Abstract
Bone integration on the surface of titanium prosthesis is critical to the success of implant surgery. Good Bone integration at the contact interface is the basis of long-term stability. TiO2 nanotubes have become one of the most commonly used modification techniques for artificial joint prostheses and bone defect implants due to their good biocompatibility, mechanical properties and chemical stability. TiO2 nanotubes can promote F-actin polymerization in bone mesenchymal stem cells (BMSCs) and osteogenic differentiation. The possibility of F-actin as an upstream part to regulate GCN5 initiation of osteogenesis was discussed. The results of gene loss and functional acquisition assay, immunoblotting assay and fluorescence staining assay showed that TiO2 nanotubes could promote the differentiation of BMSCs into osteoblasts. The intervention of TiO2 nanotubes can make BMSCs form stronger F-actin fibre bundles, which can drive the differentiation process of osteogenesis. Our results showed that F-actin mediated nanotube-induced cell differentiation through promoting the expression of GCN5 and enhancing the function of GCN5 and GCN5 was a key regulator of the osteogenic differentiation of BMSCs induced by TiO2 nanotubes as a downstream mediated osteogenesis of F-actin, providing a novel insight into the study of osteogenic differentiation on surface of TiO2 nanotubes.
Introduction
Osseointegration refers to the orderly structure and functional connection between the surface of the implant and the host bone [Citation1]. After implantation, good osseointegration at the interface is the basis of long-term stability of the prosthesis and the key to the success of the implantation [Citation2]. At present, TiO2 nanotubes have become one of the most commonly used modification techniques for prosthesis and bone defect implants due to their good biocompatibility, mechanical properties and chemical stability [Citation3]. On the one hand, titanium is used as a metal material to maintain a good balance between mechanical properties and corrosion resistance [Citation4]. On the other hand, the unique surface porosity and substrate stiffness of TiO2 nanotubes have significant effects on cell adhesion, survival, differentiation and growth [Citation5]. For example, the new studies shows that as the diameter of the TiO2 nanotube increases, the integration effect of the prosthesis and the host bone will be better. However, a excessive diameter will reduce the adhesion ability of cells [Citation6,Citation7]. The dense arrangement of nanotubes promotes the spreading and mineralization of osteoblasts on the surface, resulting in producing new bone faster [Citation8]. The microstructure of TiO2 nanotubes can be accurately controlled by adjusting the parameters of electrolyte composition, voltage and oxidation time in electrochemical anodic oxidation [Citation9]. Such TiO2 nanotubes morphology can induce bone marrow mesenchymal stem cells (BMSCs) to differentiate into osteoblasts and promote bone tissue formation, but the specific regulatory mechanisms have not been fully elucidated.
Cytoskeleton is a kind of cell scaffold in cytoplasm. It maintains cell shape, provides mechanical strength, directs movement, regulates chromosome segregation during mitosis and meiosis, and intracellular organelle transport [Citation10,Citation11]. Actin microfilament is the main structure of cytoskeleton. Actin exists in cells in the form of globular/monomer actin (G-actin) or fibrous actin (F-actin), in which the F-actin is the main part of the cytoskeleton, which can quickly aggregate and depolymerize and is directly related to the adhesion, extension and migration of cells. F-actin provides mechanical support for cells, influences the tension in the cytoskeleton, and provides pathways through the cytoplasm to help signal transduction [Citation12,Citation13]. F-actin has been shown to induce lipid differentiation in cultured BMSCs [Citation14]. The role of actin dynamics in the differentiation of chondrocytes from BMSCs has also been supported by numerous experiments. F-actin interfering compounds, such as Cytochalasin D, can induce cartilage differentiation [Citation15]. Previous studies have found that F-actin in the cytoskeleton is very sensitive to external mechanical and physical signal stimulation. On the metal surface, the aggregation of F-actin is more obvious, forming a stronger cytoskeleton. On the surface of the hydrogel, the polymerization of F-actin is weakened. And the change of cytoskeleton is accompanied by the change of cell morphology and the expression of differentiation-related markers [Citation16]. Therefore, F-actin itself can be used as a bridge for mechanical signals to regulate cell biological behaviour.
As one of the most common epigenetic modifications in organisms, histone acetylation, catalysed by the histone acetyltransferase family (HATs), is an important mode of gene expression regulation in eukaryotes [Citation17]. As an important member of the histone acetyltransferase HAT family, GCN5 catalyses acetylation of many transcription factors, regulates and controls the activity and expression level of many transcription factors, ligands and receptors [Citation18]. GCN5 activity has been shown to significantly up-regulate the activity of classical Wnt signalling pathways (a major signalling pathway in BMSCs osteogenesis), inducing BMSCs to differentiate into osteogenesis and trabeculae formation in mouse lower extremity bone [Citation19]. Whether F-actin, as a receiver of physical signals, has a regulation effect on GCN5 under the stimulation of external mechanical signals, such as TiO2 nanotubes, has not been reported in the literature. In this study, we tried to explore the mechanism of TiO2 nanotubes regulating BMSCs osteogenic differentiation and to provide new evidence and clues for F-actin as a cytoskeleton to regulate histone acetyltransferase and Histone acetylation. It could provide a novel insight for the osteogenesis mechanism of TiO2 nanotubes.
