Abstract
This work describes a general method for the encapsulation of enzymes with albumin as wall material and the enzyme catalase as prime example. Care was taken for the preparation of biochemically active sub-micrometer particles in order to prevent oxygen toxicity induced by artificial oxygen carriers of any type. In cell culture experiments, capsules containing catalase did not exhibit any harmful activities in the absence of peroxides. In the presence of hydrogen peroxide application of low and medium dosed capsules below 0.05 vol% (final concentration 0.001 vol%) even increased the cell damaging process. However, a higher dosage of capsules (>0.05 vol%) prevented completely cellular disruption induced by 5 mM hydrogen peroxide and decreased up to 90% of cellular damage at higher peroxide concentrations. These results demonstrated that encapsulated catalase was enzymatically active and the over-all activity of prepared catalase capsules was determined to be >1900 U mL−1 vol%−1.
Introduction
In order to counteract the rising shortness on blood donations all over the world, the development of artificial blood substitutes has been investigated with several different approaches [Citation1–3]. Possible blood substitutes were created using either hemoglobin-based oxygen carriers (HbOCs) [Citation4,Citation5] or perfluorocarbon-based oxygen carriers (PFCOCs) [Citation6]. HbOCs as well as PFCOCs come with a shell of either biodegradable polymers or albumin [Citation5,Citation7,Citation8] whereas free haemoglobin has widely been ruled out due to nephrotoxicity [Citation9].
Promisingly, all these approaches have the drawback, that more intracellular components, e.g. enzymes or co-factors, are needed for the adequate replacement of red blood cells (RBCs). In order to reduce damage induced by free radicals, RBCs naturally contain various additional enzymes, such as catalase and superoxide dismutase. In 1984 Turrens has shown that encapsulation of RBC-enzymes into liposomes is principally possible [Citation10]. In this work we prove that the encapsulation of RBC-enzymes is possible with the more modern method of encapsulation into a shell of albumin. We take advantage of organic, inorganic and physical chemistry for the preparation of biochemically active sub-micrometer particles to prevent oxygen toxicity induced by artificial oxygen carriers of any type.
In 1913 Heard already proposed a coherence between metal cations, carbonates and the precipitation of proteins [Citation11]. In this work, the co-precipitation of catalase together with the hardly soluble MnCO3 template was used according to a protocol introduced by Johnson [Citation12]. The subsequent addition of BSA to these MnCO3-catalase generated an albumin wall thus leading to particles consisting of a MnCO3-catalase core and a BSA shell. Mn2+ was chosen as the metal ion over commonly used Ca2+ because of the lower binding of Mn2+ to albumin, thus being easier to remove from the final sample by consequent washing [Citation13].
Encapsulation of enzymes is already being used in biocatalysis but has not yet been successfully applied to the creation of artificial, biocompatible cells. For technical purposes, enzymes can be encapsulated in polymers or sol-gels [Citation14] or immobilized on carbon nano-tubes [Citation15,Citation16]. One possible encapsulation technique for biomedical application is liposome-encapsulation of enzymes [Citation17] but this bears the disadvantage that efficiency depends on interaction between the protein and the phospholipid bilayer [Citation18]. The encapsulation of proteins into a shell of albumin has already successfully been performed by different research groups [Citation5,Citation4,Citation19], so the encapsulation of functional enzymes should be the consistent next step in creating artificial RBCs.
Experimental
Materials
Bovine serum albumin fraction V (BSA), Manganese(II) chloride tetrahydrate, Ethylenediaminetetraacetic acid disodium salt dihydrate (Na2EDTA) and Triton X-100 were purchased from Sigma-Aldrich (Darmstadt, Germany); Sodium carbonate anhydrous and Sodium hydroxide were purchased from Merck (Darmstadt, Germany); Disodium phosphate dehydrate was purchased from Carl Roth (Karlsruhe, Germany); Ringer’s solution was purchased from Fresenius Kabi (Bad Homburg, Germany); Gibco Minimum Essential Medium (MEM), Gibco Foetal bovine serum (FBS), Gibco Pen Strep and Gibco 0.05% Trypsin-EDTA were purchased from Thermo Fisher Scientific (Schwerte, Germany); Catalase from bovine liver (ca. 11,000 U/mg) was purchased from SERVA (Heidelberg, Germany); Genipin was purchased from TCI Chemicals (Eschborn, Germany). All materials were of highest quality available.
