?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Arthritis of joints remains a hard-to-treat disease due to the low drug exposure to the articular cavity. Present study was intended to develop a Tripterine (TRI) and all-trans retinoic acid (ATRA)-loaded lipid-polymer hybrid nanoparticles (ATLP) for enhanced antiarthritic efficacy in arthritis conditions. We have showed that two drugs could be loaded with high loading capacity and control the release kinetics in a pH-responsive manner. The ATLP showed strong inhibitory effects on the expressions of TNF-α, IL-6 and IL-1β in lipopolysaccharide (LPS)-stimulated RAW264.7 cells at the in vitro conditions. Compared to individual drugs (TRI and ATRA), ATLP significantly reduced the paw thickness exhibiting potent inhibition of inflammation. Consistently, ATLP resulted in lowest clinical score compared to that of individual drug indicating the remarkable improvement in the recession of inflammation. We have clearly demonstrated that the nanoparticulate based co-delivery of drugs could abolish the adverse effects of free drug as indicated by the body weight changes. Importantly, ATLP resulted in significant reduction of mRNA of TNF-α, IL-6, IFN-ϒ and IL-17 compared to either free drugs or CIA mice. Overall, ATLP represent a promising therapeutic strategy for the treatment of arthritis conditions.
Introduction
Arthritis of joints, rheumatoid arthritis (RA) is an autoimmune disorder of joints [Citation1]. As of today, disability of RA has substantial effect on the 3.85 million people across the world and expected to affect many million people by the year 2050 [Citation2]. RA is typically characterized by chronic inflammation of synovial joints that leads to the activation and excessive proliferation of synoviocytes, differentiation of osteoclasts and excessive generation of pro-inflammatory cytokines in the synovial joints [Citation3]. This leads to severe cartilage damage, erosion of bone index and eventual loss of joint function [Citation4]. The important causes of pathogenesis of RA includes the dysregulated activation of innate and adaptive immune system in the body that activates the B cells and contribute to the systemic inflammation in the joints [Citation5]. Present treatment option for includes the disease modifying anti-rheumatoid drugs (DMARDS) such as methotrexate, hydroxychloroquine, leflunomide or TNFα inhibitors such as infliximab or IL-6 blocking agent such as tocilizumab [Citation6,Citation7]. Most of these drugs prevents the progression of disease but did not result in the complete halt in the symptoms of RA. Besides, these drugs are associated with severe adverse effects and drug resistance [Citation8]. Present study is an attempt to improve the treatment of RA by employing a new treatment strategy.
Tripterine (TRI) is a phytomedicine extracted from the roots of Tripterygium Wilfordii [Citation9]. TRI has been demonstrated to possess excellent antioxidant, antitumor, anti-inflammatory and immuneregulatory effect [Citation10]. However, poor aqueous solubility (∼15 µg/mL), poor cellular permeability and poor systemic circulation properties hamper its further development and clinical translation [Citation11,Citation12]. Nanomedicine offers significant benefits towards the treatment of several clinical disorders such as cancer by overcoming the limitations of free drugs and by improving the drug pharmacokinetics [Citation13,Citation14]. In this regard, several studies have utilized lipid-based nanoparticles [Citation15,Citation16], polymer nanoparticles [Citation17], silica nanoparticles or albumin nanoparticles [Citation18] to improve the physicochemical properties of TRI in order to improve its therapeutic efficacy. Nevertheless, need to develop a better delivery carrier to enhance the systemic performance of TRI never end. Two major class of materials used for drug delivery includes the liposome and polymers. Recently, lipid-polymer hybrid nanoparticles have been reported as efficient carriers for systemic applications [Citation19]. The lipid-polymer nanoconstruct essentially consists of single or bilayer surrounding the polymer core and thereby combines the unique benefits of polymers as well as lipid nanocarriers [Citation20].
Recently, combination therapy employing the two or more functional therapeutics in a single nanocarrier is reported to be more efficient compared to that of monotherapy. Co-delivery of two agents is expected to possess synergistic effect which is normally not observed with single drug or physical mixture [Citation21]. In this regard, we have employed all-trans retinoic acid (ATRA) as a second functional component along with TRI. The biological function of ATRA is transmitted through the nuclear receptors (RARs and RXRs) and reported to possess both anti-inflammatory effect and pro-inflammatory effect [Citation22,Citation23]. Especially, ATRA could significantly suppress the inflammatory responses and inhibit the autoimmune inflammatory disorders such as RA or inflammatory bowel disease (IBD) [Citation24]. ATRA could enhance the Treg growth and differentiation and inhibit the Th17 functions and thereby regulate the adaptive immune responses [Citation15]. Therefore, we hypothesize that combination of TRI and ATRA could synergistically enhance the anti-inflammatory effect in the treatment of rheumatoid arthritis.
In this study, TRI was loaded in the polymer core while ATRA was loaded as a part of lipid bilayer shell in the lipid-polymer hybrid nanoparticles. Detailed physicochemical characterizations were carried out and in vivo studies have been performed on arthritis animal model and RT-PCR analysis was performed to evaluate the pro-inflammatory markers.
