?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chitosan/gelatine-based materials have been widely used as biocompatible scaffolds in the tissue engineering field. Chitosan suppresses the inflammatory activities of macrophages whereas gelatine induces inflammatory cytokine production by these cells. Cryogel form of the scaffolds created an effect that was mostly dominated by chitosan activity. Since independent of chitosan to gelatine ratio, the cryogels eliminated the inflammatory cytokine production by the activated macrophages. This will enable suppression of inflammatory reactions by macrophages during implant procedure while enabling a nest of the matrix for the macrophages to reside. Determining the immunomodulatory effect of these materials during the decay is crucial to assess their biocompatibility and safety. Our results suggest that when the chitosan ratio was higher than that of gelatine the materials had anti-inflammatory activity in their powder forms based on TNFα production levels by LPS activated macrophages, whereas higher gelatine to chitosan ratio eliminated this effect. To our knowledge, this is the first study to assess the powder vs. gel forms of the chitosan/gelatine-based materials for their immunomodulatory potentials as well as how the ratio of chitosan to gelatine might affect these materials immunomodulatory effects on the activated macrophages.
Chitosan/gelatin composite cryogels have anti-inflammatory activities.
Different ratios of chitosan to gelatin content altered the immunomodulatory activities.
They can be safely and effectively used as implant materials for tissue engineering applications.
They will also reduce the use of anti-inflammatory drugs during implantation.
HIGHLIGHTS
Introduction
Scaffolds are biomaterials produced with an engineering approach that functions to create and maintain a space for tissue growth, provide mechanical stability, support cell adhesion, migration, and differentiation, and are at the heart of the tissue engineering strategy [Citation1]. These materials should have physical and chemical properties similar to the extracellular matrix of natural tissue to prevent toxicity and enable biocompatibility. It is a great advantage that natural polymers can be used to achieve this unique property.
Natural materials, such as chitosan, gelatine, alginate, collagen, pectin, and fibrin are biocompatible in nature, support cell adhesion, degrade to allow new tissue formation, and their degradation products are non-toxic. To date, we have designed many series of composite cryogel scaffolds based on chitosan for potential tissue engineering applications [Citation2–4]. Chitosan is a cationic polysaccharide, which is derived from chitin, used in regenerative medicine due to its biocompatibility, biodegradation, antimicrobial, antioxidant, and immunogenic properties [Citation5]. However, due to disadvantages, such as uncontrolled degradation, cell binding ability, and insufficient mechanical properties, the use of chitosan alone still cannot meet tissue engineering requirements. To overcome these problems and minimize the disadvantages of single-component chitosan materials, two or more biopolymers have been combined to develop hybrid biocomposites with superior properties. One of the strategies was based on loading the chitosan materials with other biocompatible and functional biomaterials, such as curcumin. Curcumin loading of chitosan film leads to better wound healing activities compared to unloaded ones [Citation5]. In another example, chitosan loaded with liposomes of Vitamin E exerted high efficacies as myocardial repair materials. In one study reported by Kumar et al., the gelatine was added to chitosan scaffolds produced by the freeze-drying method to eliminate the deficiencies caused by chitosan [Citation5]. In addition, it has been reported earlier in another study that the combination of chitosan and gelatine effectively promotes cell differentiation and proliferation [Citation6,Citation7]. In differentiation studies mostly the cells with osteogenic backgrounds are utilized in the presence of chitosan/gelatine-based materials. In those studies, a cell line with an osteogenic background (such as rat bone marrow stromal cells, osteogenic progenitor cells, etc.) is utilized in flow perfusion bioreactors to enable nutrients, oxygen, and mechanic stimuli for cell differentiation. Then the formation of bone extracellular matrix is followed to determine the level of differentiation [Citation6,Citation7].
Besides all these, the immunomodulatory roles of chitosan-based scaffolds prepared with different proportions of gelatine have not been demonstrated before [Citation8]. Since the immune response is one of the most important problems encountered, especially in implanting biomaterial scaffolds, it is important to decipher these materials' effects on the immune cells. Medical devices and tissue-engineered constructs may induce an inflammatory reaction, termed foreign body reaction (FBR), after their in vivo implantation. Moreover, some of the materials have a slow decay process which can also alter the function of the immune cells. In most cases, steroids or non-steroid-based anti-inflammatory drugs are given to patients who have gone through implant procedures. Therefore, having biocompatible materials that can suppress the inflammatory reactions might become advantageous in this sense and may prevent excessive usage of anti-inflammatory drug molecules [Citation9–12].
