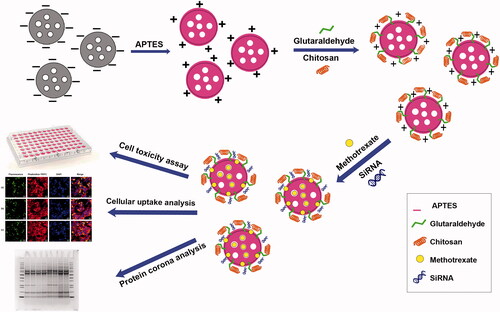
Abstract
Co-delivery of anticancer drugs and biologics can provide synergetic effects and outperform single delivery therapies. Here, a nanoparticle (NP) system for co-delivery of methotrexate (MTX) and STAT3 siRNA has been developed and tested in vitro. Mesoporous silica nanoparticles (MSNs) were functionalized with chitosan (ch) by covalent grafting mediated by aminopropyl triethoxysilane (APTES) via glutaraldehyde as the linker. Co-delivery of MTX and STAT3 siRNA to MCF7 cells was demonstrated in cells by flow cytometric analysis and confocal laser scanning fluorescence microscopy for use in breast cancer treatment. MTX either competitively inhibits the dihydrofolate reductase (DHFR) receptor or suppresses the STAT3 metabolic pathway. STAT3 protein plays an essential role in cell division, proliferation and survival. Reduction of the protein by both MTX and STAT3 siRNA, achieved by chMSNs, significantly decreased the viability of breast cancer cells compared to single treatments alone. Cellular uptake of modified NPs was increased over time when additional free MTX was added implicating the DHFR receptor in uptake. In addition, protein corona compositions coated the NPs outer surface, were different between the NPs with and without drug potentially modulating cellular uptake. This study is the first report on co-delivery of MTX and STAT3 siRNA by chitosan modified MSNs.
Introduction
Although cancer therapy has developed over the recent decades, the delivery of hydrophobic chemotherapeutic agents is still one of the main challenges for clinical applications in the treatment of cancers [Citation1,Citation2]. Therefore, novel drug delivery systems based on nanomaterials have been synthesized to carry different types of hydrophobic anticancer drugs [Citation3–5]. A recent drive has been to develop nanoparticle (NP) systems for the co-delivery of drugs [Citation6]. One of the most attractive nanocarriers for high capacity loading of hydrophobic anticancer drugs are mesoporous silica nanoparticles (MSNs) due to their large pore volume, controllable pore size, uniform particle size, high surface area and biodegradability [Citation7]. Functionalized MSNs that can simultaneously carry an anticancer drug and siRNA to the intended tumour site represents a promising method for cancer treatment [Citation8]. In addition, the internalization of modified MSNs by tumour cells can be affected by the attachment of serum proteins, the “protein corona”, on the external surface of NPs [Citation9].
Methotrexate (MTX) is a promising anticancer drug for use in breast cancer therapy due to competitive inhibition of the dihydrofolate reductase (DHFR) receptor, which suppresses DNA synthesis. MTX is a structural analogue of the natural DHFR-binder folate, but with a 1000 folds higher affinity [Citation10]. The structural similarity between MTX and folic acid has been reported to allow MTX in loaded NPs to act as a targeting agent to tumour cells [Citation11]. MTX produces several other modulations of cell function via suppression of a number of metabolic pathways, it can significantly induce apoptosis by inhibiting the signal transducer and activator of transcription factor 3 (STAT3) in the JAK/STAT pathway in tumour cells [Citation12]. In addition, increasing doses of MTX leads to overexpression of the DHFR gene in cancer cells, which affects cellular uptake [Citation13].
STAT3 is a transcription factor that plays an essential role in cell division, proliferation and survival [Citation14]. The activation of STAT3 protein as oncogenic protein is frequently detected in breast cancer cells and inhibition of STAT3 by a relatively new system using short RNA interference (siRNA) provides a novel approach to inhibit human tumour cells [Citation15]. Nanodelivery systems can co-deliver anticancer drugs and nucleic acids cancer therapy [Citation16]. Meng et al. [Citation17] demonstrated amine-functionalized MSNs for the delivery of dual drug/siRNA to overcome doxorubicin resistance in a xenograft.
MSNs with surface silanol groups are not suited to capture directly many drug molecules [Citation18], but MSNs can be modified by various functional groups including amines, carboxylic acids and thiols. Several recent reports have considered amine-modified MSNs as promising structures for the delivery of a range of negatively charged drug molecules [Citation19]. Several surface chemistries at MSNs, e.g. allowing pH sensitivity, have been applied for carrying different types of drugs and biomolecules with controllable drug release [Citation20].