Materials and methods
Materials
Poly (d,l-lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-PEG) was purchased from Daigang Biotechnology C. Ltd, Jinan, China. 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG-2000) and Lecithin was purchased from Avanti Polar Lipids, China. Tripterine and All-Trans Retinoic Acid (ATRA) were purchased from Sigma-Aldrich, China. TRIzol was purchased from Invitrogen, China. PrimeScript RT reagent kit was purchased from Takara, Dalian, China. The Absolute Blue SYBR Green ROX mixes was obtained from Thermo Fisher (Waltham, MA, USA). Primers were designed with Primer Premier 5.0 and synthesized by Bao Biological Engineering Co. Ltd, Dalian, China. Data were analysed by the Applied Biosystems StepOneTM software v2.0. Primers were designed with an online primer design tool of Primer 3, and synthesized by Invitrogen Company (Shanghai, China).
Preparation of ATRA/TRI-loaded lipid-polymer hybrid nanoparticles
The drug-loaded nanoparticles were prepared by double emulsion method. Briefly, PLGA-PEG was dissolved in 2.5 mL of methylene chloride solution containing 7.5 mg of TRI. The organic solution was added to 0.5 mL of water and emulsified by sonication for 5 min. The so-formed emulsion was added to 3 mL of 2% polyvinyl alcohol (PVA) solution and again emulsified by sonication for 5 min (amplitude 60). The emulsion was slowly and gently added to 10 mL of 0.5% PAV and mechanically stirred for 10 min at room temperature. The methylene chloride was evaporated using vacuum evaporator and nanoparticles were collected by centrifugation at 10,000 rpm at room temperature. Followed by, phospholipid mixture consisting of lecithin, DSPE-PEG-2000, and cholesterol in a mass ratio of 60/11/16 and ATRA (10% w/w of lipid) were dissolved in DCM and lipid film was formed using a rotary evaporator [Citation25–27]. To the thin-lipid film, above formed polymer emulsion was added and sonicated using a probe sonicator (Sonics-VCX 130 FSJ, USA) for 8 min (amplitude 60). The resulting lipid-polymer hybrid nanoparticles co-loaded with ATRA/TRI was collected by centrifugation at 10,000 rpm.
Size distribution, zeta potential and morphological characterizations
The particle size and surface charge (zeta potential) were determined using ZetaSizer Nano Series Nano-ZS (Malvern Instruments, UK) equipped with He-Ne Laser beam at a fixed scattering angle of 90°. The morphology was examined by transmission electron microscopy (TEM, JEM-200CX, Japan) after negatively staining the nanoparticles with 2% uranyl acetate solution. The nanoparticles are deposited on carbon-coated copper grid.
High performance liquid chromatography (HPLC) analysis
Waters HPLC system containing an auto sampler, binary pump and UV photodiode array detector (model 996) was used. The drugs were eluted using C18 reverse phase column. The mobile phase for ATRA consisted of acetonitrile: methanol (65:35, v/v) at a flow rate of 1 mL/min and detected at 360 nm. Mobile phase for TRI consisted of methanol:water (90:10, v/v) at a flow rate of 1 mL/min and detected at 426 nm.
Drug loading analysis
The entrapment efficiency and drug loading were evaluated after HPLC analysis. In brief, ATLP was incubated with methanol in 1:1 ratio at −20 °C for 1 h. The mixture was vigorously vortexed and centrifuged at 15,000 rpm for 30 min. The supernatant was used for the HPLC analysis and following equations were used:
In vitro drug release study
In order to mimic different conditions in the body, pH 7.4 (PBS), pH 6.5 (PBS) and pH 5.0 (acetate buffer) buffer systems was prepared to evaluate the pH-responsive drug release rate at in vitro conditions. Briefly, 5 mL of ATLP formulation containing at least 2 mg/mL of TRI and ATRA was packed in a dialysis bag (MW 3500) and immersed in a 25 mL of respective buffer solution at 37 °C. The sample was collected at respective time interval and replaced with equivalent volume and amount of respective drug released was quantified by HPLC method.
Cell culture
Caco-2 cells were obtained from Chinese Academy of Sciences, Shanghai, China. RAW 264.7 cells were purchased from American Type Culture Collection (Rockville, MD, USA). Cells were grown at 37 °C in a humidified atmosphere of 95% air and 5% CO2. The media used were DMEM supplemented with 10% FBS and 1% penicillin/streptomycin antibiotic mixture.
Cellular uptake and cellular internalization
The cellular uptake of ATLP was evaluated in Caco-2 cells using flow cytometer and CLSM. For this purpose, ATLP was loaded with coumarin-6 (10 µg/mL) as a fluorescent alternative to the small molecules. Coumrin-6 was loaded instead of TRI and rest of the formulation process was similar as to that of ATLP [Citation28]. Based on trial and error, we have optimized 10 µg/mL of coumarin-6-loaded ATLP for both the cellular uptake studies. The Caco-2 cells were seeded in 12-well plate and incubated for 24 h. The cells were then treated with ATLP and incubated from 30 min to 3 h and then washed thrice to remove all the surface-bound or floating nanoparticles. The cells were then extracted and centrifuged at 2000 rpm for 4 min. The cells were then analysed through flow cytometer (BD FACS) for a minimum of 10,000 counts. For CLSM, similar procedure was followed and then incubated for 2 h. The cells were washed twice and then fixed with 4% paraformaldehyde and then stained with DAPI for 10 min. The cells were washed and visualized using TCS SP8 microscope (Leica, Germany).