Synthesis of albumin-catalase capsules (ACCs)
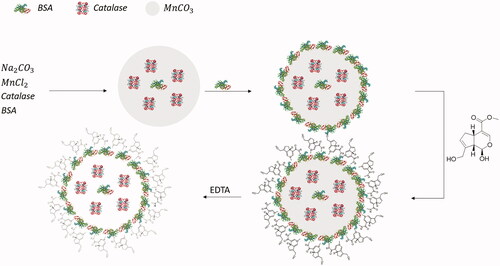
ACCs were created by a method first presented by Xiong et al. [Citation5,Citation19,Citation20] and later modified by Schakowski et al. [Citation4]. Cross-linking of the BSA molecules by genipin as described in previous works [Citation4,Citation21–23] results in a stable shell of BSA around the core particle. Dissolution of MnCO3 from the core by EDTA finally leads to the release of formerly entrapped catalase to the inside of the created capsule ().
Figure 1. Synthesis of ACCs by co-precipitation of MnCO3 and catalse, followed by adsorption of BSA and cross-linking by genipin. The MnCO3 core is then dissolved using EDTA. .
Two aqueous solutions of 25 mL, each containing 10 mg mL−1 Catalase, 1 mg mL−1 BSA and either 0.25 M Na2CO3 or 0.25 M MnCl2, respectively, were prepared. The Na2CO3 solution was placed in a 100 ml Erlenmeyer flask and the MnCl2 solution was added slowly under continuous vigorous stirring. After total merging of the two solutions, stirring was continued for two more minutes. Subsequently 125 mg BSA was carefully added in portions and after total dissolution of the BSA, stirring was continued for five more minutes. The resulting suspension was equally split into two 50 mL reaction vessels and centrifuged at 1,000 × g for two minutes. The supernatant was discarded and the residue was re-suspended in 40 mL of Ringer’s solution each using shaking and ultrasonic bath. Centrifugation and washing were repeated two more times, before the residue was re-suspended in 40 mL of MilliQ water each. The resulting suspensions were then merged into a 200 mL Erlenmeyer flask and under continuous stirring 20 mL of 940 μM Genipin solution in MilliQ water were added dropwise, resulting in a final concentration of 188 μM Genipin. The aperture of the Erlenmeyer flask was then covered by aluminium foil and the suspension was stirred for 24 h at room temperature.
The suspension was equally split into two new 50 mL reaction vessels and again centrifuged for two minutes at 1000 × g before the supernatants were discarded. 40 mL of Na2EDTA solution of pH 7.4 were added to each reaction vessel and the closed reaction vessels were shaken and kept in ultrasonic bath until all residue had fully dissolved. After centrifugation for two minutes at 1,500 × g and discarding of the supernatants, this step was repeated once more. Emerging foam on the top of the supernatants was skimmed off and residues were suspended in 1 mL of Ringer’s solution before being merged into a new suitable reaction vessel.
The final concentration of ACCs was measured by using a haematocrit centrifuge. For further assays, ACCs were diluted to suitable concentrations using appropriate media.
The amount of residue Mn was determined by atomic absorption spectrometry (AAS). The amount of residue Mn ranged from 0.0 to 31.2 mg L−1 for all samples.
Prepared ACCs can be stored at 4 °C or −20 °C in Ringer’s solution for more than six months maintaining a useful level of enzymatic activity.
This procedure has also been performed using MnCl2 under N2-atmosphere and using ZnCl2 instead of MnCl2. For preparation under N2-atmosphere, all solutions needed for the synthesis until dissolution of MnCO3 by EDTA have also been purged by N2 in order to minimize the presence of O2. The amount of genipin was tripled. AAS yielded in no detectable residue Mn (detection limit 0.26 mg L−1).
Characterization
Transmission electron microscopy (TEM)
Transmission Electron Microscopy was performed using a JEM 1400 Plus TEM (JEOL, Freising, Germany).