Various chemical structures and polymers have been investigated for amine functionalization of silica nanostructures [Citation21]. PEI, APTES and chitosan (ch) are cationic polymers used for coating NP for packaging siRNA through electrostatic attraction [Citation21,Citation22]. APTES is commonly used for amine functionalization of MSN because it has both silane and amino groups in the structure that can be couple with silanol groups of NPs [Citation22]. Amongst them, chitosan, an amine-rich natural polysaccharide, is a good biopolymer for drug delivery due to its biocompatibility, nontoxicity, high density of cationic charges, hydrophilic properties and pH sensitivity [Citation23]. Several anti-cancer drugs and biomolecules have been delivered by chMSNs, in in vitro experiments [Citation24]. Nhavene et al. [Citation25] demonstrated the ability of chitosan-functionalized MSNs for gene delivery, supporting passive uptake in HeLa cells. Co-delivery of the anticancer drug, doxorubicin and the p53 gene by dendronized chitosan derivative functionalized MSNs induced significant apoptosis in breast cancer cells due to the synergistic effects of the two delivery concepts [Citation26].
Several silica-based nanocarriers have been designed for the delivery of MTX [Citation27]. However, these systems showed large particle sizes, demanded a high concentration of the drug being loaded or exhibited low biodegradability [Citation28]. MTX is a hydrophobic drug that acquires a negative charge when exposed to physiological pH and can be carried by positively charged NPs through ionic attraction [Citation29].
In this study, a nanoparticulate system for co-delivery of MTX and STAT3 siRNA was developed based on functionalized MSN () motivated by the hypothesis that effective co-delivery may synergistically reduce the level of STAT3 by combining two separate inhibition actions. APTES and grafted chitosan provided an amine-rich surface coating allowing electrostatic loading of both MTX and siRNA. Cell uptake, cytotoxicity and STAT3 expression were studied in MCF7 cells in vitro, demonstrating effective co-delivery, inhibited cellular division and proliferation and decreased STAT3 expression at the mRNA and protein level. Finally, protein corona analysis of free modified MSNs and MTX/modified MSNs showed a lower amount of apolipoprotein on the surface of MTX loaded NPs that potentially influences the cellular uptake.
Materials and methods
Materials
Hexadecyltrimethylammonium bromide (CTAB), pluronic®F-127, 3-triethoxysilylpropylamine (APTES), tetraethylorthosilicate (TEOS), glutaraldehyde solution (50%), DAPI and phalloidin-tetramethylrhodamine B isothiocyanate were from Sigma-Aldrich (Søborg, Denmark). Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin (10,000 U/ml), trypsin–EDTA, chitosan (molecular weight: 100,000–300,000) were purchased from Fisher Scientific-ACROS Organics™ (Geel, Belgium), MTX was from Santa Cruz (Dallas, TX), fluorescein-5-isothiocyanate (FITC “Isomer I”), Superscript™ VILO™ cDNA synthesis kit, pierce protease and phosphatase inhibitor mini tablets, Bolt™ 4–12% bis-tris plus gels, Pierce™ BCA protein assay kit and lipofectamine RNAiMAX from Thermo Fisher Scientific (Waltham, MA), Direct-zol™ RNA miniPrep plus was from Zymo Research (Irvine, CA), LightCycler® 480 SYBR green I master was from Roche Life Science (Basel, Switzerland), STAT3 (79D7) rabbit mAb (#4904) was from Cell Signaling Technology (Boston, MA), goat anti-rabbit immunoglobulins/HRP (affinity isolated, P0448) was from Dako (Santa Clara, CA), Corning® collagen I, Rat tail was from Corning (Corning, NY).
Synthesis procedure and modification
Synthesis and functionalization of MSNs
MSNs were synthesized using a modified protocol from a previously reported sol–gel procedure [Citation29,Citation30] and surface-modified with 3-aminopropyltriethoxysilane (APTES) [Citation31].
MSN-APTES was functionalized with chitosan through glutaraldehyde covalent crosslinking. Here, MSN-APTES (3 mg) was dispersed in 300 µl deionized water and sonicated for 5 min. The suspension was then added dropwise to glutaraldehyde (1%, 150 µl) solution under stirring at 4 °C. Free glutaraldehyde was washed away with water after one hour. Chitosan (1.5 mg) was then dissolved in acetic acid (1% w/v, 300 µl) by stirring for 2 h and the pH set to 5.0 using NaOH. MSN-APTES-glutaraldehyde (3 mg) were dispersed in water (300 µl) and added dropwise to the chitosan solution containing 1.5 mg of chitosan under stirring at room temperature for one day. Free chitosan was washed away with acetic acid (1% w/v), then water.
Characterization of MSNs and modified MSNs
Bare MSNs and MSNs coated with APTES/chitosan were characterized by transmission electron microscopy (TEM) for size and morphology analysis on a Tecnai G2 Spirit Electron Microscopy (120 kV, FEI Company, Hillsboro, OR, via two cameras) with samples mounted on carbon coated copper grids and stained with uranyl formate. NP surface charge (zeta potential) was measured using Malvern Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). Material refractive index (RI) value, absorption and water dispersant RI was defined to be 1.54, 0.00 and 1.330 respectively at 25 °C.
MTX and siRNA loading in chitosan-modified MSNs
Loading of MTX in MSN-APTES-chitosan was achieved by 1:1 w/w ratio. Free MTX was detected by HPLC and compared with a standard calibration curve to calculate the drug loading efficiency (LE%) and loading capacity (LC%) [Citation29].