Effect of ATLP on the pro-inflammatory cytokine expression – RT-PCR analysis
The effect of individual drugs and ATLP was determined in RAW264.7 cells. The RAW264.7 cells were first treated with 500 ng/mL of LPS and after 6 h incubation, cells were treated with ATRA (10 µg/mL), TRI (1 µg/mL) and ATLP (equivalent concentrations of ATRA and TRI) and incubated for additional 24 h [Citation29,Citation30]. After 24 h, cells were extracted and total RNA was collected from cells using TRIzol reagent. The RNA was quantified using spectrometer at A260/280 ratio. The extracted RNA (1 µg) was reverse transcribed to cDNA using PrimeScript RT reagent kit. The obtained cDNA was used to quantify the mRNA expression of β-actin, TNF-α, IL-6, IFN-ϒ, and IL-17A. The PCR reaction was performed using Absolute Blue SYBR Green ROX mixes as per the manufacturer’s instruction along with corresponding forward and reverse primers for β-actin, TNF-α, IL-6, IFN-ϒ, and IL-17A. The reaction was carried out using a Veriti Thermal Cycler (Applied Biosystems, USA). Relative quantification (RQ) was obtained using the 2−ΔΔCt method (21) by adjusting the mRNA cytokine expression to the expression of GAPDH mRNA. Data were analysed by the Applied Biosystems StepOneTM software v2.0. The primer sequence information is provided in . Primers were designed with an online primer design tool of Primer 3, and synthesized by Invitrogen Company (Shanghai, China).
Table 1. Primer sequence for RT-PCR analysis.
Table 2. Optimization of different lipid mass ratio.
In vivo arthritis model development and treatment
All the protocols for animal experiment were reviewed and approved by the Institutional Experimental Animal Ethical Committee of Orthopaedic Hospital of Henan Province, Luoyang (No. 20201016-27 A). Briefly, 2 mg/mL of bovine type II collagen was emulsified with 2 mg/mL of complete Freund’s adjuvant (CFA) and administered to mice at 0.05 mg to the base of the tail and 0.1 mg at the ankle joint via intradermal injection. After immunization at day 5, mice were injected with type II collagen/incomplete Freund’s adjuvant by the same administration protocol. The treatment was started 3 days after the second CFA dose (i.e. at day 8). At day 8, treatment was started with a frequency of once every 3 days for 5 cycles of administrations. Day 0 is marked from the onset of treatment and mice were evaluated every 2 days throughout the study period until day 24. The mice were then administered with 10 mg/kg of TRI or 10 mg/kg of ATRA or equivalent concentration of ATLP with a frequency of once every 3 days [Citation31–33]. Mice were monitored every other day to measure degree of inflammation and clinical manifestation of arthritis and sacrificed on day 24. The severity of arthritis was scored on a scale of 0–4. The tissues were collected from hind paw and processed to collect the total RNA and RT-PCR analysis was performed to analyse the expression levels of pro-inflammatory cytokines as described above.
Histopathology analysis
At the end of the experiment, the animals were sacrificed ankle joints were excised and fixed in 4% paraformaldehyde for 48 h. After decalcification, the ankle joints were made into paraffin
sections and stained with Haematoxylin & Eosin (H&E) dye for histopathological check. The inflammatory progression in the joint was evaluated by the high power light microscope (Nikon, Japan).