In this study, we demonstrated mean pore size, porosity, mechanical properties, and degradation behaviour of chitosan:gelatine cryogels prepared at different chitosan and gelatine ratio, and then investigated the immunomodulatory properties of the secryogels both in gel and powder forms. Gel form represents the material in its final form to be used or implanted whereas powder form was used to determine the immunomodulatory activities during decay (although would occur in small scale and slow rate). Mammalian macrophages were seeded either on different cryogels or incubated with the powder form of these different cryogel formulations. Macrophages are well-known for their inflammatory potentials and primary roles during implant rejection [Citation13]. Pro-inflammatory cytokines TNFα and IL6 were analyzed and their production levels were compared to only LPS stimulant activated groups as positive controls. TNFα cytokine is well-known for its role in implant failures and rejections [Citation14]. IL6 is another pro-inflammatory cytokine that has a major role in implant and organ rejection reactions [Citation15–17].
Our results suggest that these cryogels have anti-inflammatory activities and different ratios of chitosan to gelatine content altered the immunomodulatory activities of the cryogels. These materials can be safely and effectively used as implant materials that can suppress the inflammatory activities of the macrophages so that the implant would not be rejected and would decrease the usage of anti-inflammatory drugs during the procedures.
Materials and methods
Materials
Chitosan with low molecular weight (75–85% deacetylated, Catalog number: 448869) and ethanol (absolute, Catalog number: 1.00983.2511) were purchased from Sigma Aldrich, USA. Gelatine (Microbiology, 1.04070.0500), glutaraldehyde (25%, Catalog number: 354400), and glacial acetic acid (100%, Catalog number: 1.00063.2511) were received from Merck, Germany. Aqueous solutions and dilutions in experiments were prepared with distilled water.
Fabrication of chitosan-based cryogels
Plain chitosan cryogels were prepared by dissolving the calculated amount of chitosan (2%, w/v) in 10 ml of 6% acetic acid solution. 2.5 ml of 6% (v/v) glutaraldehyde solution was added to the obtained homogeneous mixture and the solution was rapidly poured into the 2.5 ml of plastic syringes and incubated at −16 °C for 2 h. At the end of the incubation time, all samples were stored for 24 h in the freezer at −16 °C. After 24 h, cryogels were formed and removed from the syringes safely which were immersed in the distilled water to remove the unreacted ingredients including polymer and crosslinking agent residues. The several times washed samples were then freeze-dried. The dried specimens were prepared in two different ways for later in vitro analysis: cylindrical samples with a height of 3 mm to fit into each well of 24 well and 96 well plate, and powder form prepared by grinding in a mortar. The prepared samples were kept in the refrigerator at +4 °C.
For the fabrication of chitosan:gelatine composite cryogels, gelatine was added at different ratios to the prepared chitosan solutions in acetic acid. Composite cryogels were prepared as described above after the mixture of chitosan:gelatine polymer solution was prepared in the proportions presented in .
Table 1. The sample names and polymer ratios between the synthesized cryogel scaffolds.
Morphological observations and pore size measurement
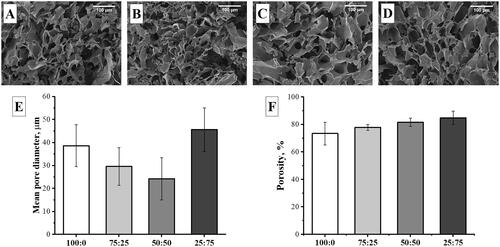
The morphology of the fabricated cryogel scaffolds was determined by scanning electron microscope (SEM, Supra 55, Zeiss, Germany) with an operating voltage of 5 kV. The dried cryogels were cut to expose their inner structure and coated with platinum using a sputter coater before SEM examinations. The pore size was quantified with Image-J software. The mean pore diameter of each sample was calculated by averaging at least 30 pore diameters measured from SEM images taken at low magnifications.