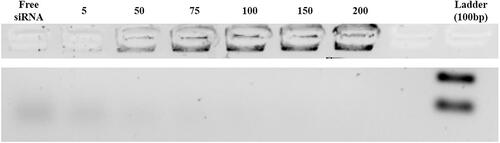
Agarose gel retardation assay was used to determine the capability of the siRNA loading in amine-modified MSN according to the optimal particle/nucleic acid (N/P) ratio.
Knockdown of the STAT3 mRNA expression
The ability of NP delivered siRNA to knock down STAT3 mRNA expression was evaluated with reverse transcriptase-polymerase chain reaction (RT-PCR). Three different concentrations of STAT3 siRNA (10, 20 and 50 µM) [Citation32] via lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) transfection according to the manufacturer’s protocol. The MCF7 cells without treatment and with treatment by NPs/siRNA (20 µM), free NPs, lipofectamine/siRNA as a positive control, and free lipofectamine were incubated for 48 h at 37 °C. The RT-qPCR primers to detect STAT3 and GAPDH, which is used for normalizing the mRNA levels, were obtained from Sigma-Aldrich (Gillingham, UK): forward: 5′-TGATCACCTTTGAGACCGAGG3′, reverse: 5′-GATCACCACAACTGGCAAGG3′.
Western blotting
STAT3 protein values in MCF7 cells were analysed by western blot after treatment with free siRNA, NPs/siRNA, free MTX, NPs/MTX, NPs/MTX/siRNA or free NPs for 48 h at 37 °C. Untreated MCF7 cells and purified STAT3 protein from HeLa cells were used as controls. GAPDH protein was used as the internal control. Western blotting was achieved as described by Mooney et al. [Citation33].
Cellular uptake analysis
ChMSNs were labelled with FITC to study cellular uptake, via isothiocyanate attachment to primary amine groups in chitosan. The FITC-labelled NPs were then dispersed in water for cellular uptake studies.
Flow cytometry
Aliquots of 5 × 104 cells (MCF7) seeded into 96-well plates were incubated at 37 °C for 48 h. Flow cytometry analysis was used to study: FITC-labelled NPs, FITC-labelled NPs/MTX and FITC-labelled NPs/MTX with additional free MTX (concentration 10 and 1000 nM) added to cells at a dose of 25 µg/ml for 1 and 4 h and FITC-labelled NPs/MTX and FITC-labelled NPs/MTX/STAT3 siRNA added to cells at a dose of 25 µg/ml for 1, 2 and 4 h.
Confocal microscopy
Sterilized rat tail collagen (10 µl) dissolved in PBS buffer (1 ml) coated eight-well chamber slides (ibidi) for 1 h. MCF7 cells (5 × 104) were cultured at 37 °C for 48 h. Thereafter, FITC-labelled NPs, FITC-labelled NPs/MTX or FITC-labelled NPs/MTX/STAT3 siRNA were added to cells at a dose of 25 µg/ml for 4 h.
Protein corona experiments
The protein corona on MSN-APTES-chitosan and MSN-APTES-chitosan/MTX were monitored by SDS-PAGE and BCA assay. Therefore, 400 µg of NPs and NPs/MTX were dispersed both in PBS (1 ml) including 10% and 90% of FBS in low-bind microtubes for 2 h.
Protein values were measured using a Pierce™ BCA Protein Assay Kit with BSA as the calibration standards. Triplicate experiments were performed and UV absorbance of samples and BSA was analysed using a 96-well plate reader (Tecan microplate reader, Männedorf, Switzerland) at 570 nm.
Cytotoxicity assay
The cytotoxicity of MSN-APTES-chitosan, free MTX, free STAT3 siRNA and NPs loaded with MTX, STAT3 siRNA and both MTX/siRNA was measured on MCF7 cells by the MTT assay.
Statistical analysis
All experiments were repeated in triplicate and statistically analysed via the SPSS software (SPSS Inc., Chicago, IL) using general linear model and Duncan's test (multiple comparison method).
Details of the methods are available in the supporting information section.
Results
Characterization of MSNs and modified-MSNs
The size, shape and structure of MSNs and amine-modified MSNs were visualized using TEM. c) shows representative images of MSNs (left panel), MSN-APTES (centre) and MSN-APTES-chitosan (right panel). The NPs were uniform and spherical with average particle size of 55.8 ± 6.4, 64.6 ± 7.4 and 74.5 ± 3.7 nm for MSNs, MSN-APTES and MSN-APTES-chitosan, respectively. Zeta potential was measured to determine the surface charges of the NPs in aqueous solutions (). The MSNs show the expected negative charge resulting from deprotonation of surface silanol groups [Citation34], while modification with APTES and then chitosan result in a switch to positive charge.
Figure 2. TEM images of (a) MSNs, (b) MSN-APTES and (c) MSN-APTES-chitosan samples. The images from left to right show MSNs, MSN-APTES and MSN-APTES-chitosan (scale bar: 200 nm).