Respirometry assay
Respirometry was performed on an Oroboros Oxygraph-2k Fluorespirometer (Oroboros Instruments, Innsbruck, Austria).
The chamber volume V of the fluorespirometer was adjusted to 2 mL and 37 °C and filled with 50 mM sodium phosphate buffer or artificial serum (AS), both of pH 7.4. After the oxygen background of the fluorespirometer had stabilized, 25 μL of freshly prepared 120 mM H2O2 in 50 mM sodium phosphate buffer of pH 7.4 were injected into the chamber using a Hamilton syringe. After the oxygen flux had stabilized due to accelerated auto-degradation of H2O2 in artificial serum, 50 μL of ACC-solution of different concentrations c was injected into the chamber and oxygen flux was measured for five minutes.
The oxygen flux was corrected for the background flux due to auto-degradation and the average corrected oxygen flux f was obtained by linear regression. The enzymatic activity A was calculated as A = (2f .V)/(c. 50 μL), taking into account the ratio of produced O2 and degraded H2O2 in 2 H2O2 → O2 + 2 H2O.
The artificial serum was created by dissolving 298.2 mg (4 mmol) KCl, 162.6 mg (0.8 mmol) MgCl2 6 H2O, 266.4 mg (2.4 mmol) CaCl2, 2016.2 mg (24 mmol) NaHCO3, 89.7 mg (0.65 mmol) NaH2PO4 H2O, 92.3 mg (0.65 mmol) Na2HPO4, 6665.1 mg (114.05 mmol) NaCl and 50 g BSA in 1000 mL MilliQ water. The pH was adjusted to 7.4 at 37 °C using NaOH and HCl, each ≤1 M. AS was split into portions of 50 mL and stored at −20 °C. AS once thawed should be kept at 37 °C and should be not be refrozen.
Cell culture
Cells were cultured in minimum essential medium (MEM) Eagle supplemented with 25 mM sodium bicarbonate, 10% FBS, 2 mM L-glutamine, and 100 units mL-1 penicillin G and 0.1 mg mL-1 streptomycin in 75 cm2 plastic flasks at 37 °C in a humidified atmosphere of 95% air and 5% CO2. Subcultures were obtained by trypsinisation (0.05% trypsin-EDTA). For experimental use, cells were transferred to 6-well plates.
Cytotoxicity assays
The standard cytotoxicity model of murine fibroblast-derived cell line L929 (American Type Culture Collection, NCTC clone 929 of strain L) was used for the experiments [Citation24]. Cells were incubated in 6-well-plates under the described conditions over night. The medium was removed and 2 mL of 50 mM sodium-phosphate buffer of pH 7.4 and 50 μL of trypan blue solution were added.
For cytotoxicity of H2O2, 50 μL of freshly prepared H2O2-solution was added, so that final H2O2-concentrations in the wells were 0, 1.25, 2.5, 5.0, 7.5 and 10 mM. Cells were counted by hand after 0, 30, 60, 90, 120, 150 and 180 min using an inverted microscope and the fraction of cells that had taken up trypan blue as a marker of loss of membrane integrity was calculated. For the duration of the experiment, cells were kept under described conditions.
For the cytotoxicity assay of ACCs, 100 μL of ACC-suspension of different concentrations was added instead of H2O2. The period of observation was prolonged to 24 h.
Protection assay
To determine the protective capacity of prepared ACCs, cells were incubated in 6-well-plates under described conditions. Again 50 μL of trypan blue solution were added. Then 50 μL of ACC-suspension were added with original concentrations of 0.001, 0.005, 0.010, 0.100, 0.250, and 0.500 vol%.
Results from H2O2 cytotoxicity assays served as control.
Results and discussion
Physical properties
Cross-linking by genipin is superior to agents commonly used, e.g. glutaraldehyde, since no special work-up is necessary to remove possible excess genipin. In presence of oxygen, excess genipin polymerizes and loses any known noxious traits [Citation23].