Result and discussion
Preparation and characterization of ATLP nanoparticles
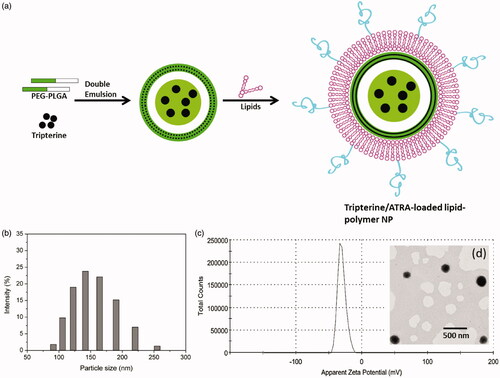
Present treatment option for arthritis includes either DMARDS or TNFα inhibitors or IL-6 blocking agents, however, these drugs prevents the progression of disease and could not inhibit the severity of RA symptoms. In this regard, we have employed a combination of TRI and ATRA to improve the therapeutic efficacy in arthritis conditions. In order to improve the pharmacokinetics profiles of free drugs, nanomedicine approach has been adopted. For example, several studies have highlighted the accumulation of nanoparticles in the leaky vasculature of inflamed joints. In this study, we have fabricated the unique lipid-polymer hybrid nanoparticles by combining the polymer core prepared by double emulsion method and self-assembly based lipid bilayer shell. The internal polymer core was constructed from PLGA-PEG block copolymer. The PLGA and PEG are one of well-characterized and biodegradable biomaterials approved for clinical purpose by Food and Drug Administration (FDA) [Citation34,Citation35]. The hydrophobic TRI could be efficiently loaded in the polymer emulsion at first by water-in-oil (W/O) emulsion and then second emulsion was generated resulting in water-oil-water (W/OW) emulsion (). A lipid mixture was then self-assembled in the polymer emulsion in the presence of ultrasonication and ATRA is loaded in the lipid bilayer shell that could yield temporal release of two components (). The different ratio resulted in different particle size and surface charge that may not be suitable for systemic applications. We have optimized a lipid ratio that has particle size between 100 and 200 nm and surface charge between −25 mV to −30 mV with uniform dispersion of particles and based on these criteria, a lipid mass ratio of lecithin/DSPE-PEG/Chol of 60/11/16 was selected with regard to small particle size, uniform PDI and acceptable surface charge (). The PEGylated lipid will prolong the systemic circulation of payload. The average hydrodynamic diameter of ATLP was 152.5 ± 1.26 nm with a narrow distribution of the particles (PDI-0.142) (). Zeta potential of ATLP was −29.2 ± 1.33 mV mainly attributed to the negatively surface charged lipid bilayer (). The particle size around 150 nm will allow the long circulation in vivo and could potentially avoid the renal clearance. Negatively surface charged nanoparticle would avoid unnecessary aggregation and serum protein absorption [Citation36,Citation37]. The particle morphology investigated through TEM revealed a clear and spherical particle with possible distinction between lipid and polymer core ().
Drug loading
The experimental results showed that ATLP showed an entrapment efficiency of 89.5 ± 1.36% and 82.1 ± 1.65% for ATRA and TRI, respectively. ATLP showed an active drug loading of 9.5 ± 0.95% and 8.4 ± 1.29% for ATRA and TRI, respectively.
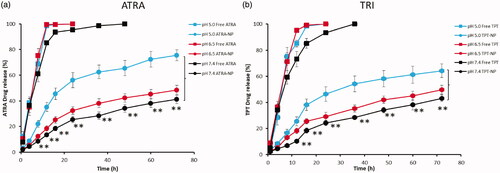
In vitro drug release
The in vitro drug release study was performed by dialysis method (). The pH value in the synovial fluid in arthritis ranges from 6.7 to 7.4 while the pH value in the endosome will be between 4.0 and 6.0, therefore, we have performed the release study is three different pH conditions of pH 7.4, pH 6.5 and pH 5.0 reflecting the physiological, acidic and endosomal conditions. ATRA as well as TRI released in a controlled manner throughout the study period. Especially, significantly lower drug release was observed in pH 7.4 conditions compared to that of pH 5.0 conditions for both ATRA and TRI. For example, ∼75% of ATRA released at the end of 72 h in pH 5.0 conditions compared to ∼40% drug release in pH 7.4 during the same time period. In the similar manner, ∼65% of TRI released after 72 h in pH 5.0 conditions compared to ∼43% in pH 7.4 conditions. Notably, no significant difference was observed between the release of ATRA and TRI in any pH conditions regardless of position of drug loading in the nanoparticles. Lower drug release in the physiological pH conditions will avoid the unnecessary drug release during the blood circulation and would minimize the associated side effects. A higher drug release in lower pH conditions will enhance the drug availability in the synovial fluid in the arthritis. The inflammation-responsive drug delivery systems might release the drug cargo in response to the low pH in inflamed synovial fluid [Citation38]. The local inflammatory reactions in and around joint tissues of patients with arthritis increases the metabolic activity, leading to oxygen insufficiency and inducing a shift towards anaerobic glycolysis and lactate formation [Citation39]. This acidifies the microenvironment; for example, pH values as low as 5.6 have been reported in the synovial tissue of patients with arthritis. These drug-loaded particles were administered systemically and entered inflamed joints from the blood stream [Citation40,Citation41].