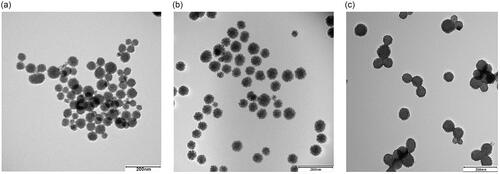
Table 1. Characterization of MSNs, MSN-APTES and MSN-APTES-chitosan samples using zeta potential, evaluation of the loading efficiency (LE), loading capacity (LC) percent of the MTX-loaded MSN-APTES-chitosan by HPLC, and calculation of loading percent of siRNA-loaded MSN-APTES-chitosan by an agarose gel retardation assay.
MTX and siRNA loading in modified-MSNs
MTX was mixed with MSN-APTES-chitosan (1:1 w/w ratio) for 24 h. MSN-APTES-chitosan was dispersed in deionized water and added dropwise to MTX-DMSO solution under stirring followed by washing. HPLC was applied to quantify free MTX and mathematically estimate the LE% and LC% of the samples according to a standard curve. The results in showed that the LE% and LC% of MTX-loaded NPs were around 13.9% and 12.2%, respectively.
The ability of NPs to load siRNA is essential for the efficient delivery of this biomolecule and can be estimated via agarose gel retardation assays. ChMSNs were complexed with STAT3 siRNA at varying N/P ratios. The data indicated that the STAT3 siRNA were loaded on the NPs in the threshold N/P ratios of 75 () corresponding to a loading percent around 1%, which remained the same with or without MTX loading.
Effect of siRNA-loaded NPs on STAT3 mRNA expression
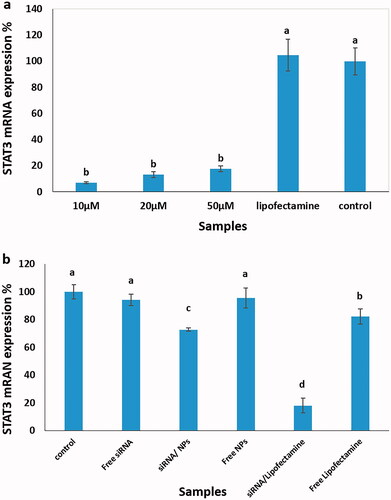
The expression of STAT3 mRNA levels was evaluated using RT-PCR. Lipofectamine, a “gold standard” for the delivery of exogenous DNA or RNA into cells, was used to confirm the accuracy of the siRNA design. Experimental details are found in section “Knockdown of the STAT3 mRNA expression”. There was no statistically significant difference between siRNA at the three different concentrations (), but these were significantly different from the control samples (p<.05). In the next step, the suppression effect of STAT3 siRNA on the expression of the STAT3 gene in transfected MCF7 cells was analysed at the mRNA levels by RT-PCR assay. Therefore, the cell samples treated with siRNA-loaded NPs were compared to cell samples exposed to siRNA-loaded lipofectamine, free siRNA, free NPs, free lipofectamine and untreated. The data revealed a decrease in the STAT3 mRNA levels of around 27% with STAT3 siRNA-loaded NPs, which was significantly different compared to control samples ().
Figure 4. STAT3 mRNA expression using the RT-PCR method. (a) Three different concentrations of STAT3 siRNA (10, 20 and 50 µM) were mixed with lipofectamine RNAiMAX, followed by adding to MCF7 cells and compared to lipofectamine treated or untreated cells as controls. (b) The cell samples treated with siRNA-loaded NPs were compared to cell samples exposed to siRNA-loaded lipofectamine, free siRNA, free NPs, free lipofectamine and untreated cells. Different letters indicate significant differences in mean values for each variable (p<.05).
Effect of siRNA-loaded NPs on STAT3 protein
The presence of STAT3 protein was determined by western blot analysis using different samples including cells treated with free siRNA, NPs/siRNA, free MTX, NPs/MTX, NPs/MTX/siRNA and free NPs compared to untreated cells and purified STAT3 protein from HeLa cells as the controls. The western blot results showed that the treated samples with both MTX and siRNA double loading in chMSNs and MTX-loaded chMSNs substantially decreased the STAT3 protein ().
Figure 5. Western blot results of the presence of STAT3 protein. The numbers were implied to (1) purified STAT3 protein from HeLa cells as the controls, the other samples were pointed to the STAT3 protein from the MCF7 cells, treated with (2) STAT3 siRNA/NPs, (3) free NPs, (4) MTX/NPs, (5) MTX/siRNA/NPs, (6) free NPs and (7) untreated cells as the second controls (8) GAPDH protein as the internal control. NPs: MSN-APTES-chitosan.

Cellular uptake analysis
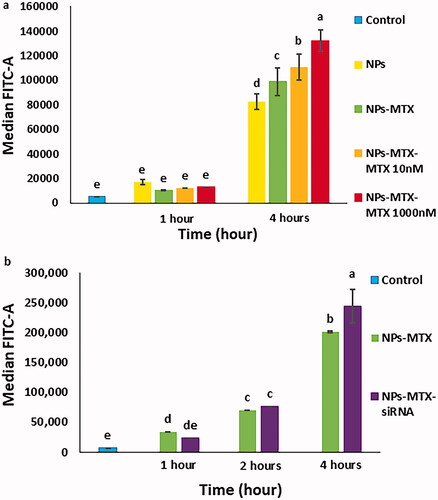
MTX and siRNA were loaded at FITC-labelled MSN-APTES-chitosan and thereafter the cellular uptake of the complexes by MCF7 cells was analysed using flow cytometry and confocal microscopy. shows that the uptake of NPs with or without MTX was increased either by the exposure time or by the addition of extra free MTX. The flow cytometry results in also indicated that the cellular uptake of NPs with MTX or with both MTX and siRNA was increased with time. Moreover, the largest uptake was seen for co-delivery of MTX and siRNA after 4 h. The overall larger fluorescence observed in versus likely related to a difference in fluorescence labelling between the NP batches used in these experiments.
