?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Plant-derived nanoparticles have multi-functionalities owing to their ecological origin and biocompatible nature. A novel and stable silver nanoparticle (AgNP) was reported here using Cyanthillium cinereum (C. cinereum) as a reducing as well as capping agent by rapid microwave-assisted green method. The synthesized nanoparticles revealed their crystalline and spherical nature with an average size of 19.25 ± 0.44 nm in HR-TEM analysis. The excitation of electrons from occupied d-bands to states above the Fermi level while employing photoluminescence studies of AgNP indicated their awesome optical properties. Rapid decomposition of dangerous organic dyes like methylene blue and fuchsine in the catalytic presence of AgNP was evidenced from simple UV–visible spectral analysis. In vitro antioxidant potential assessed by DPPH assay indicated an IC50 value of 40.80 ± 0.14 μg/mL for the new AgNP. A substantial control on the growth of pathogenic bacteria such as Staphylococcus aureus and Klebsiella pneumonia can be achieved by synthesized nanoparticles as demonstrated by the well diffusion method. AgNP was also functioned as a non-enzymatic electrochemical sensor with a sharp oxidation peak with peak potentials at 0.366 V and it has a wide application as a bio sensor in neurobiology especially in the detection of neurotransmitters like dopamine with high sensitivity.
Introduction
Nanotechnology is an emerging area which has shown an unprecedented growth that embrace diverse applications [Citation1,Citation2]. This technology mainly employs the use of particles with the dimensions of 1–100 nm [Citation3]. Nanomaterials have its usefulness in diverse fields such as electronics, biomedical, biotechnology and therapeutics. In the technology arena, silver nanoparticles (AgNPs) occupy a significant position among various nanoparticles, owing to its cost-effectiveness, non-toxic and eco-friendly nature [Citation4]. Bactericidal and bacteriostatic properties contribute towards its use as antimicrobial agents [Citation5,Citation6].
The properties of nanoparticles depend mainly on its shape, size and surface area which was inherently influenced by its mode of synthesis and tentative conditions [Citation7]. Physico-chemical methods are the most common way for the synthesis and stabilization of metal nanoparticles. Chemical methods employed includes sonochemical [Citation8,Citation9], electrochemical [Citation10], colloidal [Citation11] and thermal decomposition methods. Separate agents are required for the synthesis and also for the maintenance of their stability [Citation12]. Green synthetic methods gain much significance owing to their non-toxic, sustainable and green reductants and surfactants which eliminate the over use of hazardous chemicals [Citation13–15]. It is cost effective, uses non-toxic renewable materials, employs low temperature, and hence considered to be more environment friendly. Silver nanoparticles are synthesized through green process mainly employing various parts of plants such as leaf [Citation16], stem [Citation17], fruit [Citation18], bark [Citation19], shells [Citation20], roots [Citation21] and flowers [Citation22] as a reducing agent. Green synthetic method is known to generate particles with reasonably good morphology and stability.
Silver nanoparticles differ from all other metal-based nanoparticles in its unique optical, electrical and biological properties and wide application in bio sensing, catalysis, imaging, drug delivery and also in cancer treatment [Citation23]. It was reported that the green synthesized AgNPs using leaf extracts of Azadirachta indica [Citation24], Manilkara zapota [Citation25], Rosa brunonii Lindl [Citation26], Jatropha curcas [Citation27], etc. showed excellent antibacterial activity owing to its high surface area to volume ratio [Citation28,Citation29]. Green synthesized AgNPs are approved as efficient catalysts for photocatalytical degradation of organic pollutants [Citation30–33]. Microwave synthesized AgNPs from bio-waste (banana leaves) extract are polydispersed in nature and exhibit prominent antibacterial activity. These particles fight against lung cancer and breast cancer cells by endorsing inhibition of cell migration and proliferation on low concentrations [Citation34]. The monitoring of thiol and protein adsorption as well as the bioaffinity can be examined using the silver-nanoparticles-on-plastic sensors [Citation35].
There are various reports on photocatalytic and bactericidal effects of nanocomposites. Co3S4-SnO2/PVPCS composites work better than SnO2 nanoparticles, and Co3S4-SnO2 nanocomposites in lidocaine degradation and photocatalysis. This composite also showed a good antibacterial effect against Staphylococcus aureus, and Escherichia coli and showed antifungal effect against Candida albicans [Citation36]. Likewise, Cr2O3/cellulose composites showed an efficacy in photo-degradation of crystal violet and bactericidal effect against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus pyogenes [Citation37]. There are also reports on efficacy of CuS/polyvinyl alcohol–chitosan (CuS/PVACS) in photo-degradation with malachite green solutions in which 96.51% of malachite green was found to be degraded by CuS/PVACS upon UV-irradiation in 60 min. CuS/PVACS was also evaluated for its antibacterial activity against gram positive and gram negative bacteria in which the nanocomposite has shown bacteriostatic behaviour versus Escherichia coli, Pseudomonas syringae, Staphylococcus aureus, and Streptococcus pneumonia [Citation38]. Silver sulphide-magnesium oxide/graphene oxide (Ag2S-MgO/GO) nanocomposite synthesized via sol–gel/ultrasound method showed the highest rate of photo-degradation of rhodamine B (RhB) under UV light (98.8%) and visible light (64.8%) owing to the enhanced charge transfer efficiency via decreasing band gap amount; reduced e–/h + recombination of MgO with the Ag2S crystal and an enhanced removal efficiency with the supported on grapheme oxide. This showed a good antibacterial and antifungal activity against Bacillus vallismortis, Escherichia coli, Aspergillus flavus and Trichoderma viride [Citation39]. AgO, CoO, CdO nanoparticles and AgO-CoO-CdO heterometal oxides synthesized by the chemical method showed a substantial degradation of dye and also showed good antibacterial effect against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and B. cereus [Citation40]. Silver–platinum (Ag–Pt) nanoparticles synthesized using the Crocus sativus L. plant ethanolic extract, showed highest antioxidant properties compared to the Ag nanoparticles and ascorbic acid (AA) and demonstrated the substantial antimicrobial and cytotoxic activities against pathogenic microbes and MCF-7 breast cancer cell line. The environmental chemistry analysis depicts that methyl orange can be degraded from water by catalytic degradation process with sodium borohydride (NaBH4) [Citation41].