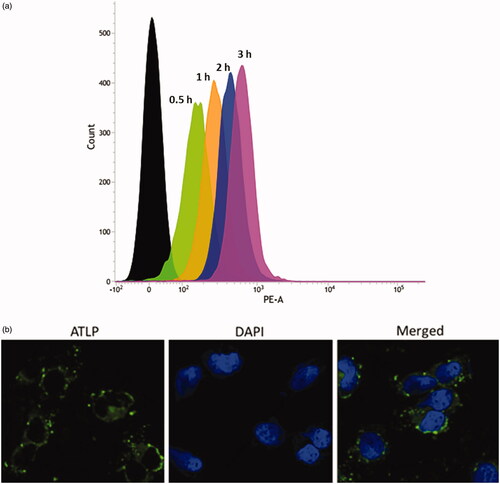
Cellular uptake of ATLP
Flow cytometric analysis of uptake of ATLP in Caco-2 cells is presented in . As shown, typical time-dependent cellular uptake was observed in Caco-2 cells with a maximum uptake observed at 3 h. The excellent cellular uptake of ATLP was due to the natural affinity of phospholipid towards biomembrane of Caco-2 cells. The cellular internalization was further studied by CLSM. Data from flow cytometer showed a linear increase in the fluorescence with the increase in the incubation time (0–3 h). Appreciable cellular uptake was observed after 2–3 h of incubations; therefore, we have incubated the samples for 2 h for CLSM analysis. The nanoparticles were loaded with Coumarin-6 as a fluorescent tracker. As seen (), definite green fluorescence was observed in the cell cytoplasm after 2 h of nanoparticle incubation. No distribution of nanoparticle was observed in the nucleus indicating an endocytosis-mediated cellular uptake of the nanoparticles. Such higher cellular uptake of ATLP may suggest the possibility of high intracellular concentrations of encapsulated payload. Following internalization of nanoparticles, they might be sequestered within the acidic endolysosomes, which might lead to cargo disintegration. In other words, influx of protons and chloride ions along with water molecules (proton sponge effect) in the endosomes might cause the vesicle to swell and expand with the subsequent membrane rupture, leading to release of drugs which will bind to the respective pharmacological targets [Citation42].
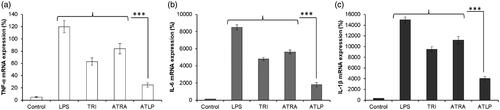
ATLP inhibits the pro-inflammatory cytokine expression in LPS-treated RAW264.7 cells
Anti-inflammatory effect of individual ATRA and TRI and nanoparticles-based ATLP were examined (). To this end, RAW264.7 cells were activated with LPS and then treated with respective formulations. The treatment with LPS significantly increased the expression of pro-inflammatory cytokines (TNF-α, IL-6 and IL-1β). As expected, treatment of TRI and ATRA significantly decreased the expression of all three tested pro-inflammatory cytokines. Both TRI and ATRA showed strong inhibitory effects on the expressions of TNF-α, IL-6 and IL-1β. ATLP exhibited the strongest inhibitory effects than that of free TRI or ATRA implying the synergistic effect of dual components in the lipid-polymer hybrid nanoparticles. It has been reported that TRI controls the inflammation via the regulation of NF-κB, JAK/STAT, and PI3K/AKT pathways [Citation43,Citation44]. Whereas, ATRA has shown anti-inflammatory responses in several autoimmune disorders such as RA. ATRA is oxidized into retinoic acid by retinal dehydrogenase. The biological function of retinoic acid is mediated by nuclear receptors RARs and RXRs. Consistent with this published information, we have observed in our study that ATRA could potentially inhibit the expression of pro-inflammatory cytokines contributing to the anti-arthritic effect [Citation45]. The combination of ATRA with TRI produced a strongest anti-inflammatory effect in the LPS-stimulated RAW264.7 cells. It is possible that co-loading of the two therapeutics in a lipid-polymer hybrid nanoparticles that would allow a controlled and pH-responsive release of drugs could contribute to the synergistic anti-inflammatory effect [Citation46].
In vivo antiarthritic activity of ATLP in mouse model
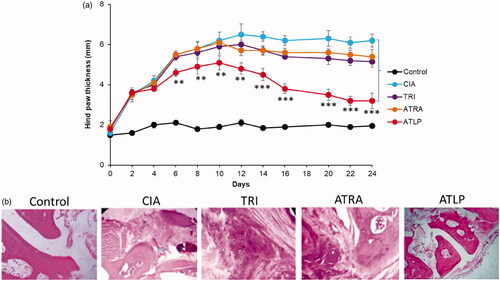
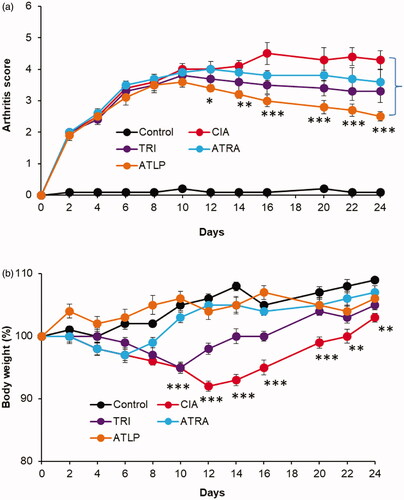
Following the excellent in vitro efficacy of ATLP, we have examined the anti-inflammatory effect of individual drugs and ATLP in mouse model. After 3 days of onset of CIA mice, ATRA, TRI and ATLP were administered. As expected, hind paw thickness continue to increase in the untreated CIA mice and developed serious inflammation (). Mice treated with TRI or ATRA did show some sign of reduction of inflammation, however, no significant decrease in paw thickness was observed compared to CIA mice indicating that free drug may be successful in the in vivo conditions. Part of the reason might be the rapid elimination of free drug from the systemic circulation. Compared to individual drug, ATLP showed the potent inhibition of inflammation [Citation47]. ATLP significantly reduced the paw thickness from day 10 after the onset and nearly 2-fold reduction in paw thickness was observed in CIA mice. Consistent with the paw thickness