Figure 6. Flow cytometry analysis of MCF7 cells treated with (a) FITC-labelled NPs with or without MTX as well as the cells exposed to FITC-labelled NPs/MTX with extra 10 and 1000 nM of free MTX added for 1 or 4 h, compared to untreated cells. (b) FITC-labelled NPs/MTX and FITC-labelled NPs/MTX/STAT3 siRNA after 1, 2 and 4 h, compared to untreated cells. NPs: MSN-APTES-chitosan. Different letters indicate significant differences in mean values for each variable (p<.05).
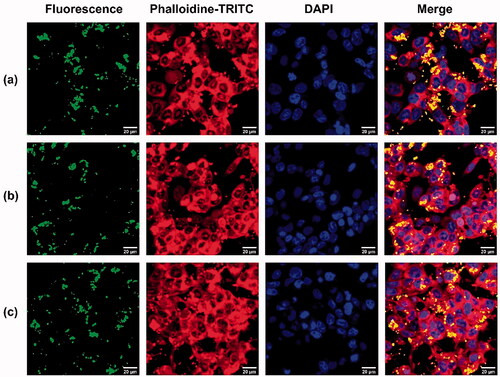
The cellular uptake and intracellular distribution of the NPs with or without loading drugs were analysed using confocal microscopy. After 4 h of incubation of MTX-loaded NPs, MTX/STAT3 siRNA-loaded NPs and free NPs with MCF7 cells, increased intracellular FITC fluorescence was observed in the cytoplasm, the most obvious for the cells treated with MTX/STAT3 siRNA-loaded NPs (). These results were in line with the results from flow cytometry.
Figure 7. Confocal microscopy images of MCF7 cells (a) MCF7 cells treated with NPs/FITC, (b) MCF7 cells treated with MTX/NPs/FITC, (c) MCF7 cells treated with MTX/siRNA/NPs/FITC at 37 °C for 4 h. FITC was shown as green fluorescence, actin filaments (red) were stained with phalloidin-TRITC and cell nuclei (blue) were stained with DAPI. NPs: MSN-APTES-chitosan.
Protein corona analysis
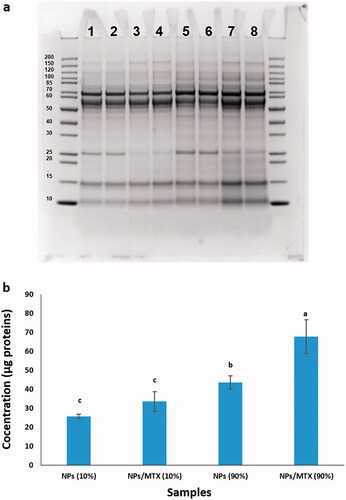
Protein coronas formed on the chMSNs surfaces with or without MTX after incubation in 10% or 90% of FBS were analysed by SDS-PAGE and the BCA assay. The results presented in , indicated a broad distribution of proteins that interacted with free NPs and MTX/NPs. Specific bands showed a major contribution of proteins around 10, 15, 25, 55 and 70 kDa. The overall amount of protein was increased for a higher percentage of FBS. In addition, the total protein band frequency was increased by the presence of MTX, more clear for the 90% FBS samples, while the amount of the proteins at around 25 kDa was decreased. This band has been related to apolipoprotein A-I-like protein [Citation35]. The results of the BCA assay are shown in , indicating that the total values of protein corona were higher for the increased percentage of FBS or with the presence of MTX. The BCA assay data confirmed the SDS-PAGE analysis.
Figure 8. Protein corona analysis. (a) SDS-PAGE gel electrophoresis. The first and end lines implied two repeats of the markers. The adsorbed proteins on the surface of (1 and 2) two repeats of NPs incubated in 10% of FBS, (3 and 4) two repeats of MTX/NPs incubated in 10% of FBS, (5 and 6) two repeats of NPs incubated in 90% of FBS and (7 and 8) two repeats of MTX/NPs incubated in 90% of FBS. The intensity of the bands revealed the abundance and relative composition of the protein corona that covered the surface of the nanoparticles. (b) BCA assay analysis of the total values of protein corona. The adsorbed proteins on the surface of NPs incubated in 10% of FBS, MTX/NPs incubated in 10% of FBS, NPs incubated in 90% of FBS, and MTX/NPs incubated in 90% of FBS. NPs: MSN-APTES-chitosan. Different letters indicate significant differences in mean values for each variable (p<.05).