Materials and methods
Isolation and culture of BMSCs
Four-week-old male Sprague-Dawley (SD) rats were purchased from the Experimental Animal Centre of Anhui Medical University (SPF grade, weighed 100–130 g)(Hefei, China). A total of ∼50 rats were used for various experimental approaches in the current study. The rats were housed in groups (four to five rats per cage). All rats received a standard rodent diet and tap water ad lib. A constant temperature of 21–23 °C and humidity of 50 ± 5% were maintained. Following feeding for one week, the rats were killed by cervical dislocation and they were confirmed that have no spontaneous breathing and heartbeat. All efforts were made to minimize suffering. The rats were immersed in 75% ethanol and sterilized for 20 min. After removing the femur under aseptic conditions, used scissors to cut both ends of the femur. Use a 5 ml syringe equipped with a 25-gauge needle to rinse BMSCs with α-modified minimum essential medium (α-MEM, HyClone). The washed cells were collected, cultured in α-MEM supplemented with 10% foetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco), and incubated at 37 °C with 5% CO 2. All experiments used cells from passage 3–5. 3 × 105 Cells were seeded on ordinary culture dishes with a diameter of 6 cm (Control group), pure titanium plates (Ti group) or TiO2 specimens (TiO2 group). Before changing the medium, the cells were pre-treated with Cytochalstin D (Sigma) (20 uM) or Phalloidin (Sigma) (10 uM) for 3 h. The study has been approved by the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University.
Specimen preparation of TiO2 nanotubes
A pure titanium plate with a thickness of 2 mm was cut into a square with a length of 35 mm and used as a substrate. Sanded the top and bottom surfaces with silicon carbide sandpaper. Soaked the pure titanium plate in absolute ethanol and cleaned it with an ultrasonic cleaner. After washing again with deionized water, then air-dried at room temperature. Anodizing was performed by using a potentiostat, and formed in an electrolyte solution containing 0.15 M NH4F at 20 V for 30 min to form nanotubes. The cathode was a pure platinum plate. After the anodization was completed, the sample was rinsed with deionized water, then dried at room temperature. Scanning electron microscopy (SEM450, FEI Nova Nano SEM; Thermo Fisher Scientific, Waltham, MA, USA) was used to characterize surface structure.
Lentiviral vector construction and transduction
Three shRNA targets were designed based on the transcript of the wild Rat GCN5 gene and primer synthesis was arranged. qRT-PCR was used to amplify rat GCN5. XbaI and NotI were used to digest the amplicons before insertion into the pCDH lentiviral vector. The sequences of the primers were: forward: AGCACTCCCATCTTCAGTCC; reverse: GCTTCCTCTTCTCTCCTGGCAT. The primers for GCN5 shRNA were: forward: CCGGGCAGGGTGTTCTGAACTTTCTCAAGAGAAAAGTTCAGAACACCCTGCTTTTTTG; reverse: ATTCAAAAAAGCAGGGTGTTCTGAACTTTTCTCTTGAGAAAGTTCAGAACACCCTGC.
The restriction enzymes AgeI and EcoRI were used to digest the qRT-PCR product before incorporation into the pLKD vector (Invitrogen). Sanger sequencing was performed to verify the inserted fragments. To produce the lentivirus, the 293 T cell line was co-transfected with two packaging vectors (psPAX2 and pMD2.G) and two plasmid vectors. Supernatant was centrifuged at 1,000 rpm for 10 min to remove cell debris, then filtered through a 0.45-μm polyethersulfone low protein-binding filter. The titre of the lentivirus was 115 IFU/mL, assessed by a quantification kit (Clontech Laboratories). A vector containing scrambled GCN5 was used as a negative control. To produce stable high expression group and low expression group, BMSCs were infected with lentivirus expressing either GCN5 or three shRNA. The expression of GCN5-related mRNA was detected by qRT-PCR and the GCN5 shRNA lentivirus with the highest silencing efficiency was selected (Supplementary Figure 1, Supplementary Table 1).
Determination of ALP content
The alkaline phosphatase (ALP) content was measured with Alkaline Phosphatase Assay Kit (Abcam). The BMSCs were harvested from the substrate and were washed with cold PBS. Cells resuspended in 50 µL of Assay Buffer. Then centrifuge samples at 4 °C at top speed for 15 min in a cold microcentrifuge to remove any insoluble material and kept them on ice. Add 50 µL of 5 mM pNPP (Abcam) to each well containing Sample and Background Sample Controls and 10 µL of ALP enzyme (Abcam) to each pNPP Standard well. Incubate plate at 25 °C for 60 min protected from light. Stop reaction in Sample wells and Standard wells by adding 20 µL of 3 mol/l NaOH (Abcam). Then measure output at OD 405 nm on a microplate reader. Plot standard curve readings and measure ALP content in the sample.