Porosity
Porosity was calculated by dividing the volume of the cryogel (Vc) by the pore volume (Vp) using the ethanol infiltration method. While the cryogel volume was calculated using the height and diameter of the sample, the pore volume was determined as follows: The dry cryogel sample was weighed (Wi) and then incubated in absolute ethanol (with density at room temperature, ρe = 0.805 mg/mL) for at least 15 min. After that time, the excess ethanol on the surface of the sample was removed by a filter paper and weighed immediately (Wf). Vp was defined according to the following equation:
(1)
(1)
Finally, the porosity of the cryogels was determined as follows:
(2)
(2)
Mechanical properties
The mechanical stability of cryogels was determined using a texture analyzer (TA.XT. Plus Texture Analyzer, Stable Micro Systems, Surrey, UK). The measurement was carried out at room temperature in a dry state according to the method used in previous studies [Citation3,Citation18,Citation19]. Before measurements, the cryogels with a diameter of 0.9 cm were cut transversely at a height of 3 cm. The tests were performed at a compression speed of 0.1 mm/s and compression force of a 5 kg load cell. The samples were compressed 2 mm in a longitudinal direction after having reached a trigger load of 1 g. Mechanical properties were evaluated with calculated compressive strength data.
In vitro degradation
The in vitro degradation behaviour was studied based on the weight loss of cryogels after immersion in distilled water for 28 days. For this, firstly, the completely dried gels were weighed (Wo). Afterwards, the samples were placed in plastic tubes filled with 10 ml of distilled water, and the tubes were placed in a water bath (Daihan, WiseBath WB-22, Korea) at 37 °C. The samples were withdrawn at 14 and 28 days, dried using lyophilization and weighed (Wt). Using the data obtained, the degradation rate of cryogels was calculated with the following equation:
(3)
(3)
In vitro experimental studies
Murine macrophage cell line RAW 264.7 was previously purchased from ATCC. The cell growth was achieved in 1640 RPMI media containing 10% FBS and 1% Pen/Strep antibiotics. An incubator with 37 °C 5% CO2conditions was utilized to grow the cells and conduct the experiments. 24 well plates containing 1 × 106 cells/mL concentration per well were used for the stimulations. Negative and positive groups lacked any chemicals. 1 µg/mL of the final concentration of LPS (Isolated from E.coli and purchased from ENZO) was used for the positive control group and also to stimulate the cells with cryogels or powder form of the cryogels. Ten and 100 µg/mL of powder form of the cryogels were used to treat the cells. In a separate set of experiments gel form of each cryogel type was used to cover the bottom of each well of 24 well plates. 1 × 106 cells/mL was added to each well-covered with cryogel and the cells were rested on these cryogels overnight. Later on, the cells were activated by 1 µg/mL of LPS for 24 h. After the incubation period, the media were collected for TNFα and IL6 ELISAs (BD kits) by following the procedures described in our previous publications [Citation20].
The cells were further analyzed by SEM (SEM, Supra 55, Zeiss, Germany). Before SEM analysis, cell-seeded scaffold samples were fixed with glutaraldehyde solution (2.5%, v/v) for 2 h at 4 °C in a refrigerator. Then, the glutaraldehyde solution was removed and samples were washed with sterile PBS. Later on, PBS solution was eliminated and dehydration procedure was done with a series of alcohol solutions for 10 min (50, 70, 90, and 100%, v/v). Afterward, they were dried in a desiccator at room temperature. Finally, they were covered with platinum and analyzed with SEM with the following conditions: an operating voltage of 5 kV at different magnifications. All of these experimental set ups were repeated three times (N = 3) and GraphPad Prism version 5 program was used to run one way ANOVA test on the data [Citation21].
Results and discussion
Synthesis of chitosan:gelatine composite cryogels
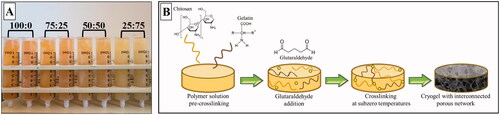
The composite cryogels were fabricated at different polymer ratios (100:0, 75:25, 50:50, and 25:75, chitosan:gelatine, w:w) using cryotropic gelation method (). In the cryogelation process, a crosslinked polymer network forms around the ice crystals while gelation occurs under semi-frozen conditions. Subsequent thawing of ice crystals formed at sub-zero temperatures leaves behind a polymeric network with an interconnected and macroporous structure surrounded by a highly dense polymer wall and this material is called cryogels [Citation22]. The crosslinking mechanism that occurred between chitosan:gelatine and glutaraldehyde at subzero temperature was summarized in .
Figure 1. (A) The image of composite cryogels prepared at different polymer ratios. (B) The schematic representation of the cryogelation process steps occurred between chitosan and gelatine in the presence of glutaraldehyde.