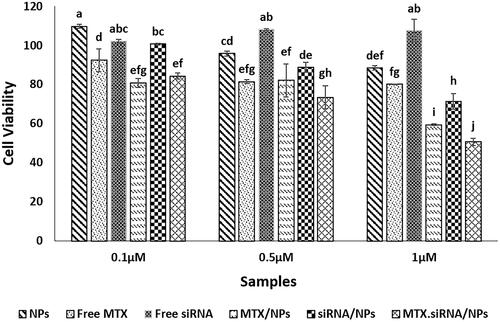
Cell cytotoxicity assay
An MTT assay was used to analyse the cytotoxicity of MTX/siRNA-loaded NPs to breast cancer cells. The cytotoxicity for cells treated with MTX/siRNA-loaded NPs was significantly higher than others at 1 µM concentration of MTX (), giving a viability of MCF7 cells around 50% while the value for free NPs was around 88%. Co-delivery of MTX and STAT3 siRNA showed a stronger effect than the delivery of either MTX or STAT3 siRNA at this concentration suggesting that co-delivery of MTX and STAT3 siRNA is a more efficient method for use in the treatment of breast cancer cells. Higher overall concentrations showed a lower cytotoxicity for both free MTX and the MTX/siRNA NPs (see supplementary figure).
Discussion
In this study, co-delivery of MTX and STAT3 siRNA by chMSNs was revealed as an efficient method for inducing cytotoxicity in breast cancer cells and with a potential for use in breast cancer treatment. One of the major challenges in NP based drug delivery relates to maintaining the particle size of the final functionalized delivery vehicle between 10 and 100 nm, as the ideal size for EPR effects [Citation33,Citation36]. Importantly in this study, the size of the synthesized MSNs could be decreased below 100 nm using Pluronic F127 () and kept below 100 nm during subsequent functionalization with APTES, chitosan (). Surface functionalization of MSNs by APTES and chitosan molecules was confirmed by zeta potential analysis (). Chitosan as a positive biopolymer was chosen to give the potential for the pH-sensitive properties of the NPs [Citation37]. In addition, the surface positive charge of chMSNs was the effective factor in drug loading due to electrostatic interactions with negatively charged drug molecules.
MTX and siRNA with a negative charge in physiological pH can be attracted to the surface of chMSNs with the loading capacity of MTX and siRNA being around 12% and 1%, respectively (, ). Several studies have shown that the drugs and biomolecule loading capacity of functionalized MSNs could be significantly improved compared to un-functionalized NPs [Citation38,Citation39]. Recently, different nanocarriers have been studied for co-delivery of various types of drugs and nucleic acids [Citation40]. Meng et al. [Citation17] reported that the doxorubicin and the Pgp siRNA loading capacity of PEI-modified MSNs was about 3.3% (w/w) and 1%, respectively. The high loading capacity for MTX achieved here indicates that the mesopores of NPs have been largely filled with the drug molecules providing an effective loading. The loading step with siRNA gives a smaller loading as expected as the siRNA will be unable to enter the mesopores because of its larger size and likely remains on the outer surface. The MTX also appears to functionalize the outer surface since the adsorption of proteins (too large to enter the mesopores) from the media is somewhat altered ().
Co-delivery of all drug components by modified NPs improved cellular uptake which simultaneously led to enhanced the cellular uptake of siRNA-loaded NPs [Citation41].
Cellular uptake of FITC-labelled chMSNs with or without MTX that was measured by flow cytometry analysis was increased with both MTX/NPs exposure time and also by the addition of additional free MTX values (). Increasing the dose of the MTX leads to overexpression and the increase of the levels of DHFR on the surface of cancer cell membranes [Citation13]. Hence, we propose that the uptake of MTX/NPs resulted at least in part from receptor mediated interactions with DHFR explaining the increased cellular uptake of MTX-loaded chMSNs compared to chMSNs after 4 h. However, the main uptake route likely does not involve DHFR. Moreover, the values of cell uptake were enhanced using both MTX and siRNA-delivered by NPs (). The results of confocal microscopy were also confirmed the flow cytometry data and show that MTX loading leads to more internalization of the NPs ().
MTX-loaded chMSNs were exposed to different FBS concentration (10% and 90%) and were then analysed by SDS-PAGE and the BCA assay. The lower concentration of FBS is routinely used in cell culture studies while the 90% FBS media better represents the concentrations of protein expected in vivo (albeit without the full set of plasma proteins). The protein corona contains a limited set of the FBS proteins which are up-concentrated from the media as has been shown for other particle types and indicating that after exposure to proteins the NP gains a specific protein corona profile, the so called biological identity proposed to modulate cellular uptake [Citation42,Citation43]. The highly positive surface charge of the NPs allows it to interact strongly with the most net negatively charged serum proteins. BSA (mass ∼66 kDa protein) appears to be well represented in that corona likely as a result of its high bulk concentration allowing it to interact with the NP early with the electrostatic attraction preventing it from being replaced by other serum proteins. Interestingly, the total amount of protein was increased for MTX loading particles compared to unloaded NPs (), suggesting that the interaction of proteins was enhanced by the presence of MTX. Shahabi et al. [Citation44] reported that endocytic uptake of doxorubicin-loaded silica NPs that were covered with protein corona facilitated the cellular accumulation of the drug. In the current study, in the MTX-loaded chMSNs samples, the presence of proteins around 25 kDa was significantly decreased compared to free chMSNs. This band was related to apolipoprotein A-I-like protein [Citation45]. Apo A1 is seen to be part of the protein coronas of many NP types and in particular at bare silica NPs. This indicates that the MTX may prevent interaction of the Apo A1 with the core particle surface and perhaps alter the availability of the chitosan for other proteins. Ritz et al. [Citation46] showed that the cellular uptake of the NPs decorated with some apolipoproteins was significantly decreased. Therefore, a decrease in this protein value due to the presence of the MTX may explain the observed increase of the cellular uptake of the MTX/chMSNs compared to unloaded NPs.