analysis, ATLP resulted in lowest clinical score compared to that of individual drug indicating the remarkable improvement in the recession of inflammation (). The mice treated with ATLP greatly restored the daily mobility and joints became flexible with greater detumescence compared to individual drug treated mice which still existed the joint swelling and higher arthritic scores. In terms of body weight, non-treated control mice continue to gain wait while CIA mice showed a significant reduction in the body weight possibly due to the spike in the inflammation and reduction in the appetite and food consumption (). Although, TRI was relatively effective in curbing the paw thickness, however, systemic administration of TRI resulted in significant loss of body weight before regaining the wait after 12 days. It has been reported that free drug administration of TRI might cause severe systemic toxicity and remains an obstacle in the successful treatment. In contrast, dual drug-loaded lipid-polymer hybrid nanoparticles (ATLP) did not show any sign of body weight loss indicating the excellent safety feature of nanocarrier based drug delivery in RA [Citation48]. The site-specific delivery of TRI and ATRA with higher anti-inflammatory effect along with no systemic side effects will be an excellent alternative in RA management. The pore sizes of vasculature produced by inflammatory mediators are usually uniform and can be as large as 0.5 μm [Citation49]. Furthermore, transcellular holes of the endothelium may also facilitate the transport of nanoparticles at the inflamed joint tissues. As a result, particles with a diameter between 100 and 300 nm are usually preferred for arthritic tissue penetration [Citation50].
Histopathological analysis
Histopathological studies were performed by H&E. As seen, control mice shows normal bone with no signs of infiltration while arthritic control mice shows marked infiltration of inflammatory cells, erosion of bone tissue and sever connective tissue proliferation was observed. As expected, ATLP treated mice groups had reduced pathological features including cartilage destruction, bone erosion and minimal connective tissue proliferation indicating the potential of combinational drug-loaded nanoparticle system.
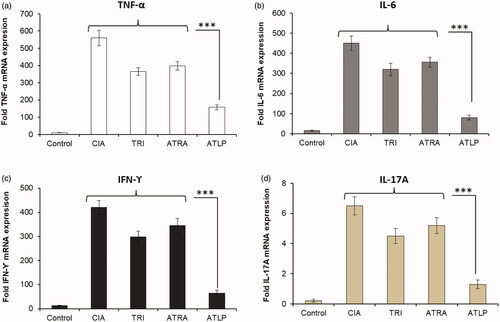
Effect of ATLP on the expression of pro-inflammatory cytokines in CIA mice
It is well-known that activated macrophages will increase the production and accumulation of pro-inflammatory cytokines (TNF-α and IL-6) in the synovial joints in the RA conditions [Citation51]. Similarly, higher levels of IFN-ϒ and IL-17 are reported in the synovial fluids of RA subjects contributing to the severity of disease progression [Citation52]. The paw tissue was collected from all the experiment animals and subjected to RT-PCR analysis (). As seen, expression levels of TNF-α, IL-6, IFN-ϒ and IL-17 were markedly elevated in CIA mice whereas free drugs, TRI and ATRA significantly decreased the expression levels of these markers exhibiting a moderate inhibitory effect. Importantly, ATLP resulted in significant reduction of mRNA of TNF-α, IL-6, IFN-ϒ and IL-17 compared to either free drugs or CIA mice. The synergistic suppression of inflammatory responses by TRI and ATRA resulted in the superior anti-inflammatory effect. The generation of cytokines such as TNF-α and IL-6 regulated by transcription factor nuclear factor κB (NF-κB) which is a pivotal event leading to chronic inflammation in arthritis [Citation53]. Pro-inflammatory cytokines have been reported as potential therapeutic targets for arthritis, as these cytokines stimulate inflammatory responses in arthritic joints and synovial tissues [Citation54]. At the same time, systemic inhibition of these cytokines with therapeutic might also suppress the whole immune system and increase infection risk. In this regard, nanoparticle-based drugs could potentially accumulate in the inflammatory region and release the encapsulated drugs [Citation55,Citation56]. The results of the present study demonstrated that ATLP decreased TNF-α, IL-6, IFN-ϒ and IL-17 in the serum of experimental animals. It is of clinical significance to reduce the inflammatory markers and relieve the inflammatory reactions. Present design of ATLP showed remarkable inhibition of several inflammatory mediators and has potential to arthritis conditions and other inflammatory disorders [Citation57,Citation58].
