Abstract
Acute respiratory distress syndrome (ARDS) features pulmonary dysfunction capable of causing life-threatening hypoxaemia. Ventilation and hyperoxic therapies force oxygen through dysfunctional alveoli but risk exacerbating damage. Ox66™ is an ingestible, solid-state oxygen product designed for oxygen supplementation. Eighteen anaesthetized, ventilated rats were subjected to a 40% reduction in tidal volume to produce a hypoventilatory simulation of the hypoxia in ARDS (HV-ARDS). After 60 min, animals were randomized to receive either normal saline (Saline; volume control) or Ox66™ gavage. Cardiovascular function and blood oximetry/chemistry were measured alongside interstitial oxygenation (PISFO2) of the peripheral spinotrapezius muscle. HV-ARDS reduced mean arterial pressure by ∼20% and PISFO2 by ∼35% for both groups. Ox66™ gavage treatment at 60 min improved PISFO2 over Saline (p < .0001), restoring baseline values, however, the effect was temporary. A second bolus at 120 min repeated the OX66™ PISFO2 response, which remained elevated over Saline (p < .01) until study end and was supported by systemic parameters of lactate, PaO2, SO2, and base deficit. Saline remained hypotensive, whereas Ox66™ became normotensive. Vasoconstriction was observed in the Saline, but not Ox66™ group. Supplemental oxygenation through Ox66™ gavage increased peripheral tissue oxygenation, warranting further study for disorders featuring dysfunction of pulmonary perfusion like ARDS.
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening complication of disease or injury to the lung. Onset can be rapid (hours) or progressive (days), and is clinically identified as dyspnoea, moderate to severe hypoxaemia, and diffuse damage to alveoli or pulmonary microvasculature appearing as bilateral opacities on chest radiography. Between 1999 and 2013, ARDS killed an average of 155,000 people annually in the United States with a disproportionate impact on elderly populations [Citation1]. With the emergence of COVID-19, ARDS has taken centre stage carrying a 20–40% in-hospital mortality rate as a secondary complication of infection [Citation2,Citation3].
Clinically-assessed hypoxaemia, which arises from a mismatch in the ventilation-to-perfusion ratio, is determinant in ARDS severity [Citation4]. Alveolar damage and pulmonary edoema due to injury and hyperactive inflammatory processes limit gas exchange (see [Citation5] for review) leading to tissue (gastrointestinal) hypoxia, sepsis, and multi-organ failure [Citation6]. Standard treatments for ARDS-induced hypoxaemia include lung-protective ventilation (e.g. low tidal volume and low pressure) [Citation7], supplemental oxygen [Citation8], prone positioning [Citation9], and extracorporeal membrane oxygenation [Citation10,Citation11]. However, interventions dependent on the respiratory pathway are limited in efficacy by the pulmonary damage associated with ARDS and also introduce the risk of further injury—ventilator-induced lung injury [Citation12,Citation13] and hyperoxic acute lung injury [Citation14]. Extracorporeal membrane oxygenation bypasses the lungs but induces a prothrombotic state that increases thromboses requiring anticoagulants that increase haemorrhages [Citation15–17]. Additionally, these approaches to supplemental oxygenation require specialized equipment and personnel to titrate dosing and operation in response to a dynamic patient status.
Ox66™ (Hemotek, Plano, TX) is a powdered (solid-state) form of oxygen. Its molecular structure is a polyoxygenated aluminium hydroxide product composed of approximately 66.2% diatomic oxygen organized as a true clathrate with oxygen molecules captured in a lattice structure. When administered orally, it has improved skeletal muscle oxygen delivery for both rodents and swine in the pilot investigations of haemorrhagic shock (data unpublished). Gavaged Ox66™ has also been evaluated for safety in healthy rats at Charles River Laboratories, and mesenteric oxygen-transfer efficacy at Song Biotechnologies with encouraging results [Citation18].