Western blot
The cells were washed three times with phosphate buffer saline (PBS) and lysed with RIPA buffer supplemented with protease and phosphatase inhibitors for 30 min at 4 °C. For western blotting analysis, 15 mg of sample was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electro-transferred onto nitrocellulose membranes. The primary antibodies used were rabbit polyclonal anti-F-actin (Abcam); rabbit polyclonal anti-GCN5 (Abcam); rabbit monoclonal anti-H3K9AC (Abcam); rabbit monoclonal anti-RUNX2 (Abcam); rabbit monoclonal anti-OCN (Abcam); and rabbit monoclonal anti-OPN (Abcam). For normalization of protein loading, GAPDH antibodies (Abcam) were used. Image J software was used to provide semi-quantitation and statistic analysis for the western blot.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the cells with TRIzol (Invitrogen) and reverse-transcribed into cDNA using a qScript cDNA Synthesis kit (Omega), according to the manufacturer’s instructions. qRT-PCR was performed with SYBR® Premix Ex Taq™ (Takara). The house-keeping gene GAPDH was used as an internal control. Data were analysed using the comparison Ct (2−ΔΔCt) method and expressed as fold changes compared to the respective control. The primer sequences are listed in .
Table 1. Sense and anti-sense primers for quantitative real-time PCR.
Immunofluorescence
BMSCs were fixed with 4% paraformaldehyde for 20 min at room temperature and then washed three times with PBS. The cells were permeabilized with 0.3% Triton-X 100 for 30 min, washed three times with PBS and blocked for 30 min in 10% normal goat serum. The BMSCs on the surface of nanotubes were stained with rabbit polyclonal anti-CD29 and rabbit polyclonal anti-CD90 antibody (Invitrogen). The cytoskeleton was stained with rabbit polyclonal anti-F-actin antibody (Abcam). The cells were stained with primary antibodies overnight at 4 °C and then washed three times with PBS. Used absorbent paper to absorb the excess liquid and added the diluted fluorescent secondary antibodies (goat anti-rabbit Alexa 594). Then cells were rinsed with PBS, and counterstained with DAPI (Beyotime Institute of Biotechnology) for 10 min at room temperature. Following three additional washes with PBS, samples were fixed on a glass slide and observed by Olympus IX70 confocal microscope. All pictures were acquired with the same contrast and brightness parameters. Used the confocal microscope’s built-in software to measure the fluorescence intensity value of a single cell in the image (6 cells with clear borders can be selected in each group).
Statistical analysis
Statistical analysis was carried out using SPSS 20.0 software (IBM). Data are representative of at least three independent experiments using triplicate samples unless otherwise indicated. Data are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using Prism software (GraphPad Software). Comparisons were analysed by one-way ANOVA and followed by Tukey’s post hoc test; p < .05 (*) and p < .01 (**) was considered as significant.
Results
TiO2 nanotubes can promote BMSCs osteogenic differentiation and up-regulate F-actin expression
As in our previous study, in order to better study the osteoinductive effects of nanotubes, ordinary culture dishes (control group) and pure titanium plates (titanium group) were used as controls. The surface morphology and related physical parameters of electrochemically modified TiO2 nanotubes have been measured before, which are about 80 nm in diameter and 2000 nm in height () [Citation20]. showed the surface morphology of pure titanium plates and ordinary culture dishes. CD29 and CD90 were used to determine that the cells on the surface of the nanotubes are the BMSCs which we need to cultivate [Citation21,Citation22] (). The content of alkaline phosphatase (ALP) in two groups of BMSCs was determined after 7 days of surface culture on ordinary culture dishes, pure titanium plates and TiO2 nanotube specimens. The content of ALP in BMSCs of TiO2 group was significantly higher than that of control group and pure titanium group (). The results of qRT-PCR and Western Blotting showed that the expression of osteoblastic marker molecules, such as osteopontin (OPN), osteocalcin (OCN) and runt-related transcription factor 2 (RUNX2), in BMSCs of TiO2 group was higher than that of control and pure Ti group (). At the same time, we further examined the expression of F-actin in three groups. The results from Western blotting show that the level of F-actin in TiO2 BMSCs is upgraded (). In the control group, the cytoskeleton can only show the basic outline of the cell. However, the cytoskeleton was remodelled and the F-actin fibre bundles began to thicken and strengthen the cell surface in the Ti group. In the TiO2 group, the expression of F-actin was further up-regulated and F-actin fibre bundles concentrated in the cell membrane (). These results demonstrate that TiO2 nanotubes can promote the osteogenic differentiation of BMSCs and significantly up-regulate the expression of F-actin.
Figure 1. Surface morphology of TiO2 nanotubes and identification of mesenchymal stem cells. (A) The morphology of nanotubes observed under a SEM. The diameter of the TiO2 nanotube is about 80 nm. Scale bars = 800nm (left) and 200 nm (right). (B) The morphology of pure titanium plate observed under a SEM. Scale bars = 800nm (left) and 200 nm (right). (C) The morphology of ordinary culture dish observed under a SEM. Scale bars = 800nm (left) and 200 nm (right). (D) CD29 (green) and CD90 (red) were used to determine that the cells on the surface of the nanotubes are the BMSCs which we need to cultivate. Scale bars = 100 μm.