As a result of cryogelation method, cryogels were successfully fabricated with an interconnected open mac porous structure as seen in SEM images given in . The mean pore diameter calculated by using SEM images was 38.60 ± 9.11, 29.60 ± 8.15, 24.23 ± 9.20, and 45.63 ± 9.49 μm for 100:0, 75:25, 50:50, and 25:75 cryogels, respectively (). According to the porosity percentage results, the porosity increased from ∼85 to ∼75%, with increasing gelatine content as shown in .
Mechanical stability and degradation behaviour of cryogels
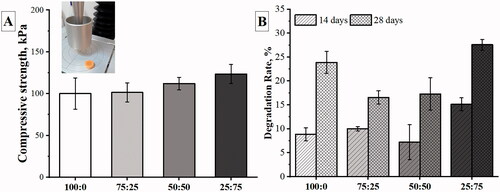
The mechanical performance of cryogels was investigated on the behaviour of dry samples under compression. According to results obtained in terms of compressive strength as presented in , the cryogels showed increased mechanical stability with the increase in gelatine ratio. The mean compressive strength value of samples was measured between 100.15 and 123.56 kPa. Increasing compression strength from 100.15 ± 18.75 to 123.56 ± 11.32 kPa resulted in a flexible but also stronger biomaterial. Therefore, mechanically compatible implantation sites for the chitosan and gelatine-based cryogel scaffolds produced in this study can be identified as rigid but not fragile tissues, as also described for nanocellulose/bioactive glass cryogels by Ferreira et al. [Citation23].
The degradation rate of the cryogels is demonstrated in . At the end of 28 days, about a quarter by weight of the gels degraded. For the samples with a higher ratio of gelatine, the mean pore diameter and porosity of gels increased and more water was absorbed by the gels, which resulted in a faster degradation rate. In addition, the faster degradation of the sample with a high amount of gelatine can be explained by the degradation of the gelatine by hydrolysis. While N-acetylglucosamine groups of the chitosan chains degrade as a result of enzymatic degradation in the presence of lysozyme, gelatine is easily hydrolyzed in the presence of water [Citation24].
Interaction of cryogel scaffolds with macrophages
Although chitosan and gelatine as natural polymers have been previously used for scaffold preparations and it demonstrated being biocompatible, we wondered if our structures would exert any cytotoxic effects on RAW 264.7 macrophage cultures and how the macrophage would react at the cryogel surface. Therefore, the macrophage cells were cultured on cryogels prepared at different polymer ratio combinations for 24 h. The SEM images given in show that these cells adhere well on all cryogel scaffolds with heterogeneous populations and preserve their round morphologies described in a previous study reported by Arbez and Libouban [Citation25]. In plain chitosan (100:0) cryogels, most cells appear to retain their round shape, while some cells appear to be flattened (). Although the cell incubation time was 24 h, as the amount of gelatine increased, the RAW cells spread elongated filopodia to be fixed to the polymeric walls. This behaviour means that the cells show a tight anchoring capacity to the scaffold [Citation25].
Figure 4. SEM images of RAW 264.7 cells cultured on macroporous cryogel scaffold surfaces for 24 h (cells are highlighted by yellow arrows): (A) 100:0, (B) 75:25, (C) 50:50, and (D) 25:75.The samples were prepared after 24 h incubation of macrophages with a gel form of # 1, 2, 3, and 4 () covering the bottom of each well. The cells were rested on the gel sheets overnight before stimulations. Cell concentration was 1 × 106 cells/mL in a final volume of 1 ml. The cells were incubated without 1 µg/mL LPS for 24 h.
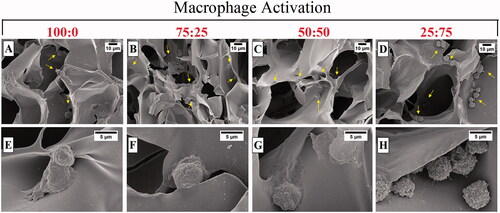
Similar images were detected in LPS-induced macrophage cells (). The cells often exhibit rounded morphology, as well as clustering and linked together by fibres (). RAW 264.7 cells seeded on 25:75 cryogel sample () showed a more elongated spindle shape compared to the cells seeded on groups containing high amounts of chitosan ().The shape difference of cells seeded on the different ratios of chitosan:gelatine composite revealed that the polymer type could change the morphology of anchoring and possibly the function of the cells. Both chitosan and gelatine are known well as materials that macrophages can interact with [Citation26,Citation27].