The results of RT-PCR to analyse the effect of siRNA-loaded NPs on STAT3 mRNA expression, compared to free siRNA, showed that the adsorption of the siRNA to the NPs may preserve it from the effects of endonuclease enzymes [Citation47]. Transfection of STAT3 siRNA leads to degradation of the STAT3 mRNA and inhibits breast cancer cell division and proliferation [Citation48]. Western blot data showed that the STAT3 protein levels were decreased due to treatment of cells with MTX/NPs and MTX/STAT3 siRNA/NPs (). Although co-delivery of MTX and STAT3 siRNA by chMSNs caused a greater reduction in STAT3 protein content, the use of MTX/NPs also significantly reduced the protein compared to the control sample, some metabolic pathways, especially the JAK/STAT pathway, were shown to be suppressed by MTX [Citation49,Citation50]. Therefore, the presence of the MTX in the MTX/STAT3 siRNA/chMSNs can cause the additive effect of MTX and STAT3 siRNA on reducing STAT3 protein content.
The results of cytotoxicity effects demonstrated that co-delivery of MTX and siRNA by chMSNs at the concentration of 1.0 µM of MTX had significant effects (p<.05) on cancer cell division and proliferation after 24 h. In addition, the cell cytotoxicity data revealed that the best “synergistic” effect was seen in higher MTX concentration, where the siRNA concentration was also higher, and this effect was not significant at the lowest concentration of MTX (0.1 µM) as well as lowest siRNA concentration (). The MTX not only inhibits DHFR but can also suppress the JAK/STAT pathway and decreased the STAT3 protein levels [Citation9,Citation14]. Moreover, the presence of STAT3 siRNA and cleavage of the STAT3 mRNA led to decrease in the amounts of STAT3 proteins in tumour cells [Citation15]. Therefore, the lack of this protein due to the additive effects of MTX and STAT3 siRNA, likely contributed to the reduction of cell division, proliferation and survival.
Our study showed that the co-delivery of MTX and STAT3 siRNA loaded in the chMSNs in a better concentration dose resulted in a greater cellular uptake and a clear decrease in the viability of MCF7 cells. This co-delivery system is of interest for use in the development of breast cancer therapies.
Conclusions
Our study indicates the synthesis of MSNs and functionalization of the NPs with positively charge materials containing amine groups. The amine modified MSNs with uniform spherical morphology and nano-scale size were used for high loading of two anticancer agents including MTX and STAT3 siRNA. The co-delivery of the MTX/siRNA/NPs to the MCF7 cells was monitored by cellular uptake. Increasing the dose of the MTX leads to DHFR overexpression and an increase in cellular uptake. In addition, the abundance of the protein corona coated at the NPs could potentially change cellular uptake. The total amounts of protein corona on the surface of chMSNs were increased by the presence of MTX on the NPs. Cell cytotoxicity data indicated that the co-delivery of MTX and STAT3 siRNA by chMSNs, tested on the MCF7 cell lines (at 1.0 µM of MTX) had significant effects on cancer cell division and proliferation and reduced the viability of breast cancer cells. Co-delivery of MTX and STAT3 siRNA by this effective nano-scale NP makes them of high interest for application in breast cancer.
Supplemental Material
Download MS Excel (88.3 KB)Supplemental Material
Download TIFF Image (211.7 KB)Supplemental Material
Download PNG Image (59 KB)Supplemental Material
Download TIFF Image (733.2 KB)Supplemental Material
Download MS Word (2.9 MB)Acknowledgements
The kind support of the Nanobiointerfaces group at iNANO is acknowledged.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
- Dong K, Liu Z, Li Z, et al. Hydrophobic anticancer drug delivery by a 980 nm laser-driven photothermal vehicle for efficient synergistic therapy of cancer cells in vivo. Adv Mater. 2013;25(32):4452–4458.
- Li ZY, Hu JJ, Xu Q, et al. A redox-responsive drug delivery system based on RGD containing peptide-capped mesoporous silica nanoparticles. J Mater Chem B. 2015;3(1):39–44.
- Wang Y, Zhao Q, Han N, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine. 2015;11(2):313–327.
- Seo JW, Ang J, Mahakian LM, et al. Self-assembled 20-nm (64)Cu-micelles enhance accumulation in rat glioblastoma. J Control Release. 2015;220(Pt A):51–60.