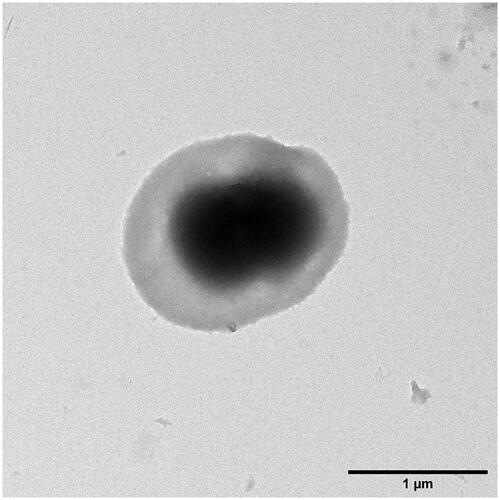
Transmission electron microscopy was used to determine shape and size of the ACCs. Measuring the longest diameter of 452 single ACCs, prepared by using a MnCO3 template, yielded in a mean diameter of 1.245 μm with a standard deviation of 0.272 μm. The overall range of diameters ranges from 0.433 to 1.951 μm. As seen in , particles are shaped ellipsoid rather than round, whereas the core displays to have a peanut-like shape.
Figure 2. A solitary ACC from MnCO3 template pictured by TEM. Its longest diameter measures about 1.65 μm.
ζ-potential was determined to be −30.64 mV ± 7.76 mV. Each sample was measured three times and the overall mean and standard deviation were calculated.
ACCs prepared under N2 present with a mean diameter of 1.040 μm with a standard deviation of 0.225 μm with a range from 0.506 to 1.605 μm (n = 180).
ζ-potential for ACCs prepared under N2 presented to be −30.45 ± 15.56 mV. Each sample was measured three times and the overall mean and standard deviation were calculated.
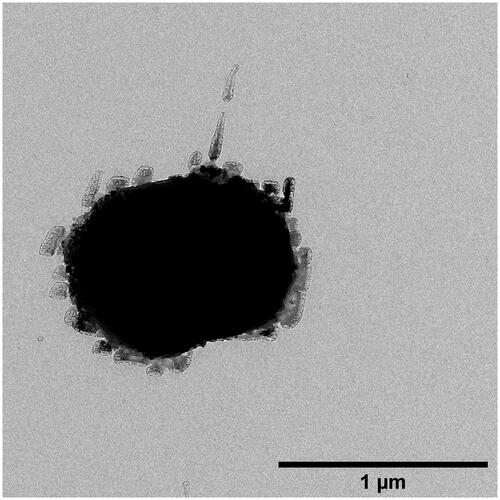
shows a typical ACC synthesized under N2-atmosphere. Though it has a similar diameter compared to ACCs prepared under normal atmosphere, it presents with nearly no visible mantle of albumin. The absence of O2 and, by association, radical-induced polymerization of genipin might lead to a slower polymerization of genipin molecules, thus leaving enough time for excess albumin to leave the surface of the pre-formed inorganic core [Citation21].
Figure 3. A solitary ACC from MnCO3 template under N2-atmosphere. It's longest diameter measures about 1.09 μm
ZnCO3 was chosen as an alternative template, due to its excellent protein-binding abilities [Citation11,Citation25,Citation26].
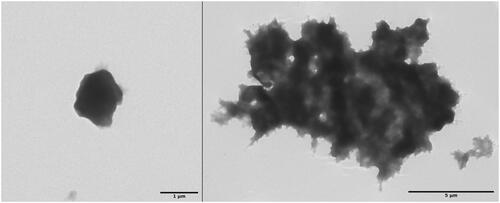
Using ZnCO3 as a template, solitary capsules observed were about 20% smaller, as seen in (left), but have the great disadvantage of forming huge bulky adducts with large amount of inter-capsular cross-linking (right). Unfortunately, the changing of various parameters (e.g. kind of enzyme, albumin and cross-linking agent) failed in decreasing the amount of inter-capsular cross-linking to insignificant yields.
Figure 4. TEM images of a solitary ACC (left) and bulky adducts prepared from ZnCO3 templates. The longest diameter of the solitary ACC measures about 1 μm, while the adduct measures 13.2 μm.
ζ-potential was determined to be −39.4 ± 2.94 mV. Each sample was measured three times and the overall mean and standard deviation were calculated.