Figure 5. SEM images of LPS induced RAW 264.7 cells cultured on macroporous cryogel scaffold surfaces for 24 h (cells are highlighted by yellow arrows): (A–E) 100:0, (B–F) 75:25, (C–G) 50:50, and (D–H) 25:75. The samples were prepared after 24 h incubation of macrophages with a gel form of # 1, 2, 3, and 4 () covering the bottom of each well. The cells were rested on the gel sheets overnight before stimulations. Cell concentration was 1 × 106 cells/mL in a final volume of 1 ml. The cells were incubated with 1 µg/mL LPS for 24 h.
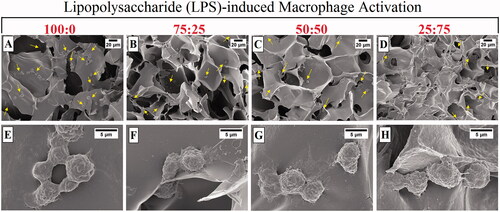
Previous studies suggest that gelatine leads to the aggregation of macrophages [Citation28]. Our results are in line with those studies, since when the gelatine content of cryogels has increased the cells clustered more together whereas in cryogels with less gelatine content the cells were more dispersed within the material ().
Higher chitosan ratio in the cryogels led to a stronger anti-inflammatory activity
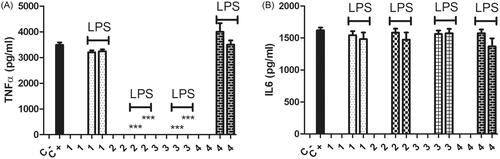
Previous studies suggest that chitosan can alter the activities of macrophages [Citation26,Citation29]. We tested the powder form of the cryogels on macrophages to determine their possible immunomodulatory activities as materials themselves independent of their structure or shape as well as to determine their effects in the patients in the future during their decay. Since the degradation of the implant materials should also be assessed to determine their biosafety [Citation30,Citation31]. The powder form of the cryogel materials did not stimulate the macrophages to produce either TNFα or IL6 in the absence of LPS. This suggests that these materials would not activate macrophages during their degradation or decay and they will be biocompatible even during their decay. When they were tested for their immunomodulatory activities in the presence of LPS as a stimulant, they acted as anti-inflammatory molecules based on TNFα production levels. Increasing the concentration of gelatine eliminated this effect (). None of the cryogel types had any effect on IL6 production in their powder forms (). Our results imply that cryogel with the highest gelatine content (4) was inert in terms of its immunomodulatory activities. Therefore, it (4) might be the safest in terms of immunotoxicity during the degradation or decay process. Depending on the chitosan to gelatine ratio the powder forms of the cryogels had differential immunomodulatory activities on mammalian macrophages. Chitosan is known for its chemotactic and NO stimulatory roles on macrophages [Citation29]. Studies also suggest that oligo chitosan has anti-inflammatory activities on LPS stimulated macrophages [Citation32], our results are in line with these observations (). Gelatine seems to have pro-inflammatory properties [Citation28], in our study having different ratios of chitosan to gelatine altered their effects on the inflammatory behaviours of LPS induced macrophages. In line with these observations, cryogels with a higher chitosan ratio had anti-inflammatory activities whereas increasing the gelatine content evened out this effect and made the material more biocompatible and immunologically inert.
Figure 6. TNFα (A) and IL6 (B) levels were measured after 24 h incubation of macrophages with 10 and 100 µg/mL powder form of materials # 1, 2, 3, and 4 (). Cell concentration was 1 × 106 cells/mL in a final volume of 1 ml. The cells were incubated with or without 1 µg/mL LPS as represented on the graphs. Control negative wells did not have any chemical or stimulant. Control positive wells had only LPS as a stimulant and did not have any of the chemicals. p < .0001 N = 3, one way ANOVA test was applied to calculate the statistical significance.