The present study sought to model the pulmonary gas-exchange insufficiency seen in respiratory distress to assess a novel, gavage route of oxygen supplementation using Ox66™. Direct measurements of peripheral microvascular oxygenation also provided insight into tissue oxygen delivery as a contrast to the systemic oximetry measurements (PaO2: indicator of hypoxaemia) typically collected in a clinical setting.
Methods
Animals
The following protocol and experimental procedures performed by Song Biotechnologies LLC were approved by the SoBran Biosciences Inc. Institutional Animal Care and Use Committee (Protocol # SON-005-2018) and are consistent with the National Institutes of Health guidelines for the humane treatment of laboratory animals. Study subjects were male, Sprague Dawley rats (N = 18; 250–300 g; 8–10 weeks old; Charles River Laboratories, Inc., Wilmington, MA, USA) housed in pairs with a 12/12 day/night cycle and provided ad libitum access to feed and water. The study was unblinded and animals enrolled into treatment groups through random selection at the time of gavage treatment. Two to three experiments (AM, PM, and late PM) typically occurred in a day to minimize variability due to animal weight gain and ageing.
Surgical preparation
All pre-operative and surgical procedures occurred under anaesthesia. Animals were inducted with 1–5% isoflurane in air for initial pre-operative preparation and cannulations. Following surgical cannulation of the femoral vein, the anaesthetic plane was maintained with a continuous intravenous infusion (0.1 mg/kg/min) of alfaxalone acetate (Alfaxan, Schering-Plough Animal Health, Welwyn Garden City, UK) regulated by a syringe pump (Genie Touch™, Kent Scientific, Torrington, CT). A femoral artery cannula was connected to a pressure transducer for continuous monitoring of cardiovascular parameters (BIOPAC MP-150, BIOPAC Systems, Goleta, CA). The same femoral artery cannula was also used to collect blood samples for analysis of blood gases and chemistry (ABL90 Flex, Radiometer, Denmark). Cannulas were kept free of clots with heparinized phosphate-buffered saline (20 IU Heparin Sodium per ml; Pfizer, New York, NY, USA), which was not infused into the animal. A tracheal tube was inserted via tracheotomy to maintain airway patency, and animals continued to inspire room air without artificial ventilation until baseline (BL) metrics had been collected. Mechanical ventilation (RoVent® Jr, Kent Scientific, Torrington, CT) with medical air (21% oxygen, 78% nitrogen: Airgas, Radnor Township, PA, USA) through the tracheal tube was used to simulate the hypoxia in ARDS through a 40% reduction in respiratory tidal volume. A stomach tube was placed down the oesophagus for gavage.
Following surgical preparation, the animal was moved to a custom, thermostabilized (37 °C) animal platform adapted for microcirculatory studies [Citation19–21] and positioned laterally on its right side. The rat spinotrapezius muscle (utilized for the investigation of microcirculatory parameters) was exteriorized as described by Grey [Citation22] and secured in situ onto the platform’s thermostable pedestal (37 °C) for measurements of arteriolar diameters and the partial pressure of oxygen in the interstitium (PISFO2).
Intravital microscopy
Observation and measurement of the exteriorized spinotrapezius preparation were carried out with an upright microscope (Axioimager2m, Carl Zeiss AG, Oberkochen, Germany) configured with a 5X/0.25 objective (N-Achroplan; Zeiss) for trans-illumination and a 20X/0.8 objective (Plan-APOCHROMATE, Zeiss) for phosphorescence quenching microscopy. Trans-illumination was used to select measurement sites, establish appropriate focal planes, and verify flow conditions. For arteriolar diameter measurements, images were focussed in the diametral plane and captured with a colour CMOS camera (Axiocam 305 colour, Zeiss, Germany) and stored digitally. Arterioles ranging from 50 to 100 µm were analyzed, which are representative of the vessels responsible for vascular autoregulation and changes in total peripheral resistance. Internal vessel diameters were measured with a digital ruler (ZEN 3.3 Blue Edition, Zeiss, Germany) calibrated to a standard microscope micrometer (Stage Micrometer, Zeiss). For vasoactivity data, each vessel’s measured diameter was normalized to its diameter at BL.
