITC conjugated porous and non-porous DPPs. The result showed us that non-porous DPPs resulted in significantly higher fluorescence intensities and uptake for Raw264.7 cells. The reason might be that non-porous DPPs had a higher density than porous DPPs. In conclusion, the uptake of non-porous DPPs was higher, as shown in , due to the release of curcumin from the porous surface. The flow cytometry results suggest that porous DPPs can escape phagocytosis.
Figure 7. Quantitative measurement of DPPs uptake by flow cytometry. (a) Confocal imaging of FITC-conjugated porous and non-porous DPPs on Raw 264.7 cells. (b) Flow cytometry analysis of FITC-conjugated porous and non-porous DPPs in Raw 264.7 cells. Cells were exposed to 100 µg/ml FITC-conjugated porous and non-porous DPPs for 12 h (*p<.05; **p<.01; ***p<.001). The scale bar equals 10 micrometres.
Conclusion
Curcumin has outstanding therapeutic benefits in various diseases and has a broad range of pharmacological activities such as anti-inflammatory, anticancer, and antioxidant. However, curcumin application has been hindered due to low water solubility leading to poor bioavailability. For this reason, in this study, the top-down fabrication method was used to prepare porous and non-porous curcumin-loaded DPPs, using ammonium bicarbonate as a porogen for the treatment of human breast cancer cells. Here, porous DPPs showed high drug loading content, faster drug release, and phagocytosis escapement characteristics. From the higher drug release and minimized phagocytic uptake, porous DPPs can be a promising drug carrier.
Acknowledgement
This research was funded by the National Research Foundation of Korea (NRF), grant numbers 2018R1D1A1B07042339, 2019K2A9A2A08000123.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Kundu M, Sadhukhan P, Ghosh N, et al. Sil, pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J Adv Res. 2019;18:161–172.
- Khalil NM, do Nascimento TCF, Casa DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–360.
- Verderio P, Bonetti P, Colombo M, et al. Intracellular drug release from curcumin-loaded PLGA nanoparticles induces G2/M block in breast cancer cells. Biomacromolecules. 2013;14(3):672–682.
- Kasi PD, Tamilselvam R, Skalicka-Wozniak K, et al. Molecular targets of curcumin for cancer therapy: an updated review. Tumour Biol. 2016;37(10):13017–13028.
- Dhivya R, Ranjani J, Bowen PK, et al. Biocompatible curcumin loaded PMMA-PEG/ZnO nanocomposite induce apoptosis and cytotoxicity in human gastric cancer cells. Mater Sci Eng C Mater Biol Appl. 2017;80:59–68.
- Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3875.
- Punfa W, Yodkeeree S, Pitchakarn P, et al. Enhancement of cellular uptake and cytotoxicity of curcumin-loaded PLGA nanoparticles by conjugation with anti-P-glycoprotein in drug resistance cancer cells. Acta Pharmacol Sin. 2012;33(6):823–831.
- Khalil S, Hatch L, Price CR, et al. Addressing breast cancer screening disparities among uninsured and insured patients: a student-run free clinic initiative. J Community Health. 2020;45(3):501–505.
- Aryal S, Park H, Leary JF, et al. Top-down fabrication-based nano/microparticles for molecular imaging and drug delivery. Int J Nanomedicine. 2019;14:6631–6644.
- Liyanage PY, Hettiarachchi SD, Zhou Y, et al. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim Biophys Acta Rev Cancer. 2019;1871(2):419–433.
- Montazerabadi A, Beik J, Irajirad R, et al. Folate-modified and curcumin-loaded dendritic magnetite nanocarriers for the targeted thermo-chemotherapy of cancer cells. Artif Cells Nanomed Biotechnol. 2019;47(1):330–340.
- Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26.
- Cartiera MS, Johnson KM, Rajendran V, et al. The uptake and intracellular fate of PLGA nanoparticles in epithelial cells. Biomaterials. 2009;30(14):2790–2798.
- Xie X, Wang H, Williams GR, et al. Erythrocyte membrane cloaked curcumin-loaded nanoparticles for enhanced chemotherapy. Pharmaceutics. 2019;11(9):429.
- Kim K-T, Lee J-Y, Kim D-D, et al. Recent progress in the development of poly (lactic-co-glycolic acid)-based nanostructures for cancer imaging and therapy. Pharmaceutics. 2019;11(6):280.
- Ahuja G, Pathak K. Porous carriers for controlled/modulated drug delivery. Indian J Pharm Sci. 2009;71(6):599–607.
- Gharse S, Fiegel J. Large porous hollow particles: lightweight champions of pulmonary drug delivery. CPD. 2016;22(17):2463–2469.
- Key J, Palange AL, Gentile F, et al. Soft discoidal polymeric nanoconstructs resist macrophage uptake and enhance vascular targeting in tumors. ACS Nano. 2015;9(12):11628–11641.
- Nguyen TDT, Aryal S, Pitchaimani A, et al. Biomimetic surface modification of discoidal polymeric particles. Nanomedicine. 2019;16:79–87.
- Park JY, Park S, Lee TS, et al. Biodegradable micro-sized discoidal polymeric particles for lung-targeted delivery system. Biomaterials. 2019;218:119331.
- Kim SH, Jeong JH, Chun KW, et al. Target-specific cellular uptake of PLGA nanoparticles coated with poly(L-lysine)-poly(ethylene glycol)-folate conjugate. Langmuir. 2005;21(19):8852–8857.
- Park J, Ha MK, Yang N, et al. Flow cytometry-based quantification of cellular Au nanoparticles. Anal Chem. 2017;89(4):2449–2456.
- Chen Q, Si X, Ma L, et al. Oral delivery of curcumin via porous polymeric nanoparticles for effective ulcerative colitis therapy. J Mater Chem B. 2017;5(29):5881–5891.