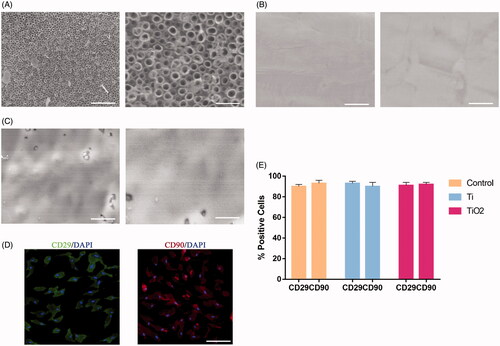
Figure 2. The effect of TiO2 nanotubes on the osteogenic differentiation of BMSCs and F-actin expression. Rat BMSCs were cultured on the surface of pure titanium and TiO2 nanotubes for 7 days. (A) The expression level of ALP in the three groups. (B) Western blot results show that TiO2 nanotubes can promote the expression of osteogenic differentiation markers (OCN, OPN, RUNX2). (C) Quantitative analysis of the results in (B). (D) The results of qRT-PCR show that TiO2 nanotubes up-regulate the gene expression levels of OCN, OPN and RUNX2. (E) Confocal laser scanning microscope observed the F-actin (red) fluorescence staining results of the three groups of BMSCs. Scale bars = 25 μm. The expression of F-actin in the TiO2 group was significantly higher than that in the pure titanium group. (F) Western blot results show that TiO2 nanotubes can up-regulate the protein level of F-actin. (G) Quantitative analysis of the results in (E). (H) Quantitative analysis of the results in (F). p < .05 (*) was considered as significant.
Osteogenic differentiation of BMSCs induced by F-actin
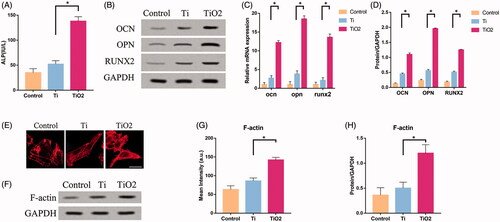
To investigate the regulatory role of F-actin in BMSCs osteogenesis, we inoculated rat BMSCs onto the surface of TiO2 nanotube specimens. Then BMSCs were treated with Cytochalasin D and phalloidin daily. Phallus ring peptides bind only to polymerized filaments, not to actin monomers. It interacts with polymerized microfilaments, destroys the dynamic balance of polymerization and depolymerization, inhibits the disintegration of microfilaments and promotes the formation of more robust cytoskeleton. Cytochalasin D exerts the opposite effect [Citation23]. validated the effects of phalloidin and cytochalasin on BMSCs which were seeded on the surface of TiO2 nanotubes from the aspect of cell morphology. F-actin in the TiO2 + Cytochalasin D group was almost depolymerized and the fibrous structures were rarely seen. But the F-actin in the TiO2 + Phalloidin group further polymerized to form thicker fibre bundles. The fibrous structures were not only concentrated at the edges of the cells, but even filled the entire cells. The content of ALP in BMSCs of TiO2 + Phalloidin group was significantly higher than that of TiO2 + Cytochalasin D group (). The results of qRT-PCR and Western blotting showed that the expression of OPN, OCN and RUNX2 in the TiO2 + Cytochalasin D group was lower than that in the TiO2 + Phalloidin () group. These results confirmed that BMSCs treated with Cytochalasin D inhibited the polymerization of F-actin, thereby inhibiting osteogenesis differentiation, manifested by decreased expression of ALP and related osteogenic differentiation markers (OPN, OCN, RUNX2). On the contrary, Phalloidin promoted the polymerization of F-actin and elevated the expression of F-actin, ALP and osteogenic differentiation markers.
Figure 3. The role of F-actin on the osteogenic differentiation of BMSCs. Rat BMSCs were cultured on the surface of TiO2 specimens, treated with cytochalasin D and phalloidin, respectively, and cultured for 7 days. (A) ALP expression levels in the three groups. (B) Western blot results show that cytochalasin D can inhibit the expression of osteogenic differentiation markers (OCN, OPN, RUNX2), while phalloidin promotes the expression of osteogenic differentiation markers (OCN, OPN, RUNX2). (C) Confocal laser microscope observation of the F-actin (red) fluorescent staining results in three groups of BMSCs. Cytochalasin D can inhibit F-actin polymerization, while phalloidin promotes F-actin polymerization. Scale bars = 25 μm. (D) Western blot results show that cytochalasin D can down-regulate the expression of F-actin, GCN5, H3K9AC, and phalloidin can up-regulate the expression of F-actin, GCN5, H3K9AC. (E) Quantitative analysis of the results in 3(C). (F) The results of qRT-PCR show that TiO2 nanotubes up-regulate the gene expression levels of OCN, OPN and RUNX2. (G) Quantitative analysis of the results in (D). p <. 05 (*) was considered as significant (compared to TiO2 in each group).