Phosphorescence quenching microscopy
Measurements of interstitial oxygen tension (PISFO2) in the exteriorized spinotrapezius muscle and the technical setup have been previously described in depth [Citation23]. Briefly, the phosphor (Oxyphore R0; Frontier Scientific, Newark, DE) was topically applied to the tissue and allowed to diffuse into the interstitium. The phosphor probe was excited by a green laser diode operating a 520 nm (NDG7475 1 W; Nichia Tokushima, Japan) in an octagonal region 300 µm in diameter at a frequency of 1 Hz. The excitation light pulse was passed through a filter cube consisting of a narrow-band filter (525 CWL Narrowband, Edmund Optics, Barrington, NJ), a dichroic mirror (567 nm DMLP Longpass, Thorlabs, Newton, NJ, USA), and a wide-band filter (Longpass Cut-on >650nm, Thorlabs, Newton, NJ, USA) for selective collection of phosphorescence emission. The phosphorescence signal was collected by a photomultiplier tube (R9110, Hamamatsu) and routed through a custom-built signal processor, collected by a data acquisition device (NI PCIe-6361, National Instruments, Austin TX, USA), and stored digitally on a computer. Decay curves were fitted to a rectangular distribution model [Citation24] and translated to discrete measurements of PISFO2. Three sites were measured longitudinally per experiment.
Treatment groups
Rats were randomly assigned to receive a gavage of either Ox66™ (N = 9; Hemotek) at 1 g/kg suspended in 2.5 ml normal saline (BD PosiFlush™, Becton, Dickinson and Company, Franklin Lakes, NJ, USA), or 2.5 ml normal saline (Saline; N = 9; volume control).
Experimental protocol
Following surgical preparation, animals were stabilized for 15–30 min on the 3D-printed thermoregulated platform used for intravital microscopy. BL measurements—spontaneous breathing at 100% tidal volume—consisted of the following systemic variables: mean arterial pressure (MAP), pulse pressure (PP), heart rate (HR), hindlimb oximetry (PulseOx), and temperature. Microcirculatory data was collected from three to five interstitial sites selected for PISFO2 per experiment. Additionally, 65 µL of arterial blood was collected for oximetry and chemical analysis via ABL90 Flex (Radiometer; Copenhagen, Denmark). Systemic and microcirculatory metrics were recorded every 15 min, and blood samples were collected at BL and 60, 90, 120, 150, and 180 min of hypoventilation (HV-ARDS).
HV-ARDS was induced by connecting the intubated animals to a mechanical ventilator (RoVent® Jr) and reducing standard tidal volume by 40% while maintaining a normal breathing rate. Tidal volumes were based on each rodent’s weight. For example, the initial tidal volume for a 300 g rat was 2.19 ml, and at the onset of the experiment, it was reduced to 1.31 ml. The 40% reduction was maintained for three hours at which time the experiment was ended.
After 60 min of HV-ARDS, animals were randomized to receive a 2.5 ml bolus of either Saline (volume control; N = 9), or Ox66™ suspended in normal saline (N = 9) by gavage. The gavage was repeated at 120 min of HV-ARDS. The observation period lasted until 180 of HV-ARDS when the study was terminated. Rats were euthanized with an IV infusion of Euthasol (150 mg/kg, pentobarbital component; Delmarva, Midlothian, Virginia, USA), while under anaesthesia.
Statistics
Data are expressed as mean ± standard error of the mean (SEM). Statistical comparisons within and between experimental groups were made using two-way ANOVA (repeated measures or multiple comparisons as appropriate; Prism 9, GraphPad Software Inc., San Diego, CA, USA). Significance was defined as p < .05. Intragroup comparisons were to BL, while inter-group comparisons were at each appropriate time point. In cases where a significant difference (p < .05) was detected, a high stringency multiple comparison test (Tukey’s or Sidak’s between groups and Dunnett’s vs. BL) was performed. An unpaired T-test was used for single metrics (weight, age, etc.).
Data sharing
For original data, please contact [email protected].