Cryogels exerted anti-inflammatory effects on the macrophages
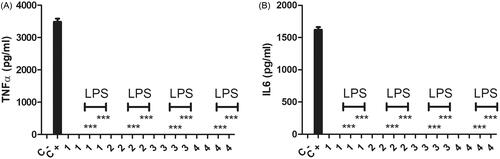
Chitosan:gelatine cryogels were previously shown to enable a proliferation and attachment haven for the macrophages [Citation33]. Chitosan and oligo chitosan are known for inducing nitric oxide (NO) and chemokine production by macrophages [Citation33–35]. Chitosan is known for its anti-inflammatory activities and gelatine is mostly known for its pro-inflammatory activities [Citation28,Citation29]. In this study, we aimed to decipher these cryogels ability to change the inflammatory TNFα and IL6 cytokine production capacities of macrophages in the presence or absence of LPS. These materials lacked immunostimulatory activities since in the absence of LPS they did not stimulate the macrophages to produce TNFα or IL6 cytokines (). When their immunomodulatory activities were tested in the presence of LPS, compared to positive control groups macrophages seeded on cryogels 1–4 completely lost their inflammatory cytokines TNFα and IL6 production capacities independent of cryogel content (). These cryogels exerted a different activity in gel form compared to their powder form (). They did not affect the pro-inflammatory cytokine IL6 production in their powder form whereas when gel forms were utilized the inflammatory activity of macropahges’ was completely knocked out (). The situation was different for TNFαproduction, materials 2 and 3 had similar anti-TNFα production effects on the activated macrophages whether they were in their powder or gel form (). Whereas, materials 1 and 4 did not alter TNFα production in their powder form while knocking out TNFΑ secretion by activated macrophages in their gel forms. This might suggest that when the cells attached to the gel form of the materials they lost their ability to generate inflammatory responses. It implies that they would behave differentially when they are intact as an implant material compared to their decaying conditions. Moreover, during the implant procedure, they enable a nest for the macrophages to anchor and stay inert in terms of their inflammatory TNFα and IL6 cytokine production. TNFα and IL6 are well-known for their role in implant rejections [Citation14–17]. No matter what ratio of chitosan to gelatine was present in the sheet or gel form of the cryogels they were able to cage the macrophages and suppress inflammatory activities of these cells (). This will prevent the usage of anti-inflammatory drugs on patients who goes through implant procedures. In terms of the fine balance of anti-inflammatory activities to prevent implant rejection vs. immunotoxicity during degradation or decay of the material cryogel 4 with the highest gelatine content would be more proper to use since it does not affect TNFα and IL6 production by LPS activated macrophages in its powder form () while it completely eliminates the production of these cytokines by LPS activated macrophages that were nested on the gel or sheet form of this cryogel ().
Figure 7. TNFα (A) and IL6 (B) levels were measured after 24 h incubation of macrophages with a gel form of # 1, 2, 3, and 4 () covering the bottom of each well. The cells were rested on the gel sheets overnight before stimulations. Cell concentration was 1 × 106 cells/mL in a final volume of 1 ml. The cells were incubated with or without 1 µg/mL LPS for 24 h. Control negative wells did not have any chemical or stimulant. Control positive wells had only LPS as a stimulant and did not have any of the chemicals. p < .0001 N = 3, one way ANOVA test was applied to calculate the statistical significance.
Conclusion
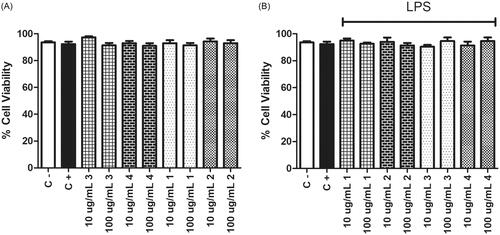
Natural polymer-based scaffolds, such as chitosan and gelatine are widely used in tissue regeneration/formation due to their high cell compatibility; however, their poor mechanical properties and little knowledge of the interaction between materials and host cells have limited the practical applications of these materials. In addition, during or after the implantation of scaffolds, inflammation and local tissue damage can occur through the activation of macrophages. Therefore, the interaction between implanted scaffolds and the immune response should be considered. With this aim, the present work focussed on the evaluation and comparison of immunomodulatory activities of the chitosan:gelatine cryogel scaffolds manufactured with different polymer ratios. The produced scaffolds showed an interconnected macroporous structure surrounded by highly dense polymer walls as a result of cryogelation method. The immunomodulatory properties of the cryogels were investigated with mammalian macrophages in both gel and powder forms to fully represent the material during implantation and to represent its fragmented form due to the degradation over time, independently. To do this, the production levels of TNFα and IL6 cytokines were analyzed. Our results showed that the chitosan:gelatine cryogels produced in this study have anti-inflammatory activities, and depending on the ratio of polymers, cryogels exerted differential effects on the inflammatory potential of the macrophages by changing the pro-inflammatory TNFα and IL6 production levels. Our results suggest that increasing the gelatine ratio should be under control, if not it may alter the behaviour of the material in its powder form. In terms of TNFα production, there was a decrease in the production of this cytokine by the activated macrophages when gelatine was mixed with the chitosan. But above a certain level, it led to a change in the behaviour of the material and the powder form of it could not suppress the TNFα production anymore. Moreover, the cryogels did not show any cytotoxic effects on RAW 264.7 macrophage cultures according to morphological analysis on the gel form as well as according to Trypan blue staining with the powder forms of the materials (). The results suggest that chitosan and gelatine-based cryogels have the potential to be involved in future applications in the fight against scaffold rejection, and may also have the potential to reduce the use of anti-inflammatory drugs used to reduce inflammation at the scaffold implantation site. To our knowledge, this study is the first example of examining this kind of materials gel and powder form as well as the impact of the change in chitosan/gelatine ratio on the inflammatory responses of the activated macrophages. We should also highlight that these behaviours may change if primary cells are used since RAW 264.7 cells are immortalized cell lines and more investigations are required to determine the effect of these materials with ex vivo and in vivo analysis of a larger panel of cytokines.