TiO2 nanotubes can regulate the expression of GCN5 in BMSCs
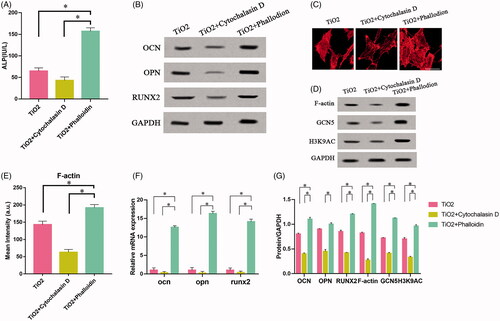
HATs has many members and is involved in the regulation of transcription, metabolism, signal pathway, nervous system, cytoskeleton remodelling and other biological processes [Citation24]. In order to explore the relationship between the changes of F-actin and the expression of HATs during the osteogenic differentiation in vitro, we further screened six enzymes in the HATs. During the 7 days of culturing BMSCs on the surface of TiO2 nanotubes, the detection was carried out on the 0th, 3rd, and 7th days respectively. It was found that the expression level of only GCN5 in the HATs increased steadily and continuously (). Previous studies have shown that GCN5 gene is closely related to osteogenic differentiation, and over-expression of GCN5 can significantly improve the osteoporosis of mouse femurs [Citation25]. In BMSCs, GCN5 can regulate the Wnt/β-catenin signalling pathway and the Wnt/β-catenin signalling pathway is an important pathway for bone development and homeostasis. It also plays a key role in regulating the differentiation of BMSCs [Citation26]. GCN5 increases the acetylation of histone 3 lysine 9 (H3K9AC) of the Wnt gene promoter promotes the osteogenic differentiation of BMSCs [Citation27]. Our experiments further prove that TiO2 nanotubes can also promote the expression of GCN5, and have a certain specificity. At the same time, the changes of F-actin were detected on the 0th, 3rd, and 7th days of TiO2 nanotube surface culture. The results of Western blot indicated that F-actin in BMSCs also increased steadily and continuously (). The results of fluorescent staining indicated that TiO2 nanotubes can promote F-actin polymerization in BMSCs, resulting in the production of stronger F-actin fibre bundles with the extension of culture time (). The increasing trend of F-actin and GCN5 expression levels is highly consistent, suggesting that the two may be closely related. Therefore, we suspect that F-actin and GCN5 may have an upstream and downstream regulatory relationship and play a role in the osteogenic differentiation of BMSCs.
Figure 4. The effect of TiO2 nanotubes on the continuous expression of HAT family and F-actin. Rat BMSCs were cultured on the surface of TiO2 specimens for 7 days. The expressions of F-actin and HAT family in BMSCs were detected on the 0th, 3rd, and 7th days respectively. (A) Western blot results showed that only the expression of GCN5 continued to increase, Consistent with the changes in the expression level of F-actin. (B) Confocal laser microscopy observed the F-actin (red) fluorescence staining results on the 0th, 3rd, and 7th day. Scale bars = 25 μm. The expression level of F-actin increased with the increase of culture time. (C) The results of qRT-PCR show that TiO2 nanotubes stably up-regulate the gene expression level of GCN5. (D) Quantitative analysis of the results in (A). p < .05 (*) was considered as significant (compared to 0d in each group). (E) Quantitative analysis of the results in (B).
F-actin is involved in regulating the expression of GCN5
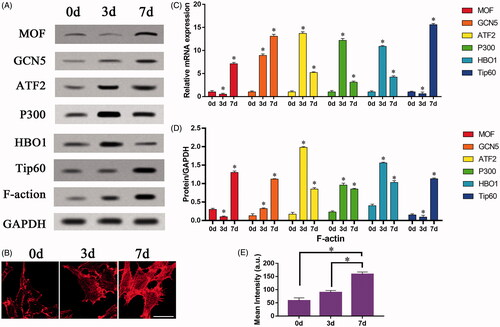
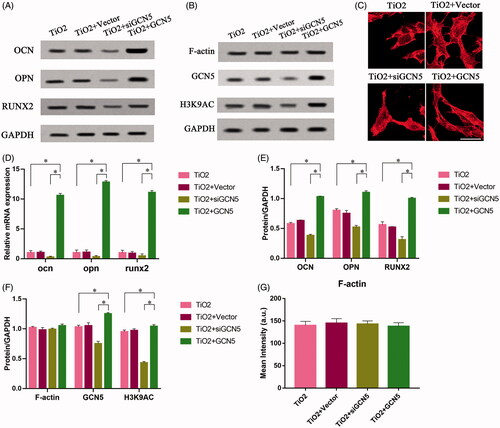
To further verify our conjecture, we used lentiviral vectors to infect BMSCs to construct cell models, and perform overexpression/silencing experiments. There are four groups: TiO2 group, TiO2 + Vector group, TiO2 + siGCN5 group, TiO2 + GCN5 group. After each group of cells were cultured on TiO2 specimens for 7 days, the expression levels of the marker molecules for osteogenic differentiation of BMSCs (such as OPN, OCN, RUNX2), GCN5 and F-actin were detected at the protein level. The nuclear localization and F-actin cytoskeleton in BMSCs were observed by immunofluorescence staining experiments. The results of Western blot showed that GCN5 can regulate the osteogenic differentiation level of BMSCs. Overexpression of GCN5 can significantly drive BMSCs into osteoblasts and increased the expression levels of OPN, OCN and RUNX2. Down-regulation of GCN5 expression inhibited the expression of OPN, OCN, and RUNX2 (). The results of qRT-PCR also proved this (). However, the intervention of GCN5 could not regulate the expression level of F-actin (). The expression level of F-actin is relatively stable and is not affected by overexpression of GCN5 or siGCN5. The results of F-actin staining verified this point from the perspective of cell morphology. The regulation of GCN5 cannot affect the fibrous structures in BMSCs (). At the same time, through the intervention of F-actin, the expression levels of GCN5 and H3K9AC can be regulated (). Phalloidin can increase the expression of GCN5 while promoting the expression of F-actin, while cytochalasin D has the opposite effect. These results all prove our hypothesis that F-actin is involved in regulating the expression of GCN5 and plays a role at upstream of GCN5.