Cyanthillium cinereum (Less.) H. Rob. (Asteraceae) commonly called as little ironweed has been consistently known for its medicinal properties and also get its recognition in the Ayurveda’s [Citation42]. This plant has possessed excellent antibacterial, antiviral, analgesic antipyretic and anticancer activities [Citation43,Citation44]. The current work reports the synthesis of AgNPs via eco-friendly green route microwave-assisted synthesis utilizing the leaf extract of Cyanthillium cinereum as the reducing as well as stabilizing agents. The formation of AgNPs at different concentrations and also their stability at different intervals of time were evaluated using UV–vis spectroscopic technique. The obtained nanoparticles were properly characterized using XRD, FE-SEM and TEM techniques. The antioxidant property of the noble metal nanoparticles could be evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The ecological importance of the AgNPs was assessed in the degradation of polluting organic dyes and thus proved to be a material of immediate relevance in the contemporary era. The in vitro antibacterial scopes of the AgNPs were also tested. Optical properties and electrochemical properties as sensor for the detection of dopamine (DA) were evaluated.
Materials and methods
Materials
All the chemicals used were of analytical grade. Silver nitrate (AgNO3; 99.8%), methylene blue, fuchsine and NaBH4 were purchased from Merck India Ltd. (Bengaluru, India).
Preparation of plant extract
Fresh and healthy leaves of Cyanthillium cinereum (C. cinereum) were collected and washed well with distilled water and dried in air. Then, 25 g of dried leaves were cut into small piece and boiled with 200 mL of deionized water taken in a round bottom flask fitted with water condenser for 20 min. It was cooled and filtered using Whatman No 1. filter paper. The plant extracts thus obtained were stored at 4 °C in refrigerator and used as a reducing agent within two days.
Synthesis of silver nanoparticles
Ten millilitres of the plant extract was added to 100 mL of the varied concentrations of aqueous AgNO3 solution (1 M to 1 mM) at a ratio of 1:10 (v/v). The resulting mixture was continuously stirred and subjected to microwave irradiation in an oven operating at a power of 800 W and frequency 2450 MHz until the solution changed its colour. The bioreduction of Ag+ ions to Ag0 was monitored by analysing samples at 3, 4, 5 and 6 min intervals of reaction time using UV–vis spectrophotometer [Citation45]. The synthesized nanoparticle was then dispersed in double distilled water and centrifuged. The separated particles were dried and used for further analysis.
Characterization of silver nanoparticles
The absorption spectra of the synthesized nanoparticles were analysed using Shimadzu UV-1800 spectrophotometer (Kyoto, Japan) at a wavelength of 300–700 nm [Citation46]. XRD measurement was made on a Bruker AXSD8 advanced powder X-ray diffractometer (Billerica, MA). Cu-Kα (λ = 1.54 Å) radiation was used as the X-ray source (40 kV, 35 mA) and 2θ range from 2 to 800 and the scanning rate used was 0.05°/s. The XRD sample was prepared by drop coating the nanoparticle solution on a glass slide followed by drying under ambient condition. The mean particle diameter of AgNP was calculated from the XRD pattern according to the line width of the plane, reflection peak using Scherrer formula. D = 0.9λ/βcos θ where D is the average crystalline domain size perpendicular to the reflection planes, λ is the X-ray wavelength; β is the full width at half maximum (FWHM) and θ is the diffraction angle [Citation15]. HR-TEM images were recorded using JEOL JEM-2100 microscope (JEOL Ltd., Tokyo, Japan) to analyse the size and shape of nanoparticles [Citation13]. To find out the excitation and emission maxima for the AgNPs, prescan was performed using fluorescence spectrometer (Fluoromax 4-Horiba Instruments, Kyoto, Japan) which recorded the spectra with a scan speed of 240 nm/min with excitation slit width of 5 nm and emission slit width of 5 nm.
Catalytic degradation
The AgNPs synthesized were used for the removal of organic dyes, causing ecological pollution. For analysing the degradation reaction, two prominent cationic dyes methylene blue and fuchsine were used. Two millilitres (0.08 × 10−3 M) of the dye, 0.5 mL of freshly prepared NaBH4 (0.06 M) and 0.5 mL of AgNP-Cinereum (0.02 mg/mL) were taken in a quartz cuvette of 1 cm path length. The UV–vis absorption spectra of the reaction mixture were recorded at definite intervals of time in the range of 200–700 nm. Complete disappearance of the colour in the reaction mixture was the direct indication of the degradation of the dye. The kinetics of the reaction was scanned by measuring the absorbance at specified wavelength for both the dyes. A control reaction was also setup without nanoparticles to ascertain the dye degradation is brought about by nanoparticles.
Antioxidant capacity using the DPPH assay
The antioxidant activity of the synthesized nanoparticles was evaluated based on their ability to trap DPPH radical. Ascorbic acid is used as a positive control. AgNP without DPPH was used as blank. Colloidal solution of AgNP (2 mL) and 0.1 mM DPPH solution in ethanol (2 mL) were mixed and shaken vigorously for 2 min. The solution was incubated in the dark at room temperature for 30 min followed by absorbance reading at 517 nm using UV–vis. Results were conveyed as percentage reduction of the initial DPPH absorption in relative to the control. The inhibition ratio can be calculated according to the equation:
where A0 is the absorbance of the control and Ac is the absorbance at the addition of the analytical sample.