Conclusion
In conclusion, we have successfully synthesized a TRI and ATRA-loaded lipid-polymer hybrid nanoparticles for enhanced antiarthritic efficacy in arthritis conditions. We have showed that two drugs could be loaded with high loading capacity and control the release kinetics in a pH-responsive manner. ATLP showed strong inhibitory effects on the expressions of TNF-α, IL-6 and IL-1β in LPS-stimulated RAW264.7 cells at the in vitro conditions. Compared to individual drugs (TRI and ATRA), ATLP significantly reduced the paw thickness exhibiting potent inhibition of inflammation. Consistently, ATLP resulted in lowest clinical score compared to that of individual drug indicating the remarkable improvement in the recession of inflammation. We have clearly demonstrated that the nanoparticulate based co-delivery of drugs could abolish the adverse effects of free drug as indicated by the body weight changes. Importantly, ATLP resulted in significant reduction of mRNA of TNF-α, IL-6, IFN-ϒ and IL-17 compared to either free drugs or CIA mice. Overall, ATLP represent a promising therapeutic strategy for the treatment of arthritis conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Carvalho CS, Andrade LEC, Keusseyan SP, et al. Study of advanced rheumatoid arthritis rev. Bras Eng Biomed. 2014;30:54–63.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361.
- O’dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350:2591–2602.
- Onuora S. Rheumatoid arthritis: vagus nerve stimulation reduces RA severity in patients. Nat Rev Rheumatol. 2016;12(9):500–500.
- Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289.
- Pandey S, Rai N, Rawat P, et al. Nanofacilitated synergistic treatment for rheumatoid arthritis: a ‘three-pronged’ approach. Med Hypotheses. 2016;92:44–47.
- Arora S, Rafiq A, Jolly M. Management of rheumatoid arthritis: review of current guidelines. JAJS. 2016;3(2):45–50.
- Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874.
- Ng SW, Chan Y, Chellappan DK, et al. Molecular modulators of celastrol as the keystones for its diverse pharmacological activities. Biomed Pharmacother. 2019;109:1785–1792.
- Dai W, Wang X, Teng H, et al. Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int Immunopharmacol. 2019;66:215–223.
- Qi X, Qin J, Ma N, et al. Solid self-microemulsifying dispersible tablets of celastrol: formulation development, charaterization and bioavailability evaluation. Int J Pharm. 2014;472(1–2):40–47.
- Song J, Shi F, Zhang Z, et al. Formulation and evaluation of celastrol-loaded liposomes. Molecules. 2011;16(9):7880–7892.
- Freag MS, Saleh WM, Abdallah OY. Self-assembled phospholipid-based phytosomal nanocarriers as promising platforms for improving oral bioavailability of the anticancer celastrol. Int J Pharm. 2018;535(1–2):18–26.
- Li W, Zhang T, Ye Y, et al. Enhanced bioavailability of tripterine through lipid nanoparticles using broccoli-derived lipids as a carrier material. Int J Pharm. 2015;495(2):948–955.
- Cao X, Hu Y, Luo S, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9(3):575–589.
- Choi JY, Gupta B, Ramasamy T, et al. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf B Biointerfaces. 2018;165:56–66.
- Guo L, Luo S, Du Z, et al. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat Commun. 2017;8(1):878.
- Zhao Y, Tan Y, Meng T, et al. Simultaneous targeting therapy for lung metastasis and breast tumor by blocking the NF-κB signaling pathway using Celastrol-loaded micelles . Drug Deliv. 2018;25(1):341–352.
- Ramasamy T, Ruttala HB, Gupta B, et al. Smart chemistry-based nanosized drug delivery systems for systemic applications: a comprehensive review. J Control Release. 2017;258:226–253.
- Ramasamy T, Munusamy S, Ruttala HB, et al. Smart nanocarriers for the delivery of nucleic acid-based therapeutics: a comprehensive review. Biotechnol J. 2021;16(2):e1900408.
- Ruttala HB, Ramasamy T, Poudel BK, et al. Multi-responsive albumin-lonidamine conjugated hybridized gold nanoparticle as a combined photothermal-chemotherapy for synergistic tumor ablation. Acta Biomater. 2020;101:531–543.
- Kwok SK, Park MK, Cho ML, et al. Retinoic acid attenuates rheumatoid inflammation in mice. J Immunol. 2012;189(2):1062–1071.
- Racke MK, Burnett D, Pak SH, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J Immunol. 1995;154(1):450–458.
- Hong K, Zhang Y, Guo Y, et al. All-trans retinoic acid attenuates experimental colitis through inhibition of NF-κB signaling. Immunol Lett. 2014;162(1 Pt A):34–40.
- Li F, Zhao X, Wang H, et al. Multiple layer-by-Layer Lipid-Polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv Funct Mater. 2015;25(5):788–798.
- Mukherjee A, Waters AK, Kalyan P, et al. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: state of the art, emerging technologies, and perspectives. Int J Nanomedicine. 2019;14:1937–1952.
- Zhang W, Wang Z, Wu C, et al. The effect of DSPE-PEG2000, cholesterol and drug incorporated in bilayer on the formation of discoidal micelles. Eur J Pharm Sci. 2018;125:74–85.
- Liu Y, Zhou C, Wei S, et al. Paclitaxel delivered by CD44 receptor-targeting and endosomal pH sensitive dual functionalized hyaluronic acid micelles for multidrug resistance reversion. Colloids Surf B Biointerfaces. 2018;170:330–340.
- Chen Y, Qu D, Fu R, et al. A Tf-modified tripterine-loaded coix seed oil microemulsion enhances anti-cervical cancer treatment. Int J Nanomedicine. 2018;13:7275–7287.