Figure 5. The effect of F-actin on the osteogenic differentiation of mesenchymal stem cells regulated by GCN5. BMSCs infected with lentiviral vectors were cultured on the surface of TiO2 nanotubes for 7 days, and BMSCs that inhibited GCN5 expression (TiO2 + siGCN5) and overexpressed GCN5 (TiO2 + GCN5) were constructed. (A) Western blot results showed that the expression levels of GCN5 and osteogenic differentiation markers (OCN, OPN and RUNX2) in the TiO2 + siGCN5 group decreased and in the TiO2 + GCN5 group increased. (B) Western blot results verified the intervention effect of siGCN5 and GCN5 overexpression and also showed that F-actin is not regulated by GCN5. (C) Confocal laser microscope observation of the F-actin (red) fluorescent staining results on 7 day show that F-actin is not regulated by GCN5. Scale bars = 25 μm. (D) The results of qRT-PCR showed that the gene levels of GCN5 and osteogenic differentiation markers (OCN, OPN and RUNX2) in the TiO2 + siGCN5 group decreased, and in the TiO2 + GCN5 group increased. (E) Quantitative analysis of the results in (A). (F) Quantitative analysis of the results in (B). (G) Quantitative analysis of the results in (C). p < .05 (*) was considered as significant.
Discussion
The cytoskeleton is an important part of the cell. It involves many aspects of cell life, such as differentiation, replication, signal transmission, maintenance of cell morphology, cell movement and apoptosis. The cytoskeleton has three basic structures: (1) microfilaments, which are composed of actin in all eukaryotic cells; (2) microtubules, which are composed of tubulin; and (3) intermediate filaments, which are the most stable supporting parts in the cytoskeleton. The protein composition depends on the cell type. Actin is a structural protein of microfilaments, which exists in two states: free G-actin and polymerized F-actin in the cell [Citation28]. F-actin is fibrous and is the main part of the cytoskeleton. F-actin has more obvious biological functions. To form F-actin, G-actin monomers are first aggregated into oligomers. This step is called nucleation. After nucleation, the process of further polymerization of oligomers depends on the concentration of effective G-actin in the cytoplasm. The actin polymer aggregates and assembles in a double helix to form F-actin. Under normal circumstances, the two types of actin in the cell are in a balanced state. When they are interfered by the outside situation, especially stimulated by mechanical signals, this balanced state will be broken [Citation29]. The free G-actin in the cell tends to aggregate with each other to form F-actin. Afterwards, microfilaments are formed through self-helix, which is the process of cytoskeleton rearrangement. Cytoskeleton rearrangement is directly related to cell adhesion, migration, proliferation and differentiation. Considering that F-actin participates in cell morphology maintenance and a variety of cell functions, the dynamic changes of its aggregation and depolymerization are closely related to the functional status of BMSCs [Citation30]. Through the intervention of TiO2 nanotubes, we proved that promoting the polymerization of F-actin can significantly increase the level of osteogenic differentiation of BMSCs. The stronger F-actin skeleton is the morphological manifestation of osteogenic differentiation.