Figure 8. Cell viabilities were measured after 24 h incubation of macrophages with 10 and 100 µg/mL powder form of materials # 1, 2, 3, and 4 (). Trypan Blue staining was utilized for this purpose. Cell concentration was 1 × 106 cells/mL in a final volume of 1 ml. The cells were incubated with (A) or without (B) 1 µg/mL LPS as represented on the graphs. Control negative wells did not have any chemical or stimulant. Control positive wells had only LPS as a stimulant and did not have any of the chemicals.
Disclosure statement
The authors do not have any financial or non-financial conflict of interest to declare.
Data availability statement
All data is included in the submission/manuscript file.
References
- Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239–254.
- Demir D, Öfkeli F, Ceylan S, et al. Extraction and characterization of chitin and chitosan from blue crab and synthesis of chitosan cryogel scaffolds. J Turkish Chem Soc Sect A Chem. 2016;3;131-144.
- Demir D, Ceylan S, Göktürk D, et al. Extraction of pectin from albedo of lemon peels for preparation of tissue engineering scaffolds. Polym Bull. 78, 2211–2226, 202.
- Bölgen N, Demir D, Yalçın MS, et al. Development of hypericum perforatum oil incorporated antimicrobial and antioxidant chitosan cryogel as a wound dressing material. Int J Biol Macromol. 2020;161:1581–1590.
- Kumar P, Dehiya BS, Sindhu A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int Nano Lett. 2017;7(4):285–290.
- Nagahama H, Maeda H, Kashiki T, et al. Preparation and characterization of novel chitosan/gelatin membranes using chitosan hydrogel. Carbohydr Polym. 2009;76(2):255–260.
- Martins AM, Alves CM, Kurtis Kasper F, et al. Responsive and in situ-forming chitosan scaffolds for bone tissue engineering applications: an overview of the last decade. J Mater Chem. 2010;20(9):1638–1645.
- Argüelles-Monal WM, Lizardi-Mendoza J, Fernández-Quiroz D, et al. Chitosan derivatives: Introducing new functionalities with a controlled molecular architecture for innovative materials. Polymers. 2018;10(3):342.
- Etikala A, Tattan M, Askar H, et al. Effects of NSAIDS on periodontal and dental implant therapy. Compendium; 2019 [cited 2020 Oct 14]. Available from: https://www.aegisdentalnetwork.com/cced/2019/02/effects-of-nsaids-on-periodontal-and-dental-implant-therapy
- Yaghini J, Abed AM, Izadi M, et al. Effect of short-term steroid use (prednisolone) on bone healing around implants: an experimental study on dogs OHDM- Vol. 16- No.2-April, 2017 .
- Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988;34(2):101–107.
- Ericsson I, Persson LG, Berglundh T, et al. Different types of inflammatory reactions in peri-implant soft tissues. J Clin Periodontol. 1995;22(3):255–261.
- Kzhyshkowska J, Gudima A, Riabov V, et al. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol. 2015;98(6):953–962.
- Lechner J, Noumbissi S, von Baehr V. Titanium implants and silent inflammation in jawbone-a critical interplay of dissolved titanium particles and cytokines TNF-α and RANTES/CCL5 on overall health? EPMA J. 2018;9(3):331–343.