Results
Animal characteristics and HV-ARDS profile
Animal weights (Ox66™: 311 ± 9 g; Saline: 323 ± 7 g; p = 0.47) and all baseline (BL) metrics were equivalent between groups. Arteriolar diameters were 60 ± 3 and 67 ± 4 µm for Saline and Ox66™, respectively (p = .64). A femoral cannula clotted in one Ox66™ animal preventing the collection of systemic and blood chemistry, but microcirculatory data and overall physiological status were unaffected. Hypoventilation to produce HV-ARDS involved a tidal volume reduction by 40% (Saline: 1.4 ± 0.03 and Ox66™: 1.4 ± 0.04 ml per breath) while maintaining normal breath rates at 71 ± 0.5 and 72 ± 0.5 BPM for Saline and Ox66™, respectively. Within 15 min of HV-ARDS, reductions in oxygen delivery to the spinotrapezius muscle were noted for both groups (). PISFO2 remained depressed as hypotension manifested, becoming significant for Ox66™ by 15-min and both by 30-min (). Drops in PaO2 were detected at 60 min for Saline (p = .04) and trending for Ox66™ (p = .43), corresponding to the PISFO2 deficits. Other blood gases and chemistry were not significantly affected by HV-ARDS before treatment (). PaO2/FiO2, a clinical calculation to normalize blood oxygen tension to the fraction of inspired oxygen (21% for all experiments), decreased for both groups with Saline (p = .042) crossing the threshold for significance versus Ox66™ (p = .422) by the 60-min mark. However, PaO2/FiO2 remained above the human threshold for ARDS for both groups ().
Figure 1. Oxygen delivery to the spinotrapezius muscle. Measurements were collected at 15 min intervals. Baseline (BL) followed stabilization from surgery and was collected during normal breathing. Hypoventilation (60% tidal volume with normal breath rate) was then initiated and maintained for the duration of the experiment. The first gavage occurred following the 60 min measurement and the second after 120 min as demarcated by the dotted lines. Of note, the second Ox66™ gavage had a longer duration of impact on oxygen delivery than the first, but both produced significant PISFO2 responses within 15 min of treatment.
Data are mean ± SEM; N = 26 sites/group.
*Indicates p<.05 compared to BL.
β Indicates p<.05 between groups.
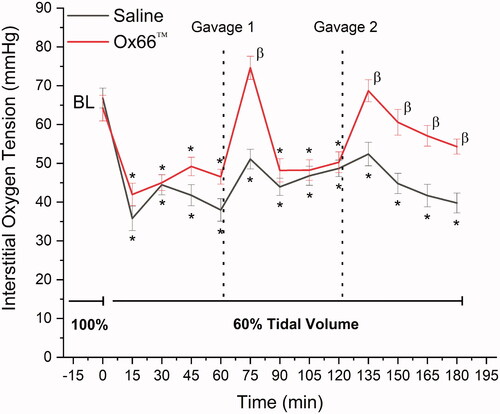
Table 1. Systemic variables.
Table 2. Arterial blood gases and chemistry.
Gavage 1 (G1)
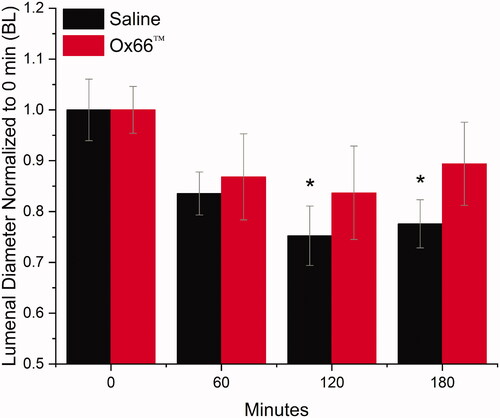
Gavage with Ox66™ at 60 min of HV-ARDS significantly increased oxygen delivery compared to Saline (p < .0001), returning PISFO2 to BL (). The Ox66™ spike in PISFO2 was temporary, returning to pre-treatment levels by the 90-min time point (30 min after G1). MAP remained mostly hypotensive for both groups with Ox66™ returning briefly to BL at 105 min. Bradycardia was noted for Saline after 90 min and a significant drop in hindlimb PulseOx detected at 120 min (), which followed the depression in arterial SO2 first measured at 90 min. Arterial PO2 remained lower than BL for both groups, but base deficit left-shifted for Saline at the 90 min mark (p = .003) and not for Ox66™ (). PaO2/FiO2 did not show a response to G1 and continued to decline. Additionally, arteriolar vasoconstriction was observed for the Saline group at 120 min (p = .002, ).