Histone acetyltransferase is a type of protease that plays an important role in chromosome structural modification and gene expression regulation [Citation21]. The acetylation of histones facilitates the dissociation of DNA and histone octamers and relaxes the nucleosome structure. So that various transcription factors and cooperative transcription factors can specifically bind to DNA binding sites and activate gene transcription [Citation22]. In the nucleus, the processes of histone acetylation and histone deacetylation are in dynamic equilibrium, and are jointly regulated by histone acetyltransferase (HAT) and histone deacetylase (HDAC). HAT transfers the acetyl group of acetyl-CoA to specific lysine residues at the amino terminal of histones. HDAC deacetylates histones and binds tightly with negatively charged DNA. The chromatin is dense and curled and gene transcription is inhibited [Citation22]. More importantly, HAT plays an important regulatory role in promoting bone differentiation. Kim et al. demonstrated that the vitamin D receptor can recruit CBP and p300 to the promoters of Cyp and Opn. It can then be combined with 1,25-dihydroxyvitamin D3 to cause histone H4 acetylation in intact osteoblasts [Citation23]. The study by Zhang et al. proved that PCAF is also related to the osteogenesis of MSC. They can induce osteogenesis by increasing the expression level of Smad signals. At the same time, the expression of PCAF increased significantly. This stimulates the expression of BMP pathway genes by increasing the acetylation level of PCAF’s target (histone H3K9) [Citation31]. In order to explore the effect of TiO2 nanotubes on HAT in BMSCs, we screened six enzymes in the HATs family and found that only the expression level of GCN5 steadily increased over time. The results suggested that TiO2 nanotubes may have a specific regulatory relationship with GCN5.
Considering the known relationship between F-actin and osteoblast differentiation and the specific up-regulation of GCN5 by TiO2 nanotubes, we speculate that F-actin, as a mechanical structure constituting the cytoskeleton, may have a regulatory relationship with GCN5 in BMSCs. In order to further explore the specific regulatory mechanism between F-actin and GCN5 in the process of osteogenic differentiation of BMSCs, we have implemented enhancement/inhibition interventions on F-actin and GCN5 respectively. We used cytochalasin D to inhibit the polymerization of F-actin, and GCN5 was also inhibited. At the same time, the osteogenic differentiation level of BMSCs decreased. When phalloidin was used for intervention, the result was the opposite. But when we silenced GCN5, the F-actin expression level did not change significantly, but the osteogenic differentiation level of BMSCs also began to decrease. Therefore, we have reason to believe that F-actin may act as an upstream to regulate the level of GCN5 in BMSCs and then promote the osteogenic differentiation of BMSCs.
Conclusion
Our results showed that F-actin mediated nanotube-induced cell differentiation through promoting the expression of GCN5 and enhancing the function of GCN5 and GCN5 was a key regulator of the osteogenic differentiation of BMSCs induced by TiO2 nanotubes as a downstream mediated osteogenesis of F-actin, providing a novel insight into the study of osteogenic differentiation on surface of TiO2 nanotubes.
Authors’ contuibution
Yanchang Liu: writing – original draft, formal analysis, validation; Zhicheng Tong: formal analysis, validation; Chen Wang: formal analysis, validation; Runzhi Xia: data curation; Huiwu Li: validation, project administration; Haoran Yu: project administration; Juehua Jing: conceptualization, methodology; Wendan Cheng: conceptualization, methodology, funding acquisition
| Abbreviations | ||
| BMSCs | = | bone mesenchymal stem cells |
| HATs | = | histone acetyltransferase family |
| ALP | = | alkaline phosphatase |
| PBS | = | phosphate buffer saline |
| OPN | = | osteopontin |
| OCN | = | osteocalcin |
| RUNX2 | = | runt-related transcription factor 2 |
| H3K9AC | = | acetylation of histone 3 lysine 9 |
| HDACs | = | histone deacetylases |
| qRT-PCR | = | quantitative real-time reverse transcription polymerase chain reaction |
Supplemental Material
Download TIFF Image (4.3 MB)Supplemental Material
Download MS Word (12.9 KB)Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Scarano A, Carinci F, Orsini T, et al. Titanium implants coated with a bifunctional molecule with antimicrobic activity: a rabbit study. Materials. 2020;13:3613–3245.
- Shiel AI, Ayre WN, Blom AW, et al. Development of a facile fluorophosphonate-functionalised titanium surface for potential orthopaedic applications. J Orthop Translat. 2020;23:647–653.
- Qiaoxia L, Yujie Z, Meng Y, et al. Hydroxyapatite/tannic acid composite coating formation based on Ti modified by TiO nanotubes. Colloids Surf B Biointerfaces. 2020;196:3472–3486.
- Costa Valente ML, Oliveira TT, Kreve S, et al. Analysis of the mechanical and physicochemical properties of Ti-6Al-4 V discs obtained by selective laser melting and subtractive manufacturing method. J Biomed Mater Res. 2021;109:420–865.
- He P, Zhang H, Li Y, et al. 1α,25-Dihydroxyvitamin D3-loaded hierarchical titanium scaffold enhanced early osseointegration. Mater Sci Eng C Mater Biol Appl. 2020;109:1159–1168.
- Bartkowiak A, Zarzycki A, Kac S, et al. Mechanical properties of different nanopatterned TiO2 substrates and their effect on hydrothermally synthesized bioactive hydroxyapatite coatings. Materials. 2020;13:5290.