Antibacterial evaluation
The antibacterial activities of AgNP were carried out by well diffusion method [Citation47]. For that, a nutrient agar medium plate was prepared, sterilized and solidified. After solidification, bacterial cultures both Gram-positive bacteria Staphylococcus aureus (S. aureus) and Gram-negative bacteria Klebsiella pneumoniae (K. pneumoniae) were swabbed on these plates. The wells were made on the plates and AgNPs solution (10 mg/mL) along with positive and negative controls was loaded in the wells. It was then kept for incubation at 37 °C for 24 h. Zones of inhibition for control and AgNP were measured. Repeat the experiment thrice and mean values of zone diameter were presented.
Electrochemical analysis
Cyclic voltammetry (CV) was chosen as a primary mode for the development of non-enzymatic DA sensor with carbon paste electrodes (CPEs) modified with AgNP as working electrode in a three-electrode electrochemical setup using Metrohm Autolab potentiostat/galvanostat (model no. PGSTAT302N). The modified electrode was prepared by dropping 4.0 µL of the nanocomposite suspension onto pre-cleaned CPE and dried at room temperature. The electrochemical measurements were carried in electrochemical cell system by successive voltammetric cycles (130 cycles, potential range from −0.30 to 0.95 V versus Ag/AgCl (3.0 mol L−1 KCl) in 0.1 mol L−1 NaOH solution). After the optimized procedure of the electrochemical measurements, the CPE/AgNP was evaluated for the DA determination which was carried out by differential pulse voltammetry (DPV).
Results and discussion
UV–vis spectra analysis
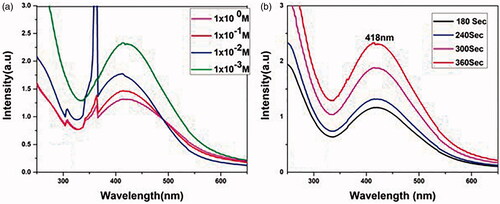
Primarily, the formation of AgNPs was confirmed by observing the colour change of AgNO3 solution. With the addition of Cyanthillium cinereum leaf extract to the AgNO3 solution, a steady change in colour from colourless to dark brown was observed, which was further confirmed by UV–vis spectroscopy [Citation48]. The intensity of extremely symmetric single-band absorption with peak maxima of surface plasmon resonance (SPR) peaks for AgNP [Citation49] synthesized at different concentration of AgNO3 solution was analysed () and found that a concentration of about 1 × 10−3 M AgNO3 was adequate for the synthesis of AgNP [Citation50]. Also, the absorption spectrum using the precursor 1 × 10−3 M AgNO3 was verified at different intervals of time such as 180 s, 240 s, 300 s and 360 s [Citation51] () and observed that at a maximum of 360 s is enough for the complete formation of AgNP where there is a broad absorption peak at 418 nm and after a specific irradiation interval of 360 s coagulation of AgNP makes it difficult to analyse it by spectral data. The size and shape of the particles, in nearby dielectric medium and the accumulation of nanoparticles affect the SPR peak of AgNP [Citation52].
Photoluminescence study
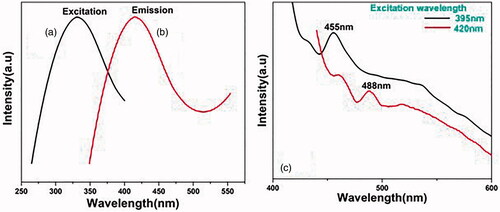
Photoluminescence occurs in noble metals due to an excitation in the electrons from occupied d bands to states above the Fermi level. The emission spectrum of synthesized AgNP is dependent on the excitation wavelength [Citation53,Citation54]. Ensuing the electron–phonon and hole–phonon scattering processes lead to energy loss and finally a photoluminescent recombination of an electron from an occupied sp band with the hole [Citation14,Citation53]. Photo excitation of AgNP at excitation wavelength of 332 nm () produced a very intense fluorescent emission peak at 416 nm (), while excitation at 395 nm and 420 nm produced fluorescence emission peak at 455 nm and 420 nm respectively with reduced intensity (). For AgNPs, the luminescence emission arises as a result of electron–hole recombination processes, i.e. the electron from sp conduction band above the Fermi level and hole from d-band below the Fermi level [Citation52–54].
XRD analysis
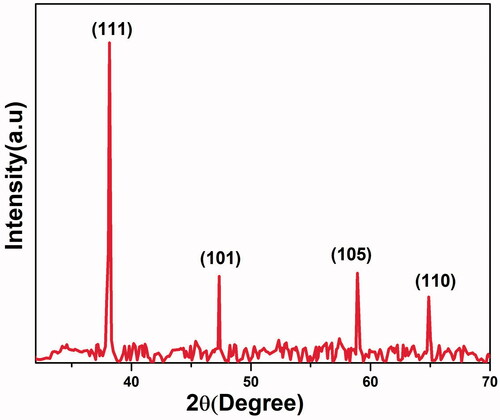
The results obtained from XRD analysis of biosynthesized AgNP was very intense and sharp and it confirms its crystalline structure [Citation55] (). The existence of strong peaks at 2θ = 38.16°, 47.33°, 58.91° and 64.88° belonged to the presence of (1 1 1), (1 0 1), (1 0 5) and (1 1 0) planes (JCPDS file no. 04-0783) (Bragg’s reflection), respectively, and confirmed the crystalline and face centred cubic (FCC) structure of AgNP [Citation48].