- Payne CM, Burke LP, Cavanagh B, et al. Evaluation of the immunomodulatory effects of All-Trans retinoic acid solid lipid nanoparticles and human mesenchymal stem cells in an A549 epithelial cell line model. Pharm Res. 2019;36(4):50.
- Du Y, Song Y, Zhang L, et al. Combined treatment with low dose prednisone and escin improves the anti-arthritic effect in experimental arthritis. Int Immunopharmacol. 2016;31:257–265.
- Zhao J, Luo D, Zhang Z, et al. Celastrol-loaded PEG-PCL nanomicelles ameliorate inflammation, lipid accumulation, insulin resistance and gastrointestinal injury in diet-induced obese mice. J Control Release. 2019;310:188–197.
- Li P, Yang X, Yang Y, et al. Synergistic effect of all-trans-retinal and triptolide encapsulated in an inflammation-targeted nanoparticle on collagen-induced arthritis in mice. J Control Release. 2020;319:87–103.
- Pino-Lagos K, Guo Y, Noelle RJ. Retinoic acid: a key player in immunity. Biofactors. 2010;36(6):430–436.
- Bazile D, Prud’homme C, Bassoullet MT, et al. Stealth me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995;84(4):493–498.
- Karmali PP, Simberg D. Interactions of nanoparticles with plasma proteins: implication on clearance and toxicity of drug delivery systems. Expert Opin Drug Deliv. 2011;8(3):343–357.
- Guerrini L, Alvarez-Puebla RA, Pazos-Perez N. Surface modifications of nanoparticles for stability in biological fluids. Materials. 2018;11:1154.
- Li C, Li H, Wang Q, et al. pH-sensitive polymeric micelles for targeted delivery to inflamed joints. J Control Release. 2017;246:133–141.
- Wang D, Miller SC, Liu X, et al. Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res. Ther. 2007;9:1–9.
- Andersson SE, Lexmüller K, Johansson A, et al. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J Rheumatol. 1999;26(9):2018–2024.
- Goetzi EJ, Rynes RI, Stillman JS. Abnormalities of respiratory gases in synovial fluid of patients with juvenile rheumatoid arthritis. Arthritis Rheum. 1974;17(4):450–454.
- Ruttala HB, Ramasamy T, Ruttala RRT, et al. Mitochondria-targeting multi-metallic ZnCuO nanoparticles and IR780 for efficient photodynamic and photothermal cancer treatments. Journal of Materials Science & Technology. 2021;86:139–150.
- Choi JK, Oh H-M, Park JH, et al. Salvia plebeia extract inhibits the inflammatory response in human rheumatoid synovial fibroblasts and a murine model of arthritis. Phytomedicine. 2015;22(3):415–422.
- Xue M, Jiang ZZ, Wu T, et al. Anti-inflammatory effects and hepatotoxicity of tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine. 2012;19(11):998–1006.
- Kim JL, Kang SW, Kang MK, et al. Osteoblastogenesis and osteoprotection enhanced by flavonolignan silibinin in osteoblasts and osteoclasts. J Cell Biochem. 2012;113(1):247–259.
- Ha YJ, Lee SM, Mun CH, et al. Methotrexate-loaded multifunctional nanoparticles with near-infrared irradiation for the treatment of rheumatoid arthritis. Arthritis Res Ther. 2020;22(1):146.
- Jokerst JV, Lobovkina T, Zare RN, et al. Nanoparticle PEGylation for imaging and therapy. Nanomedicine. 2011;6(4):715–728.
- Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–1380.
- Baluk P, Hirata A, Thurston G, et al. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am J Physiol. 1997;272(1 Pt 1):L155–L170.
- Decuzzi P, Pasqualini R, Arap W, et al. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26(1):235–243.
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344(12):907–916.
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103(9):1345–1352.
- Gupta G, Dahiya R, Singh M, et al. Role of liraglutide in a major complication of diabetes: a critical review of clinical studies. Bull Pharm Res. 2018;8(1):155–164.44.
- Gupta G, Dua K, Kazmi I, et al. Anticonvulsant activity of Morusin isolated from Morus Alba: Modulation of GABA receptor. Biomed Aging Pathol. 2014;4(1):29–32.
- Li Q, Verma IM. NF-kappaB regulation in the immune system . Nat Rev Immunol. 2002;2(10):725–734.
- Salama AH, Abdelkhalek AA, Elkasabgy NA. Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int J Pharm. 2020;578:119081.
- Hsiao HB, Hsieh CC, Wu JB, et al. Kinsenoside inhibits the inflammatory mediator release in a type-II collagen induced arthritis mouse model by regulating the T cells responses. BMC Complement Altern Med. 2016;16:80.
- Shi Q, Cao J, Fang L, et al. Geniposide suppresses LPS-induced nitric oxide, PGE2 and inflammatory cytokine by downregulating NF-κB, MAPK and AP-1 signaling pathways in macrophages. Int Immunopharmacol. 2014;20(2):298–306.