Figure 2. Diameters of resistance arterioles. Arterial diameters, as an indicator of resistance to blood flow, were imaged every hour using intravital microscopy. Reductions in diameter indicate increased peripheral resistance to blood flow. The first measurement (0 min) occurred before the onset of hypoventilation. The 60-min measurement reflects 60 min of hypoventilation without treatment. Gavages were administered following 60- and 120-min. Measurements used a digital ruler calibrated to a standard microscope stage micrometer. Raw measurements were normalized to 0 min (BL) and reported as mean ± SEM. N = 17 and 11 arterioles for Saline and Ox66™ respective.
*Indicates p<.05 compared to BL.
Intergroup comparisons did not reveal significant differences.
Gavage 2 (G2)
Following measurement at the 120 min mark, a second gavage (G2) produced similar results to the first. No PISFO2 response was detected for Saline while, Ox66™ returned to BL and remained significantly higher than Saline until the end of observation (). Hypotension and bradycardia persisted for Saline, while Ox66™ became normotensive and showed no change to heart rate. Pulse Ox dipped below the threshold for significance against BL at 135 and 180 min (). PaO2/FiO2 remained low for Saline but Ox66™ improved above the threshold for “no difference” (p = .10 and .11 at 150- and 180-min). Arteriolar diameters were reduced ∼20% relative to BL for Saline at 180 min (p = .012), while Ox66™ remained not different (p = .653).
The blood gases and chemistry profile suggested a cumulative effect of 40% tidal volume reduction in metabolic parameters for Saline, while Ox66™ showed some improvement. PaO2 and SO2 remained low for Saline but improved for Ox66™. Carboxyhaemoglobin was elevated at the end of observation for Saline, however, numerical differences between the groups were minor. Saline’s base deficit continued to worsen, and lactate became elevated at 135 min and continued to rise until the end of observation. Ox66™ showed elevated potassium (starting at 120 min) and tHb (120 and 150 min). No differences between groups were detected for any time point ().
Discussion
A rat hypoventilation model of ARDS-induced, gas-exchange insufficiency was used to test the efficacy of a novel, oral approach to supplemental oxygenation. Since pulmonary dysfunction interferes with pulmonary-based oxygenation strategies, alternate and accessible routes of administration are of great interest, especially in a time when COVID-19 surges create a massive strain on intensive care units. The gut has an acceptable surface-to-volume ratio and ingestion is the preferred route of therapeutic intervention when feasible. It was hypothesized that Ox66™, an oxygen-caged clatherate that breaks down under acidic conditions, could provide a meaningful amount of oxygen through the gastric and intestinal lining. Hence, the oxygen deficits of ARDS were targeted with a hypoventilation protocol to mitigate the confounding variables that systemic and pulmonary inflammation might add.