TEM analysis
The morphological and crystallographical information of the synthesized AgNP was obtained from the TEM analysis. represents the microscopic images (a–c) of spherical AgNPs at different magnifications. The lattice fringes and atomic columns of nanosilver were clearly seen from the HR-TEM image (d). Elastic scattering of electrons on AgNP produced the bright spots (e) in the selected area electron diffraction (SAED) pattern, which indicates that particles are highly crystalline in nature [Citation15]. The distribution of the particles’ size of synthesized AgNP was shown in the histogram (f) and the particle size is in between 5 and 40 nm with an average size of 19.25 ± 0.44 nm. Also from , it is clear that the present study emphasizes on the synthesis of very small AgNPs with enhanced property as comparing to others, which is discussed in this work.
Figure 4. Transmission electron microscopic images of AgNP. (a–c) Images under diverse magnifications, (d) HR-TEM image of AgNP, (e) SAED pattern of AgNP and (f) particle size histogram.
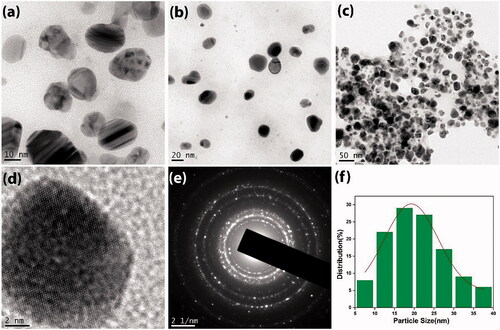
Table 1. Comparative study on the particle size and property analysed for AgNP synthesized from various sources.
Catalytic degradation
The catalytic activity of plant derived AgNP was investigated using the degradation reactions of fuchsine and methylene blue using NaBH4. These dyes were selected for our study because its absorption maximum does not overlap with the SPR band of AgNPs [Citation26]. Fuchsine or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl. The UV–vis absorption spectrum of an aqueous solution of fuchsine shows peaks at 292 nm and 546 nm. The reduction of fuchsine into leucofuchsine can be followed spectrophotometrically by monitoring the absorption maximum at 546 nm. Comparing , it is clear that when AgNP was added to the reaction mixture containing both fuchsine and NaBH4, the intensity of the peak at 546 nm began to decrease continuously with the passage of time. The kinetic studies were performed by ln[A] versus time graph ((c)). Rate constant determination proved a pseudo-first order kinetics with respect to the concentration of the dye [Citation64].
Figure 5. UV–vis absorption spectra measured at 1 min intervals for the degradation of fuchsine. (a) In the absence of AgNP. (b) In the presence of AgNP. (c) Kinetic plot (ln[A] verses time).
![Figure 5. UV–vis absorption spectra measured at 1 min intervals for the degradation of fuchsine. (a) In the absence of AgNP. (b) In the presence of AgNP. (c) Kinetic plot (ln[A] verses time).](/cms/asset/cdefd648-69ab-4be8-b1cd-9f03e9164fa4/ianb_a_1925678_f0005_c.jpg)
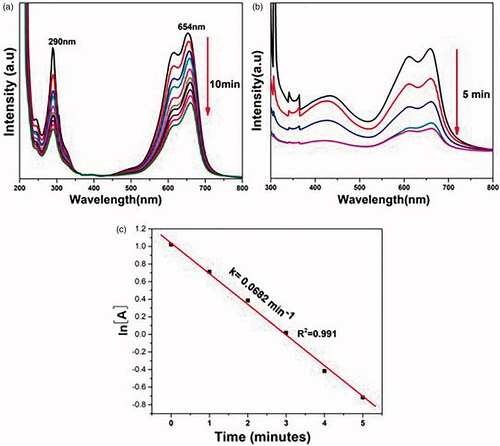
Methylene blue is a cationic thiazine dye which shows its UV–vis absorption peaks at 290 and 654 nm with hump at 612 nm due to π → π* and n → π* transitions [Citation28]. The reduction of methylene blue into its colourless form is schematically represented in [Citation65,Citation66] and further analysed by spectrophotometer using to monitor the absorption maximum at 654 nm. The kinetic plot of ln[A] versus time graph (c) verifies that it follows a pseudo-first order kinetics.
Antioxidant capacity using the DPPH assay
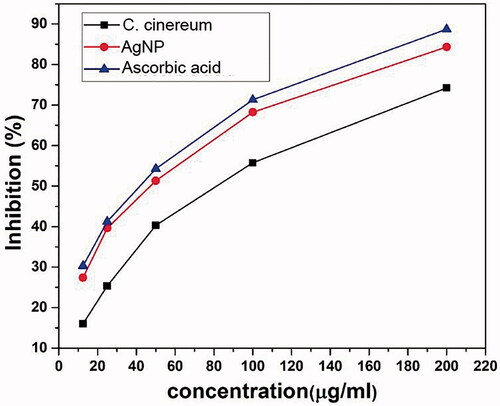
The excellent free radical capturing power of phyto-fabricated AgNP and the plant extract was tested by DPPH method. The percentage radical scavenging activity (RSA) was measured and results are shown in . The RSA increased in a dose-dependent manner of the tested samples and plant extracts (). The recorded scavenging ability for the lowest concentration of the synthesized AgNP (12.5 μg/mL) was 27.41 ± 0.09 and this scavenging ability was increased to 84.33 ± 0.02, when concentration was increased to 200 μg/mL (average IC50 − 40.80 ± 0.14 μg/mL) (). However, the scavenging ability was recorded for aqueous leaf extract at lowest concentration 16.01 ± 0.05 (12.5 μg/mL) and when concentration was increased the scavenging ability was 74.24 ± 0.09 (200 μg/mL) () with average IC50 value, 74.05 ± 0.05 μg/mL. The IC50 values of the synthesized nanoparticles are comparable with that of standard antioxidants having the IC50 value, 35.52 ± 0.12 μg/mL (). These results corroborate well with the previous reports on antioxidant property shown by the phyto-fabricated AgNP using various plant extracts depending mainly on the methods employed in the preparation of nanoparticles [Citation14,Citation26,Citation28,Citation46].