- Li Y, Song Y, Ma A, et al. Surface immobilization of TiO2 nanotubes with bone morphogenetic protein-2 synergistically enhances initial preosteoblast adhesion and osseointegration. Biomed Res Int. 2019;23:1206–1223.
- Jiang N, Du P, Qu W, et al. The synergistic effect of TiO2 nanoporous modification and platelet-rich plasma treatment on titanium-implant stability in ovariectomized rats. Int J Nanomedicine. 2016;11:4719–4733.
- Jafari S, Mahyad B, Hashemzadeh H, et al. Biomedical applications of TiO nanostructures: recent advances. Int J Nanomedicine. 2020;15:487–498.
- Yadav V, Banerjee DS, Tabatabai AP, et al. Filament nucleation tunes mechanical memory in active polymer networks. Adv Funct Mater. 2019;29:2149–2158.
- Chang Y-C, Wu J-W, Wang C-W, et al. Hippo signaling-mediated mechanotransduction in cell movement and cancer metastasis. Front Mol Biosci. 2019;6:867–878.
- Zeng Y, Zhang B, Liu X, et al. Astragaloside IV alleviates puromycin aminonucleoside-induced podocyte cytoskeleton injury through the Wnt/PCP pathway. Am J Transl Res. 2020;12:318–326.
- Wang H, Jiang Y, Li H, et al. Carbachol protects the intestinal barrier in severe acute pancreatitis by regulating Cdc42/F-actin cytoskeleton. Exp Ther Med. 2020;20:261–278.
- Qi Y, Liang X, Dai F, et al. RhoA/ROCK pathway activation is regulated by AT1 receptor and participates in smooth muscle migration and dedifferentiation via promoting actin cytoskeleton polymerization. Int J Mol Sci. 2020;21:621–631.
- Usik MA, Ogneva IV. Cytoskeleton structure in mouse sperm and testes after 30 days of hindlimb unloading and 12 hours of recovery. Cell Physiol Biochem. 2018;51:375–326.
- Rachubik P, Szrejder M, Rogacka D, et al. The TRPC6-AMPK pathway is involved in insulin-dependent cytoskeleton reorganization and glucose uptake in cultured rat podocytes. Cell Physiol Biochem. 2018;51:393–496.
- Wiese M, Hamdan FH, Kubiak K, et al. Combined treatment with CBP and BET inhibitors reverses inadvertent activation of detrimental super enhancer programs in DIPG cells. Cell Death Dis. 2020;11:257–263.
- Albaugh BN, Denu JM. Catalysis by protein acetyltransferase Gcn5. Biochim Biophys Acta Gene Regul Mech. 2020;36:1248–1259.
- Mutlu B, Puigserver P. GCN5 acetyltransferase in cellular energetic and metabolic processes. Biochim Biophys Acta Gene Regul Mech. 2020;28:618–629.
- Chang Y, Shao Y, Liu Y, et al. Mechanical strain promotes osteogenic differentiation of mesenchymal stem cells on TiO2 nanotubes substrate. Biochem Biophys Res Commun. 2019;511:840–846.
- Xia P, Wang X, Qu Y, et al. TGF-β1-induced chondrogenesis of bone marrow mesenchymal stem cells is promoted by low-intensity pulsed ultrasound through the integrin-mTOR signaling pathway. Stem Cell Res Ther. 2017;8:281–296.
- Pal B, Das B. In vitro culture of naïve human bone marrow mesenchymal stem cells: a stemness based approach. Front Cell Dev Biol. 2017;5:69–73.
- Zhang W, Xu Y, Chen G, et al. Dynamic single-vesicle tracking of cell-bound membrane vesicles on resting, activated, and cytoskeleton-disrupted cells. Biochim Biophys Acta Biomembr. 2019;1861:2331–2342.
- Michaelides MR, Kluge A, Patane M, et al. Discovery of spiro oxazolidinediones as selective, orally bioavailable inhibitors of p300/CBP histone acetyltransferases. ACS Med Chem Lett. 2018;9:28–128.
- Petty EL, Pillus L. Cell cycle roles for GCN5 revealed through genetic suppression. Biochim Biophys Acta Gene Regul Mech. 2020;21:628–637.
- Zhang E, Yang Y, Chen S, et al. Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res Ther. 2018;9:149–159.
- Koutelou E, Farria AT, Dent SYR. Complex functions of Gcn5 and Pcaf in development and disease. Biochim Biophys Acta Gene Regul Mech. 2020;53:2158–2169.
- Naumann H, Rathjen T, Poy MN, et al. The RhoGAP Stard13 controls insulin secretion through F-actin remodeling. Mol Metab. 2018;8:96–696.
- Li Z, Li M, Du M, et al. Dephosphorylation enhances postmortem degradation of myofibrillar proteins. Food Chem. 2018;45:841–853.
- Liu X, Hou W, He L, et al. AMOT130/YAP pathway in topography-induced BMSC osteoblastic differentiation. Colloids Surf B Biointerfaces. 2019;82:2337–2349.
- Kim S, Shevde NK, Pike JW. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J Bone Miner Res. 2015;20:427–439.