A key metric guiding the development of this model was the direct measurement of tissue oxygenation in the interstitial fluid (PISFO2) of peripheral skeletal muscle. The exteriorized spinotrapezius preparation has been in use for modelling terminal microvascular blood flow for almost 50 years [Citation22] and more recently, as technological progress has allowed, oxygen delivery kinetics [Citation25,Citation26]. The microcirculation largely remains a realm of research but, as demonstrated here, is nonetheless a powerful indicator of early (subclinical) disease and distress. While the HV-ARDS and gavage protocol produced longitudinal changes for clinical metrics such as SO2, PaO2, lactate, and base deficits, direct differences between groups were not detected and, in the case of PaO2, the longitudinal differences were small, if statistically valid. Indeed, it is also noted that animals did not meet the clinical PaO2/FiO2 criteria for ARDS. However, the capillary beds proved a better discriminator of subclinical oxygen deficits as both longitudinal and inter-group differences were detected. PISFO2 values of 40–50 mmHg () demonstrated decreased oxygen delivery during HV-ARDS. For reference, <30 mmHg and below is where oxygen consumption becomes limited by diffusion in rat skeletal muscle at rest [Citation20]. Treatment effect was also established between Ox66™ and volume control, indicating that PISFO2 was an effective testing platform. Further, the discrepancy between the oxygenation in the microcirculation and the blood supports our and others’ assertion that hypoxaemia may not sufficiently describe the level of hypoxia occurring in the tissue capillary beds in conditions like ARDS.
In the clinic, ARDS is quantified and stratified by the PaO2/FiO2 ratio. Values of <300 mmHg, <200 mmHg, and <100 mmHg qualify as mild, moderate, and severe, respectively. For reference, the ratios seen in this study were generally 330–350, which—without scaling between species—would suggest very mild or emergent ARDS. However, if factoring in the oxygen demand differential between rats and humans, values of 350 become more severe because oxygen consumption is fivefold higher in rats at rest [Citation27,Citation28], Thus, similarities in supply may not meet the same needs between species, which was supported by our findings of increased rodent mortality when the tidal restriction is increased to 50% (data not published, under review). Returning to the present study, another issue with the PaO2/FiO2 ratio may be oxyhaemoglobin. Since inspired air was fixed at 21% oxygen, the ratio is dependent on dissolved oxygen (PaO2), which is subject to the much larger mass of oxygen (70-fold higher) bound to haemoglobin. The slight rise in PaCO2 from hypoventilation could have right-shifted the oxygen dissociation curve of haemoglobin leading to inflated PaO2 values. While this effect was not quantified, it is posited as a confounder of arterial blood gas measurements in over-estimating oxygen delivery to tissues, especially in situations where more marked shifts in CO2 and pH occur.
Oral versus inhalation administration changes the therapeutic kinetics. Pulmonary-based treatments to improve oxygenation (e.g. mechanical ventilation and concentrated oxygen) and extracorporeal membrane oxygenation, which functions similar to a cardiac bypass by externally reoxygenating blood [Citation29,Citation30], have immediate impacts on PaO2 and oxygen delivery. The gut also offers a vascularized surface similar in size to the lungs for absorption [Citation31], which has shown promise during enteral passage of hyper-oxygenated perfluorocarbons [Citation32]. However, two key differences are blood perfusion and a rate-controlling digestive step. The lungs have their own, dedicated half of the heart to ensure gas exchange is not diffusion limited, whereas the mesenteric blood flow accounts for about 10% of cardiac output [Citation33]. The digestion step is specific to Ox66™, which creates a longer duration, time-release effect. Indeed, this means an immediate oxygen need to relieve critical organ hypoxia may not be feasible. But, neither would the specialized equipment, training, and personnel be required in such cases as enteral perfusion. Utilizing gavage, the response in oxygen delivery to the spinotrapezius muscle took approximately 15 min to manifest (a determinant in the sampling resolution). This was expected given gastric transit time into the small intestine and (likely) digestive kinetics of the clathrate structure. Correspondingly, effect durations were protracted compared to the immediacy of removing a gaseous oxygen supplement, which we attribute to a time-release effect. The time course of oral Ox66™ is not ideal for urgent rescue therapies but warrants further study as a complementary treatment or early intervention to reduce the cumulative hypoxic effects seen in ARDS.