Figure 9. IC50 values for antiradical analysis of C. cinereum and AgNP in comparison with ascorbic acid.
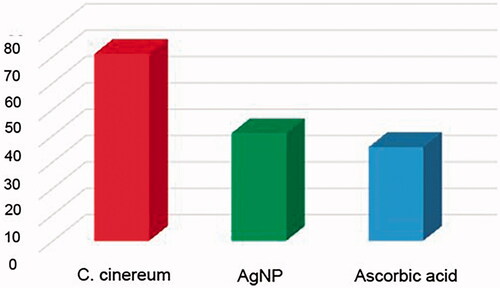
Table 2. Antioxidant activity of aqueous leaf extracts, AgNP and ascorbic acid (standard) showing scavenging % of extracts and IC50 value in C. cinereum.
Antibacterial evaluation
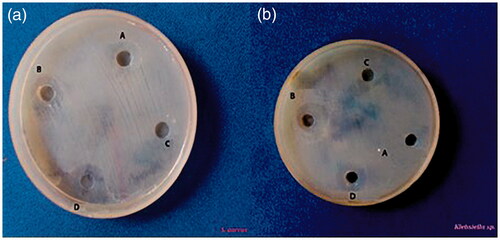
The in vitro antibacterial activity of green synthesized AgNP against the common pathogenic bacteria both Gram-positive bacteria Staphylococcus aureus (S. aureus) and Gram-negative bacteria Klebsiella pneumoniae (K. pneumoniae) was assessed by the well diffusion method in which circular inhibition zone was formed around the well impregnated AgNP [Citation67]. A maximum zone was recorded as 1.6 cm for S. aureus and 1.9 cm for K. pneumoniae when treated with AgNP. No zone of inhibition was observed for the plant extract alone (). The efficacy of AgNP in inhibiting the growth of pathogenic bacteria is attributed to its ability to enter the cell and bind to various bacterial cellular components [Citation68]. Inside a cell, the nanoparticles would interfere with the bacterial growth and signalling pathway by modulating tyrosine phosphorylation of putative peptide substrates critical for cell viability and division [Citation69]. A nanoparticle can interact with DNA, inside a bacterium and thus losing its ability to replicate which may lead to the cell death [Citation51,Citation68]. Growth of gram-negative bacteria was more profoundly affected by the AgNP than that of the gram-positive organisms since the interaction between such nanoparticles and the cell wall of bacteria would be facilitated by the relative abundance of negative charges on the gram-negative bacteria [Citation69] which was again confirmed from our results.
Electrochemical responses
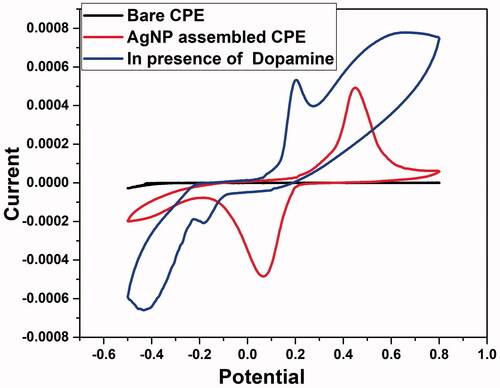
The electrochemical performance of DA having 1 × 10−3 M concentration at the CPE was investigated by CV () in 0.1 mol L−1 PBS (pH 6.5) at 20 mV s−1. Even though bare CPE does not show an oxidation or reduction peak, AgNP assembled CPE showed an anodic peak (0.45 V) which could be attributed to the oxidation of Ag to AgO. Further scanning the potential towards more negative values indicated a peak around 0.06 V which could be ascribed to the reduction of AgO to Ag [Citation70]. Due to the high surface area of AgNP-assembled-CPE, there is a significant enhancement in the peak currents that helps to increases the electro-catalytic activity of the electrode [Citation71]. Using AgNP modified CPE, a distinct redox couple for DA was observed with large increase of current height and shifting [Citation72] of the anodic peak potential to 0.21 V and cathodic peak potential to −0.178 V. Hence, it could be inferred that AgNP modified electrode is recommended for the detection of DA since it makes a significant change in potential.
Sensitivity and selectivity analysis
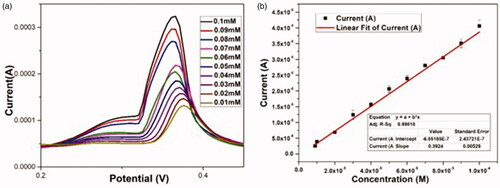
The differential pulse voltammetric (DPV) technique was carried out to determine the electrocatalytic sensitivity of DA using AgNP-assembled-CPE. The individual electrocatalytic oxidation of DA at the AgNP modified electrode was investigated in 10 mL buffer solution (pH 6.8) at a scan rate of 50 mV/s by varying the concentrations. The results showed that analytes are oxidized and show a well-defined and distinguishable sharp oxidation peaks with peak potentials at 0.366 V. With increase in the concentration of analytes, the anodic peak current increases [Citation73] () with a correlation coefficient (R2) of 0.998 indicating that analytes have been oxidized by the active AgNP modified electrode [Citation71,Citation74].
Figure 12. (a) DPV responses of the AgNP-CPE for the detection of different concentrations of dopamine (from 0.01 mM to 0.1 mM) in PBS (pH 6.8). (b) The corresponding linear calibration plots of stripping peak currents in optimized experimental conditions.