Respirometry
Respirometry assays on general functionality were performed in 50 mM sodium phosphate buffer at pH 7.4 and 37 °C (n = 4 each). Interestingly, encapsulated catalase still maintained enzymatic activity. There was a clear dependence of enzymatic activity of ACCs from the volume concentration used for the assay, which is shown in . This finding is allegeable by the high true activity of the ACCs, leading to experimental set-ups with H2O2 concentrations out of the ACCs substrate saturation. At higher peroxide concentrations any further observations were impossible due to the formation of oxygen bubbles.
Figure 5. Determination of enzymatic activity of ACCs by respirometry. Mean values are represented by the grey bars. Black bars represent the standard deviation.
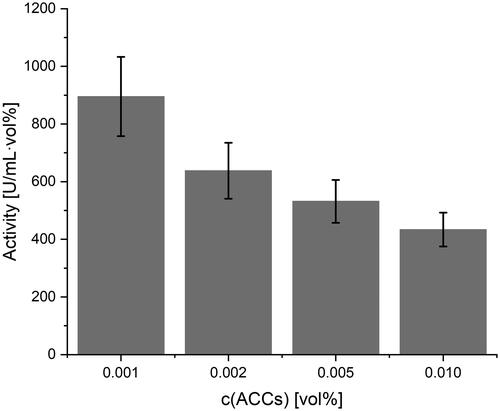
Nonetheless, ACCs from MnCO3 templates displayed enzymatic activities higher than 1000 U mL−1 vol%−1 in concentrations of 0.001 vol% of the injected suspension. ACCs prepared under N2-atmosphere even displayed activities of 1987 ± 658 U mL−1 vol%−1 at 0.002 vol% and 960 mM H2O2 (concentrations of injected solutions).
Due to the smaller volume of ACCs from ZnCO3 templates, the amount of catalase used in preparation needed to be more than tripled to reach similar enzymatic activities per volume. At the maximum enzyme-binding capacity of the ZnCO3 templates, which equals 7.32-times the amount of enzyme used for MnCO3 templates, activities of about 2000 U mL−1 vol%−1 were observed (data not shown).
For better adaption of possible clinical scenarios and application, respirometry assays on storage conditions were performed in AS instead of phosphate buffer (n = 4 each). Furthermore, concentration of injected ACC suspension was decreased to 0.0002 vol% for ACCs prepared under normal atmosphere. ACCs prepared under N2-atmosphere were measured at 0.002 vol%.
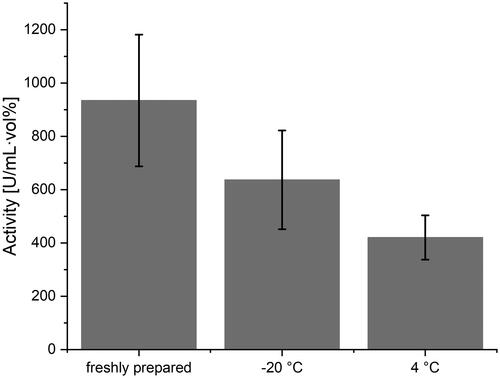
ACCs were stored over night at 37 °C, room temperature, 4 and −20 °C and compared to the activity of freshly prepared ACCs of the same concentration. For ACCs stored at room temperature and at 37 °C any enzymatic activity of H2O2 degradation was lost beyond auto-degradation, presumably accelerated by BSA present in AS. ACCs stored at 4 °C still displayed a mean activity of 45.6% with a standard deviation of 4.9% compared to freshly prepared ACCs. ACCs stored at −20 °C even maintained an activity of 67.8% with a standard deviation of 1.5% compared to freshly prepared ACCs. It is therefore tentatively suggested to storage ACCs at −20 °C after preparation.
depicts the overall activity of capsules stored under the described conditions.
Figure 6. Dependence of ACC activity from storage temperature. Mean values are represented by the grey bars. Black bars represent the standard deviation.