Another potential benefit of gut oxygenation comes from hypoxaemia leading to sepsis-induced multi-organ failure as a cause of death in ARDS [Citation34]. The catalyst is gut ischaemia [Citation6]. As blood oximetry does not always indicate deeper tissue hypoxia—shown in the setting of hypovolemic anaemia [Citation35]—a situation of “silent hypoxia” can develop in ARDS, which concentrates in the sensitive gastrointestinal tract. In response, the intestinal epithelium—barrier protecting the sterile self from the microbial-laden gut lumen—breaks down and releases an enormous endotoxin load into the body leading to sepsis [Citation36]. Previous research has shown improved mesenteric oxygenation following ingestion of Ox66™ [Citation18], an ex vivo digestion study simulating residence time from mouth through the intestine showed the efficacy of Ox66™ post- passage through saliva and gut juices (data not shown), in addition to this study’s finding of effective peripheral oxygen delivery through ingestion. While the metabolic situation of tissues and organs is multivariate and highly dependent on blood flow, there is evidence for an oxygen gradient stemming from the digestive tract. Under conditions where hypoxia concentrates in the intestinal barrier, oral oxygen supplementation may have a disproportionately large bulwarking effect compared with systemic changes and stave off sepsis.
It is of note that no significant vasoactive responses were detected in the Ox66™ group, which contrasts with other oxygen therapeutics such as haemoglobin-based oxygen carriers [Citation37]. This may be due to the oral administration route employed here, meaning only the oxygen, not the clathrate, entered the bloodstream, as well as Ox66™’s inorganic structure versus purified haemoglobin, which is a known scavenger of the vasodilator nitric oxide. Significant vasoconstriction was, however, observed over time in the Saline group. We believe this is indicative of blood flow redistribution or shunting to increase oxygenation to critical organs.
This study was designed to isolate the hypoxaemia and deficits in oxygen delivery seen in ARDS by mechanically simulating the syndrome-specific gas exchange insufficiencies. As such, the inflammatory conditions created by pulmonary disease and injury occurring over days were not reproduced. However, based on encouraging oxygen delivery results, longitudinal studies utilizing pulmonary inflammation are planned to assess survival, inflammatory and hypoxic biomarkers, organ function through end-point histochemical analysis, and to confirm speculation that Ox66™ may ward off sepsis. Additionally, induction of a true ARDS model will help determine whether this form of oxygen administration has potential for translation to the clinic.
Summary
When ingested, a solid-state, oxygen-containing compound (Ox66™) increased oxygen delivery to the peripheral tissues compared to volume control under the conditions of hypoventilation. Other metabolic metrics supported improved systemic oxygenation providing an intriguing avenue for situations where ventilation and hyperoxic therapies may be prohibitive or insufficient. The implications of an ingestible oxygenating compound also include en route care before more specialized equipment and personnel are available. Longer studies utilizing this compound in pathology models are needed to verify safety and determine efficacy in outcomes of survival and progression of hypoxia-induced sepsis.
Author contributions
William Nugent: Study design, data analysis, writing.
Danuel Carr: Data collection, writing
Rosa MacBryde: Data collection
Erica Bruce: Study design, data analysis, writing
Bjorn Song: Study design, data analysis, data collection, writing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cochi SE, Kempker JA, Annangi S, et al. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc. 2016;13(10):1742–1751.
- Nguyen NT, Sullivan B, Sagebin F, et al. Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at us academic centers. Ann Surg. 2021;274(1):40–44.
- Higgins TL, Stark MM, Henson KN, et al. Coronavirus disease 2019 ICU patients have higher-than-expected acute physiology and chronic health evaluation-adjusted mortality and length of stay than viral pneumonia ICU patients. Crit Care Med. 2021;49(7):e701–e706.
- Rawal G, Yadav S, Kumar R. Acute respiratory distress syndrome: an update and review. J Transl Int Med. 2018;6(2):74–77.
- Radermacher P, Maggiore SM, Mercat A. Fifty years of research in ARDS. Gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196(8):964–984.
- Taylor DE. Revving the motor of multiple organ dysfunction syndrome. Gut dysfunction in ARDS and multiorgan failure. Respir Care Clin N Am. 1998;4(4):611–631.
- Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14(1):R1.
- Silva PL, Pelosi P, Rocco PRM. Supplemental oxygen or something else? J Thorac Dis. 2018;10(Suppl 26):S3211–S4.
- Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168.
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42(5):889–896.
- Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975.
- Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37(4):633–646.
- Brower RG, Matthay MA, Morris A, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308.
- Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58(1):123–141.
- Durak K, Kersten A, Grottke O, et al. Thromboembolic and bleeding events in COVID-19 patients receiving extracorporeal membrane oxygenation. Thorac Cardiovasc Surg. 2021;69(06):526–536.
- Doyle AJ, Hunt BJ. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med. 2018;5:352.
- Doyle AJ, Hunt BJ, Sanderson B, et al. A comparison of thrombosis and hemorrhage rates in patients with severe respiratory failure due to coronavirus disease 2019 and influenza requiring extracorporeal membrane oxygenation. Critical Care Medicine. 2021;49(7):e663–e672.
- Zhang F, Aquino GV, Dabi A, et al. Oral ingestion of a novel oxygenating compound, Ox66™, is non-toxic and has the potential to increase oxygenation. Food Chem Toxicol. 2019;125:217–224.
- Golub AS, Pittman RN. Thermostatic animal platform for intravital microscopy of thin tissues. Microvasc Res. 2003;66(3):213–217.
- Nugent WH, Song BK, Pittman RN, et al. Simultaneous sampling of tissue oxygenation and oxygen consumption in skeletal muscle. Microvasc Res. 2016;105:15–22.
- Song BK, Nugent WH, Moon-Massat PF, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 2014;95:124–130.
- Gray SD. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res. 1973;5(3):395–400.
- Nugent WH, Cestero RF, Ward K, et al. Effects of sanguinate(R) on systemic and microcirculatory variables in a model of prolonged hemorrhagic shock. Shock. 2017;52(1S Suppl 1):108–115.
- Golub AS, Popel AS, Zheng L, et al. Analysis of phosphorescence in heterogeneous systems using distributions of quencher concentration. Biophys J. 1997;73(1):452–465.
- Lash JM, Bohlen HG. Perivascular and tissue PO2 in contracting rat spinotrapezius muscle. Am J Physiol. 1987;252(6 Pt 2):H1192–202.
- Shonat RD, Johnson PC. Oxygen tension gradients and heterogeneity in venous microcirculation: a phosphorescence quenching study. Am J Physiol. 1997;272(5):H2233–H2240.
- Demin AV, D’Iachenko AI, Ivanov AI. Oxygen consumption by resting humans at different elbrus altitudes. Aviakosm Ekolog Med. 2010;44(3):68–71.
- Shepherd RE, Gollnick PD. Oxygen uptake of rats at different work intensities. Pflugers Arch. 1976;362(3):219–222.
- Ma X, Liang M, Ding M, et al. Extracorporeal membrane oxygenation (ECMO) in critically ill patients with coronavirus disease 2019 (COVID-19) pneumonia and acute respiratory distress syndrome (ARDS). Med Sci Monit. 2020;26:e925364.
- Kowalewski M, Fina D, Słomka A, et al. COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care. 2020;24(1):205.
- Helander HF, Fandriks L. Surface area of the digestive tract – revisited. Scand J Gastroenterol. 2014;49(6):681–689.
- Okabe R, Chen-Yoshikawa TF, Yoneyama Y, et al. Mammalian enteral ventilation ameliorates respiratory failure. Med. 2021;2(6):773–783.e5.
- Fiore G, Brienza N, Cicala P, et al. Superior mesenteric artery blood flow modifications during off-pump coronary surgery. Ann Thorac Surg. 2006;82(1):62–67.
- Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532.
- Nugent WH, Sheppard FR, Dubick MA, et al. Microvascular and systemic impact of resuscitation with pegylated carboxyhemoglobin-based oxygen carrier or hetastarch in a rat model of transient hemorrhagic shock. Shock. 2020;53(4):493–502.
- Hassoun HT, Kone BC, Mercer DW, et al. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15(1):1–10.
- Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. J Am Med Assoc. 2008;299(19):2304–2312.