- Wang X, Xu X, Huang H, et al. Interleukin-6 first plays pro- then anti-inflammatory role in early versus late acute renal allograft rejection. Ann Clin Lab Sci. 2013;43(4):389–394.
- Uehara M, Li X, Sheikhi A, et al. Anti-IL-6 eluting immunomodulatory biomaterials prolong skin allograft survival. Sci Rep. 2019;9(1):1–13.
- Jordan SC, Choi J, Kim I, et al. Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: therapeutic implications of IL-6 receptor blockade. Transplantation. 2017;101(1):32–44.
- Ceylan S, Alatepeli B. Evaluation of PVA/chitosan cryogels as potential tissue engineering scaffolds; synthesis, cytotoxicity, and genotoxicity investigations. J Turkish Chem Soc Sect A Chem. 2021;8:69–78.
- Syverud K, Pettersen SR, Draget K, et al. Controlling the elastic modulus of cellulose nanofibril hydrogels—scaffolds with potential in tissue engineering. Cellulose. 2015;22(1):473–481.
- Ayaz F. Immunostimulatory and immunomodulatory effects of Nitzschia navis-varingica, Heterocapsa pygmaea and Chrysochromulina alifera whole cell extracts on mammalian macrophage cells. Nat Eng Sci. 2019;4:237–246.
- Ayaz F, Colak SG, Ocakoglu K. Investigating the immunostimulatory and immunomodulatory effects of cis and trans isomers of ruthenium polypyridyl complexes on the mammalian macrophage‐like cells. ChemistrySelect. 2020;5(37):11648–11653.
- Rogers ZJ, Bencherif SA. Cryogelation and cryogels. Gels. 2019;5(4):46.
- Marrazzo P, O’leary C. Repositioning natural antioxidants for therapeutic applications in tissue engineering. Bioengineering. 2020;7(3):104–135.
- Kartikasari N, Yuliati A, Listiana I, et al. Characteristic of bovine hydroxyapatite-gelatin-chitosan scaffolds as biomaterial candidate for bone tissue engineering. 2016 IEEE EMBS Conference on Biomedical Engineering and Sciences (IECBES); 2016.
- Arbez B, Libouban H. Comportement de lignées cellulaires de macrophages et d’ostéoblastes en contact avec le biomatériau β-TCP (phosphate bêta tricalcique). Morphologie. 2017;101(334):154–163.
- Caires HR, Esteves T, Quelhas P, et al. Macrophage interactions with polylactic acid and chitosan scaffolds lead to improved recruitment of human mesenchymal stem/stromal cells: a comprehensive study with different immune cells. J R Soc Interface. 2016;13(122):20160570.
- Phillips JM, Kao WJ. Macrophage adhesion on gelatin-based interpenetrating networks grafted with PEGylated RGD. Tissue Eng. 2005;11(5–6):964–973.
- Zhang X, Chen YR, Zhao YL, et al. Type I collagen or gelatin stimulates mouse peritoneal macrophages to aggregate and produce pro-inflammatory molecules through upregulated ROS levels. Int Immunopharmacol. 2019;76:105845.
- Peluso G, Petillo O, Ranieri M, et al. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15(15):1215–1220.
- Noam Eliaz, editor. Degradation of implant materials. New York, NY: Springer LLC; 2012.
- Elisa Tamariz and Ariadna Rios-Ramírez (June 14th 2013). Biodegradation of Medical Purpose Polymeric Materials and Their Impact on Biocompatibility, Biodegradation - Life of Science, Rolando Chamy and Francisca Rosenkranz, IntechOpen, DOI: https://doi.org/https://doi.org/10.5772/56220
- Yoon HJ, Moon ME, Park HS, et al. Chitosan oligosaccharide (COS) inhibits LPS-induced inflammatory effects in RAW 264.7 macrophage cells. Biochem Biophys Res Commun. 2007;358(3):954–959.
- Kathuria N, Tripathi A, Kar KK, Kumar A. Synthesis and characterization of elastic and macroporous chitosan-gelatin cryogels for tissue engineering. Acta Biomater. 2009;5(1):406–418.
- Razavi M, Qiao Y, Thakor AS. Three-dimensional cryogels for biomedical applications. J Biomed Mater Res A. 2019;107(12):2736–2755.
- Sultankulov B, Berillo D, Sultankulova K, et al. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules. 2019;9(9):470.