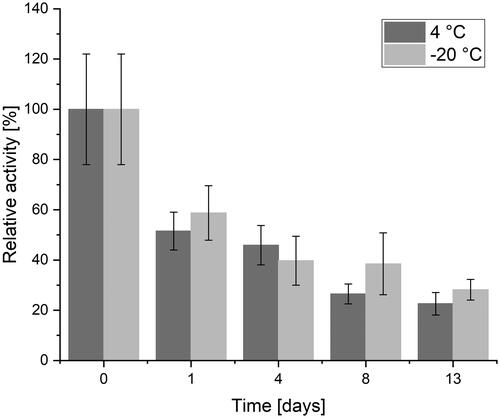
Due to the higher activity of ACCs prepared under N2 and thus associated higher interest, storage conditions were evaluated for a period of 14 days (day 0–13). After one day and four days of storage under the described conditions, there is no significant difference in loss of activity (one day p = .56, four days p = .18) but for storage longer than four days, the differences between storage at 4 °C and −20 °C become significant (p = .02) with a better outcome for storage at −20 °C. displays the described findings.
Figure 7. Maintained activity for ACCs prepared under N2-atmosphere after storage for two weeks at 4 °C and −20 °C. For short-term storage, no significant difference occurs. For storage of eight days or more, storage at −20 °C yields in maintaining significantly higher activities (p = .02).
After thawing ACCs should be applied without further delay. If delay is inevitable, thawed ACCs should be stored at 4 °C.
Cell culture
In the cytotoxicity assay (n = 3), ACCs seemed to be harmless to the L929 cells since uptake of trypan blue did not take place within the observation period of 3 h. Therefore, the observation period was prolonged to 24 h, but again any uptake of trypan blue by L929 cells did not occur.
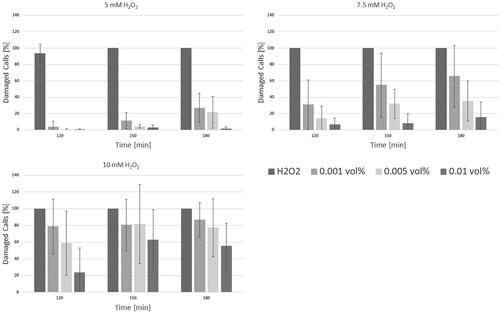
Protection assays were only performed with ACCs from MnCO3 templates under described conditions (vide supra) with 50 mM sodium phosphate buffer at pH 7.4 as solvent (n = 3). In line with previous experiments (vide supra), ACC concentrations ≥0.1 vol% did not evoke any trypan blue uptake in L929 cells, but were evident for ACC concentrations up to 0.05 vol% as shown in and .
Figure 8. Protection assay performed using low concentrations of H2O2. No protective effect can be observed using low-dose ACCs. The grey bars represent the mean values, while standard deviation is shown by the black bars.
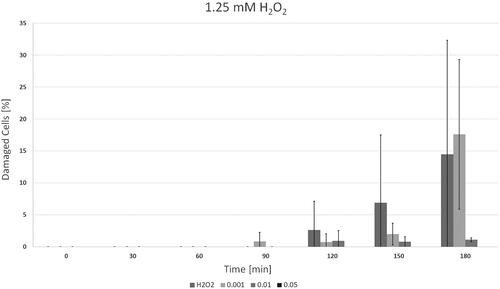
Figure 9. Results of the protection assay using higher peroxide concentrations. The grey bars represent the mean values, while standard deviation is shown by the black bars.
For final H2O2 concentrations <2.5 mM a low-dose ACCs (0.001 vol%) application failed in protecting L929 cells for more than 150 min (p = .34), whereas a medium-dose ACCs (0.01 vol%) already displayed a protective effect (p = .08) as seen in . The sharp increase in cell damage after 180 min after application of low-dose ACCs presumably occurs when self-enhancing cellular mechanisms like apoptosis could no longer be prevented due to peroxide-induced harm.
Interestingly, for H2O2 concentrations ≥2.5 mM both low-dose and medium-dose ACCs seemed to have an adverse effect, leading to increased cell damage (p < .008). High-dose ACCs (0.05 vol%) presented to have significant protective effect for all chosen H2O2 concentrations. For H2O2 concentrations <7.5 mM any trypan blue uptake by L929 cells was prevented by applying ACC concentration of 0.05 vol% or higher. If H2O2 concentration was ≥7.5 mM, only high-dose ACCs displayed significant protective effects (p < .005).