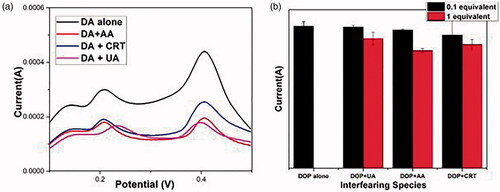
The backbone parameter for evaluating the selectivity is the performance of non-enzymatic DA sensors in the presence of known interferents. During the quantification of DA level, the interferents including uric acid (UA), AA and creatinine (CRT) are found in human blood serum. The interfering effect on adding 1 equivalent of UA, AA and CRT which can be compared with 0.1 equivalent DA at the specified potential were determined and the results were found. (b) indicates that even in the presence of interfering species the DA having a concentration of 0.1 equivalent shows a significant current using AgNP supported electrode suggesting the high selective nature of the developed DA sensor [Citation75,Citation76].
Conclusions
Plant mediated microwave synthesis offers a green and non-toxic synthetic pathway for AgNPs. The AgNPs obtained were well characterized via UV–vis, XRD and TEM analyses. It is clear from the various spectroscopic and imaging analyses, AgNP was found to be crystalline and spherical in shape with an average size of 19.25 nm Their biological significance was proven by DPPH radical scavenging assay which indicated an IC50 value of 40.80 ± 0.14 μg/mL. The progress of degradation of environment polluting dyes was monitored in situ via UV–vis spectroscopy without light irradiation. Also the AgNP shows effective antibacterial activity against Staphylococcus aureus (S. aureus) which is gram positive and Klebsiella pneumoniae (K. pneumoniae) which is gram negative having a zone of inhibition of 1.6 cm and 1.9 cm respectively which is very close to the standard streptomycin. Moreover, the AgNP modified CPE shows excellent electrocatalytic activity towards DA detection with high sensitivity and with a correlation coefficient (R2) of 0.998 which imparts its application as a non-enzymatic sensor for DA. The present research highlights the potential application of multifunctional nanoparticles for environmental protection due to their catalytic capacities.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. This article does not contain any studies with animal subjects.
References
- Sahoo M, Vishwakarma S, Panigrahi C, et al. Nanotechnology: current applications and future scope in food. Food Front. 2021;2(1):3–22.
- Varadavenkatesan T, Selvaraj R, Vinayagam R. Phyto-synthesis of silver nanoparticles from Mussaenda erythrophylla leaf extract and their application in catalytic degradation of methyl orange dye. J Mol Liq. 2016;221:1063–1070.
- Pirtarighat S, Ghannadnia M, Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J Nanostruct Chem. 2019;9(1):1–9.
- Keat CL, Aziz A, Eid AM, et al. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour Bioprocess. 2015;2(1):1–11.
- Hamouda HI, Abdel-Ghafar HM, Mahmoud MHH. Multi-walled carbon nanotubes decorated with silver nanoparticles for antimicrobial applications. J Environ Chem Eng. 2021;9(2):105034.
- Rao SS, Saptami K, Venkatesan J, et al. Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: characterization with assessment of biocompatibility and antimicrobial activity. Int J Biol Macromol. 2020;163:745–755.
- Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J Am Chem Soc. 2002;124(28):8204–8205.
- Bai J, Luo Y, Chen C, et al. Functionalization of 1D In2O3 nanotubes with abundant oxygen vacancies by rare earth dopant for ultra-high sensitive ethanol detection. Sens Actuators B. 2020;324:128755.
- Jayachandrabal B, Sivasankar T, Manickam S. Facile sonochemical synthesis of Ag2O-guar gum nanocomposite as a visible light photocatalyst for the organic transformation reactions. J Hazard Mater. 2020;385:121621.
- Park JH, Ahn HS. Electrochemical synthesis of multimetallic nanoparticles and their application in alkaline oxygen reduction catalysis. Appl Surf Sci. 2020;504:144517.
- Iravani S, Korbekandi H, Mirmohammadi SV, et al. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9:385.
- Rajeshkumar S, Bharath LV. Mechanism of plant-mediated synthesis of silver nanoparticles – a review on biomolecules involved, characterisation and antibacterial activity. Chem Biol Interact. 2017;273:219–227.
- Bindhu MR, Umadevi M, Esmail GA, et al. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J Photochem Photobiol B Biol. 2020;205:111836.
- Francis S, Joseph S, Koshy EP, et al. Microwave assisted green synthesis of silver nanoparticles using leaf extract of Elephantopus scaber and its environmental and biological applications. Artif Cells Nanomed Biotechnol. 2018;46(4):795–804.
- Joseph S, Mathew B. Microwave-assisted green synthesis of silver nanoparticles and the study on catalytic activity in the degradation of dyes. J Mol Liq. 2015;204:184–191.
- Krithiga N, Rajalakshmi A, Jayachitra A. Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J Nanosci. 2015;2015:1–8.
- Sasikala A, Linga Rao M, Savithramma N, et al. Synthesis of silver nanoparticles from stem bark of Cochlospermum religiosum (L.) Alston: an important medicinal plant and evaluation of their antimicrobial efficacy. Appl Nanosci. 2015;5(7):827–835.
- Mane-Gavade SJ, Nikam G, Dhabbe R, et al. Green synthesis of silver nanoparticles by using carambola fruit extract and their antibacterial activity. Adv Nat Sci Nanosci Nanotechnol. 2015;6(4):045015.
- Iravani S, Zolfaghari B. Green synthesis of silver nanoparticles using Pinus eldarica bark extract. BioMed Res Int. 2013;2013:1–5.
- Lateef A, Azeez MA, Asafa TB, et al. Cola nitida-mediated biogenic synthesis of silver nanoparticles using seed and seed shell extracts and evaluation of antibacterial activities. BioNanoScience. 2015;5(4):196–205.
- Al-Nuairi AG, Mosa KA, Mohammad MG, et al. Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from Cyperus conglomeratus root extracts on breast cancer cell line MCF-7. Biol Trace Elem Res. 2020;194(2):560–569.
- Aravind M, Ahmad A, Ahmad I, et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J Environ Chem Eng. 2021;9(1):104877.