The adverse effect of insufficient dosage might be explained by the mechanism of catalase-mediated peroxide degradation as proposed by Putnam et al. in 2000 [Citation27]. During the reaction, radical intermediate states occur, that themselves offer alternative reaction pathways for excess peroxide to form free radicals. This accelerated formation of free radicals advances radical-induced cell damaged. The formation of free radicals can be reduced or even prevented by increasing the amount of catalase.
displays the depicted findings.
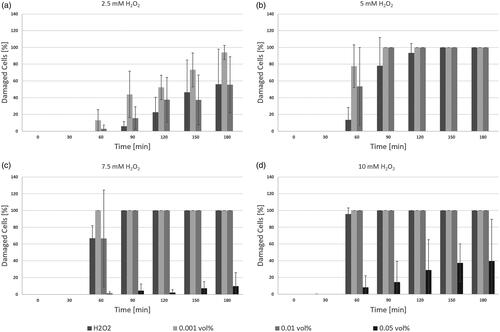
Protection assays were repeated for ACCs prepared under N2-atmosphere. Final H2O2 concentrations were chosen to be 5, 7.5, and 10 mM, ACC concentrations were 0.001, 0.005 and 0.01 vol%. ACCs in any concentration yielded in highly significant decrease of cellular damage as all p-values present to be <1.3 × 10−4. Data is shown in .
Conclusion
The presented method of co-precipitation of an enzyme together with an inorganic template followed by encapsulation into a shell of albumin, successfully led to functional microcapsules.
Catalase capsules prepared by the developed method remain enzymatically active and decreased peroxide induced cell damage up to 90% at higher concentrations, if dosed correctly. A too low dosage should be avoided because this might result in driving harmful effects. Capsules present a size of about 1.25 μm in diameter, thus being small enough for intravenous application. The ζ-potential of about −30 mV prevents rapid aggregation. The absence of O2 during preparation even increased activity and protective effects. In further assays, co-encapsulation of catalase and other intracellular RBC enzymes, e.g. superoxide dismutase or carbonic anhydrase, could be the next step in developing tools for artificial oxygen carriers. In principle, the presented method can be applied for both perfluorocarbon-based oxygen carriers and haemoglobin-based ones, but, of course, a co-encapsulation of enzymes occurring in erythrocytes together with haemoglobin might be a mandatory formulation in the near future. Another future application might be the use in biocatalysis among other encapsulation and immobilization methods.
Acknowledgements
The authors thankfully express their gratitude to Mr. Bernd Walkenfort from the Electron Microscopy Unit of the Imaging Centre Essen (IMCES) for the performance of TEM imaging and Mrs. Susanne Eitner from the Institute of Physiological Chemistry of the University Hospital Essen for counting cells. Furthermore, authors express gratitude to Mr. Robin Meya from the Labor für Mikroanalytik of the University Duisburg-Essen for performing AAS. No external funding was provided for this work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used in this work is publicly available at https://www.doi.org/10.7303/syn23519345
References
- Chung K-W, Basavaraju SV, Mu Y, et al. Declining blood collection and utilization in the United States. Transfusion. 2016;56(9):2184–2192.
- Ellingson KD, Sapiano MRP, Haass KA, et al. Continued decline in blood collection and transfusion in the United States-2015. Transfusion. 2017;57(Suppl 2):1588–1598.
- Müller MM, Geisen C, Zacharowski K, et al. Transfusion of packed red cells. Deutsches Aerzteblatt Online. 2015;112: 507–518. DOI: https://doi.org/10.3238/arztebl.2015.0507
- Schakowski KM, Linders J, Ferenz KB, et al. Synthesis and characterisation of aqueous haemoglobin-based microcapsules coated by genipin-cross-linked albumin. J Microencapsulation. 2020;37(3):193–204.
- Xiong Y, Steffen A, Andreas K, et al. Hemoglobin-based oxygen carrier microparticles: synthesis, properties, and in vitro and in vivo investigations. Biomacromolecules. 2012;13(10):3292–3300.