- Siddiqi KS, Husen A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J Trace Elem Med Biol. 2017;40:10–23.
- Ahmed S, et al. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9(1):1–7.
- Otari SV, Patil RM, Ghosh SJ, et al. Green phytosynthesis of silver nanoparticles using aqueous extract of Manilkara zapota (L.) seeds and its inhibitory action against Candida species. Mater Lett. 2014;116:367–369.
- Bhagat M, Anand R, Datt R, et al. Green synthesis of silver nanoparticles using aqueous extract of Rosa brunonii Lindl and their morphological, biological and photocatalytic characterizations. J Inorg Organomet Polym. 2019;29(3):1039–1047.
- Chauhan N, Tyagi AK, Kumar P, et al. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front Microbiol. 2016;7:1748.
- Francis S, Joseph S, Koshy EP, et al. Synthesis and characterization of multifunctional gold and silver nanoparticles using leaf extract of Naregamia alata and their applications in the catalysis and control of mastitis. New J Chem. 2017;41(23):14288–14298.
- Patil V, Mahajan S, Kulkarni M, et al. Synthesis of silver nanoparticles colloids in imidazolium halide ionic liquids and their antibacterial activities for gram-positive and gram-negative bacteria. Chemosphere. 2020;243:125302.
- Momin B, Rahman S, Jha N, et al. Valorization of mutant Bacillus licheniformis M09 supernatant for green synthesis of silver nanoparticles: photocatalytic dye degradation, antibacterial activity, and cytotoxicity. Bioprocess Biosyst Eng. 2019;42(4):541–553.
- Vinay SP, Chandrasekhar N. Facile green chemistry synthesis of Ag nanoparticles using Areca catechu extracts for the antimicrobial activity and photocatalytic degradation of methylene blue dye. Mater Today Proc. 2019;9:499–505.
- Joshi S J, S. J. G, Al-Mamari S, Al-Azkawi A. Green Synthesis of Silver Nanoparticles Using Pomegranate Peel Extracts and Its Application in Photocatalytic Degradation of Methylene Blue, Jundishapur J Nat Pharm Prod. 2018 ; 13(3):e67846.
- Singh J, Dhaliwal AS. Plasmon-induced photocatalytic degradation of methylene blue dye using biosynthesized silver nanoparticles as photocatalyst. Environ Technol. 2020;41(12):1520–1534.
- Narasimha R, et al. Microwave assisted biosynthesis of silver nanoparticles using banana leaves extract: phytochemical, spectral characterization, and anticancer activity studies. J Water Environ Nanotechnol. 2021;6(1):49–61.
- Fan M, Thompson M, Andrade ML, et al. Silver nanoparticles on a plastic platform for localized surface plasmon resonance biosensing. Anal Chem. 2010;82(15):6350–6352.
- Huang M, Zhang R, Yang Z, et al. Synthesis of Co3S4-SnO2/polyvinylpyrrolidone–cellulose heterojunction as highly performance catalyst for photocatalytic and antimicrobial properties under ultra-violet irradiation. Int J Biol Macromol. 2020;162:220–228.
- Lu M, Cui Y, Zhao S, et al. Cr2O3/cellulose hybrid nanocomposites with unique properties: facile synthesis, photocatalytic, bactericidal and antioxidant application. J Photochem Photobiol B Biol. 2020;205:111842.
- Wang G, Fakhri A. Preparation of CuS/polyvinyl alcohol–chitosan nanocomposites with photocatalysis activity and antibacterial behavior against G+/G– bacteria. Int J Biol Macromol. 2020;155:36–41.
- Wang H, Li G, Fakhri A. Fabrication and structural of the Ag2S-MgO/graphene oxide nanocomposites with high photocatalysis and antimicrobial activities. J Photochem Photobiol B Biol. 2020;207:111882.
- Zhang J, Ding E, Xu S, et al. Production of metal oxides nanoparticles based on poly-alanine/chitosan/reduced graphene oxide for photocatalysis degradation, anti-pathogenic bacterial and antioxidant studies. Int J Biol Macromol. 2020;164:1584–1591.
- Yang M, Lu F, Zhou T, et al. Biosynthesis of nano bimetallic Ag/Pt alloy from Crocus sativus L. extract: biological efficacy and catalytic activity. J Photochem Photobiol B Biol. 2020;212:112025.
- Shruthi R, Madhu KP, Krishna JG. Pharmacognostical and phytochemical evaluation of the drug Sahadevi (Cyanthillium cinereum (L.) H. Rob.). Int J Ayurveda Pharma Res. 2019;7:19–27.
- Tantengco OAG, Condes MLC, Estadilla HHT, et al. Antibacterial activity of Vitex parviflora A. Juss. and Cyanthillium cinereum (L.) H. Rob. against human pathogens. Asian Pac J Trop Dis. 2016;6(12):1004–1006.
- Shruthi R, Madhu KP. 163. In-silico analysis of the drug Sahadevī (Cyanthillium cinereum (L.) H. Rob.) in breast cancer. J Ayurveda Integr Med. 2018;9(2):S21.
- Krishnaraj C, Ramachandran R, Mohan K, et al. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim Acta A. 2012;93:95–99.
- Renuka R, Devi KR, Sivakami M, et al. Biosynthesis of silver nanoparticles using Phyllanthus emblica fruit extract for antimicrobial application. Biocatal Agric Biotechnol. 2020;24:101567.
- Mannan A, Junaate Kawser Md, Abu Ahmed AM, et al. Assessment of antibacterial, thrombolytic and cytotoxic potential of Cassia alata seed oil. J Appl Pharm Sci. 2011;1(9):56.
- Aadil KR, Pandey N, Mussatto SI, et al. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J Environ Chem Eng. 2019;7(5):103296.