- Wrobeln A, Schlüter KD, Linders J, et al. Functionality of albumin-derived perfluorocarbon-based artificial oxygen carriers in the Langendorff-heart. Artif Cells Nanomed Biotechnol. 2017;45(4):723–730.
- Bauer J, Zähres M, Zellermann A, et al. Perfluorocarbon-filled poly(lactide-co-gylcolide) nano- and microcapsules as artificial oxygen carriers for blood substitutes: a physico-chemical assessment. J Microencapsulation. 2010;27(2):122–132.
- Chang TMS, Yu W-P. 1995. Biodegradable polymer membrane containing hemoglobin for blood substitute. US Patent Number US5670173A.
- Simoni J, Simoni G, Hartsell A, et al. An improved blood substitute: in vivo evaluation. Artif Organ Res Develop. 1997;43(5):714–725.
- Turrens JF, Crapo JD, Freeman BA. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J Clin Invest. 1984;73(1):87–95.
- Heard WN. The reaction between metallic salts and the soluble carbonates and its bearing upon the precipitation of protein. J Physiol. 1913;46(2):104–128.
- Johnson KS. Solubility of rhodochrosite (MnCO3) in water and seawater. Geochim Cosmochim Acta. 1982;46(10):1805–1809.
- Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. BBA Gen Subj. 1985;840(2):163–169.
- Campás M., Marty JL. (2006). Encapsulation of Enzymes Using Polymers and Sol-Gel Techniques. In: Guisan J.M. (eds.), Immobilization of Enzymes and Cells. Methods in Biotechnology™, vol 22. Humana Press. https://doi.org/https://doi.org/10.1007/978-1-59745-053-9_7
- Feng W, Ji P. Enzymes immobilized on carbon nanotubes. Biotechnol Adv. 2011;29(6):889–895.
- Neupane S, Patnode K, Li H, et al. Enhancing enzyme immobilization on carbon nanotubes via metal-organic frameworks for large-substrate biocatalysis. ACS Appl Mater Interf. 2019;11(12):12133–12141.
- Jahadi M, Khosravi-Darani K. Liposomal encapsulation enzymes: from medical applications to kinetic characteristics. Mini Rev Med Chem. 2016;17(4):366–370.
- Colletier JP, Chaize B, Winterhalter M, et al. Protein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002;2(1):9.
- Xiong Y, Liu ZZ, Georgieva R, et al. Nonvasoconstrictive hemoglobin particles as oxygen carriers. ACS Nano. 2013;7(9):7454–7461.
- Li H, Xiong Y, Zhang Y, et al. Photo-decomposable sub-micrometer albumin particles cross-linked by ortho-nitrobenzyl derivatives. Macromol Chem Phys. 2017;218(24):1700413.
- Butler MF, Ng Y, Pudney PDA. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J Polym Sci A Polym Chem. 2003;41(24):3941–3953.
- Shahgholian N, Rajabzadeh G, Malaekeh-Nikouei B. Preparation and evaluation of BSA-based hydrosol nanoparticles cross-linked with genipin for oral administration of poorly water-soluble curcumin. Int J Biol Macromol. 2017;104(Pt A):788–798.
- Yoo JS, Kim YJ, Kim SH, et al. Study on genipin: a new alternative natural crosslinking agent for fixing heterograft tissue. Korean J Thorac Cardiovasc Surg. 2011;44(3):197–207.
- Lomonosova EE, Kirsch M, de Groot H. Calcium vs. iron-mediated processes in hydrogen peroxide toxicity to l929 cells: effects of glucose. Free Radical Biol Med. 1998;25(4–5):493–503.
- Frantzen F, Grimsrud K, Heggli DE, et al. Selective precipitation of human hemoglobin by organic solvents and metal cations. Hemoglobin. 1997;21(2):155–172.
- Lehmann H, Williamson D, Carrell RW, et al. The precipitation of hemoglobin by zinc: Its application to the isolation of a minor hemoglobin fraction (hbβ2 δ16 gly → arg) from lysed whole blood. Hemoglobin. 1982;6(2):183–186.
- Putnam CD, Arvai AS, Bourne Y, et al. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol. 2000;296(1):295–309.