- Banjare MK, Behera K, Banjare RK, et al. Interaction of ionic liquid with silver nanoparticles: potential application in induced structural changes of globular proteins. ACS Sustain Chem Eng. 2019;7(13):11088–11100.
- Anandan M, Poorani G, Boomi P, et al. Green synthesis of anisotropic silver nanoparticles from the aqueous leaf extract of Dodonaea viscosa with their antibacterial and anticancer activities. Process Biochem. 2019;80:80–88.
- Alfuraydi AA, Devanesan S, Al-Ansari M, et al. Eco-friendly green synthesis of silver nanoparticles from the sesame oil cake and its potential anticancer and antimicrobial activities. J Photochem Photobiol B Biol. 2019;192:83–89.
- Smitha SL, Nissamudeen KM, Philip D, et al. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim Acta A. 2008;71(1):186–190.
- Jia K, Wang P, Yuan L, et al. Facile synthesis of luminescent silver nanoparticles and fluorescence interactions with blue-emitting polyarylene ether nitrile. J Mater Chem C. 2015;3(15):3522–3529.
- Iqbal S, Shabaninezhad M, Abuhagr A, et al. Photoluminescence enhancement of perovskites nanocomposites using ion implanted silver nanoparticles. Chem Phys Lett. 2020;760:137995.
- Anjana VN, Koshy EP, Mathew B. Facile synthesis of silver nanoparticles using Azolla caroliniana, their cytotoxicity, catalytic, optical and antibacterial activity. Mater Today Proc. 2020;25:163–168.
- MubarakAli D, Thajuddin N, Jeganathan K, et al. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf B. 2011;85(2):360–365.
- Zarei Z, Razmjoue D, Karimi J. Green synthesis of silver nanoparticles from Caralluma tuberculata extract and its antibacterial activity. J Inorg Organomet Polym. 2020;30(11):4606–4614.
- Naseem K, Zia Ur Rehman M, Ahmad A, et al. Plant extract induced biogenic preparation of silver nanoparticles and their potential as catalyst for degradation of toxic dyes. Coatings. 2020;10(12):1235.
- Shaik M, Khan M, Kuniyil M, et al. Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability. 2018;10(4):913.
- Rao JK, Paria S. Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater Res Bull. 2013;48(2):628–634.
- Ajitha B, Ashok Kumar Reddy Y, Reddy PS. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim Acta A. 2014;121:164–172.
- Patil S, Chaudhari G, Paradeshi J, et al. Instant green synthesis of silver-based herbo-metallic colloidal nanosuspension in Terminalia bellirica fruit aqueous extract for catalytic and antibacterial applications. 3 Biotech. 2017;7(1):36.
- Kumar D, Kumar G, Agrawal V. Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall. bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol Res. 2018;117(2):377–389.
- Lin H-L, Sou N-L, Huang GG. Single-step preparation of recyclable silver nanoparticle immobilized porous glass filters for the catalytic reduction of nitroarenes. RSC Adv. 2015;5(25):19248–19254.
- Kamali M, Isabel C, Costa ME. Ultrasonic synthesis of zero valent iron nanoparticles for the efficient discoloration of aqueous solutions containing methylene blue dye. In: Nanomaterials in the wet processing of textiles; 2018. p. 261–284.
- Ghattavi S, Nezamzadeh-Ejhieh A. A double-Z-scheme ZnO/AgI/WO3 photocatalyst with high visible light activity: experimental design and mechanism pathway in the degradation of methylene blue. J Mol Liq. 2021;322:114563.
- Salam S, Butola B, Mohammad F. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. 2016;6(50):44232–44247.
- Feroze N, Arshad B, Khattak Y, et al. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc Microanal. 2020;83(1):72–80.
- Lyu Y, Yu M, Liu Q, et al. Synthesis of silver nanoparticles using oxidized amylose and combination with curcumin for enhanced antibacterial activity. Carbohydr Polym. 2020;230:115573.
- Khalaf N, Ahamad T, Naushad M, et al. Chitosan polymer complex derived nanocomposite (AgNPs/NSC) for electrochemical non-enzymatic glucose sensor. Int J Biol Macromol. 2020;146:763–772.
- Sreenivasulu V, Siva Kumar N, Suguna M, et al. Biosynthesis of silver nanoparticles using Mimosa pudica plant root extract: characterization, antibacterial activity and electrochemical detection of dopamine. Int J Electrochem Sci. 2016;11:9959–9971.
- Papi P, Caetano FR, Bergamini MF, et al. Facile synthesis of a silver nanoparticles/polypyrrole nanocomposite for non-enzymatic glucose determination. Mater Sci Eng C Mater Biol Appl. 2017;75:88.
- Shetti NP, Malode SJ, Nayak DS, et al. Fabrication of ZnO nanoparticles modified sensor for electrochemical oxidation of methdilazine. Appl Surf Sci. 2019;496:143656.
- Kaur B, Pandiyan T, Satpati B, et al. Simultaneous and sensitive determination of ascorbic acid, dopamine, uric acid, and tryptophan with silver nanoparticles-decorated reduced graphene oxide modified electrode. Colloids Surf B. 2013;111:97–106.
- Adhikari A, De S, Rana D, et al. Selective sensing of dopamine by sodium cholate tailored polypyrrole-silver nanocomposite. Synth Met. 2020;260:116296.
- Wan X, Yang S, Cai Z, et al. Facile synthesis of MnO2 nanoflowers/N-doped reduced graphene oxide composite and its application for simultaneous determination of dopamine and uric acid. Nanomaterials. 2019;9(6):847.

![Figure 6. Time-dependent UV–vis spectra for the removal of methylene blue. (a) In the absence of AgNP. (b) In the presence of AgNP. (c) Kinetic plot (ln[A] verses time).](/cms/asset/38bd4ca8-97e6-40c9-a374-218c093b5283/ianb_a_1925678_f0006_c.jpg)