- Chen Y, Chen H, Shi J. Inorganic nanoparticle-based drug codelivery nanosystems to overcome the multidrug resistance of cancer cells. Mol Pharm. 2014;11(8):2495–2510.
- Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem Res Toxicol. 2012;25(11):2265–2284.
- Chen AM, Zhang M, Wei D, et al. Co-delivery of doxorubicin and bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small. 2009;5(23):2673–2677.
- Saikia J, Yazdimamaghani M, Hadipour Moghaddam SP, et al. Differential protein adsorption and cellular uptake of silica nanoparticles based on size and porosity. ACS Appl Mater Interfaces. 2016;8(50):34820–34832.
- Gutierrez JC, Hwang K. The toxicity of methotrexate in male fertility and paternal teratogenicity. Expert Opin Drug Metab Toxicol. 2017;13(1):51–58.
- Zhang Y, Li Y, Tian H, et al. Redox-responsive and dual-targeting hyaluronic acid-methotrexate prodrug self-assembling nanoparticles for enhancing intracellular drug self-delivery. Mol Pharm. 2019;16(7):3133–3144.
- Giudicessi JR, Ackerman MJ. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl Res. 2013;161(1):1–14.
- Banerjee D, Mayer-Kuckuk P, Capiaux G, et al. Novel aspects of resistance to drugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta Mol Basis Dis. 2002;1587(2–3):164–173.
- Chiarle R, Simmons WJ, Cai H, et al. Stat3 is required for ALK-mediated lymphomagenesis and provides a possible therapeutic target. Nat Med. 2005;11(6):623–629.
- Gao L-F, Xu D-Q, Wen L-J, et al. Inhibition of STAT3 expression by siRNA suppresses growth and induces apoptosis in laryngeal cancer cells. Acta Pharmacol Sin. 2005;26(3):377–383.
- Hanafi-Bojd MY, Ansari L, Malaekeh-Nikouei B. Codelivery of anticancer drugs and siRNA by mesoporous silica nanoparticles. Ther Deliv. 2016;7(9):649–655.
- Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7(2):994–1005.
- Cauda V, Schlossbauer A, Bein T. Bio-degradation study of colloidal mesoporous silica nanoparticles: effect of surface functionalization with organo-silanes and poly (ethylene glycol). Microporous Mesoporous Mater. 2010;132(1–2):60–71.
- Walcarius A, Etienne M, Lebeau B. Rate of access to the binding sites in organically modified silicates. 2. Ordered mesoporous silicas grafted with amine or thiol groups. Chem Mater. 2003;15(11):2161–2173.
- Sun JT, Hong CY, Pan CY. Fabrication of PDEAEMA-coated mesoporous silica nanoparticles and pH-responsive controlled release. J Phys Chem C. 2010;114(29):12481–12486.
- Li X, Chen Y, Wang M, et al. A mesoporous silica nanoparticle-PEI-fusogenic peptide system for siRNA delivery in cancer therapy. Biomaterials. 2013;34(4):1391–1401.
- Hao N, Jayawardana KW, Chen X, et al. One-step synthesis of amine-functionalized hollow mesoporous silica nanoparticles as efficient antibacterial and anticancer materials. ACS Appl Mater Interfaces. 2015;7(2):1040–1045.
- Tan WB, Zhang Y. Surface modification of gold and quantum dot nanoparticles with chitosan for bioapplications. J Biomed Mater Res A. 2005;75(1):56–62.
- Lv G, Qiu L, Liu G, et al. pH sensitive chitosan–mesoporous silica nanoparticles for targeted delivery of a ruthenium complex with enhanced anticancer effects. Dalton Trans. 2016;45(45):18147–18155.
- Nhavene E, Andrade G, Faria J, et al. Biodegradable polymers grafted onto multifunctional mesoporous silica nanoparticles for gene delivery. ChemEngineering. 2018;2(2):24.
- Lin J-T, Liu Z-K, Zhu Q-L, et al. Redox-responsive nanocarriers for drug and gene co-delivery based on chitosan derivatives modified mesoporous silica nanoparticles. Colloids Surf B Biointerfaces. 2017;155:41–50.
- Rosenholm JM, Peuhu E, Bate LT, et al. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small. 2010;6(11):1234–1241.
- Rasouli S, Davaran S, Rasouli F, et al. Synthesis, characterization and pH-controllable methotrexate release from biocompatible polymer/silica nanocomposite for anticancer drug delivery. Drug Deliv. 2014;21(3):155–163.
- Shakeran Z, Keyhanfar M, Varshosaz J, et al. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater Sci Eng C Mater Biol Appl. 2021;118:111526.
- Meng H, Xue M, Xia T, et al. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano. 2011;5(5):4131–4144.
- Mohammad-Beigi H, Yaghmaei S, Roostaazad R, et al. Effect of pH, citrate treatment and silane-coupling agent concentration on the magnetic, structural and surface properties of functionalized silica-coated iron oxide nanocomposite particles. Physica E. 2011;44(3):618–627.
- Meng H, Liong M, Xia T, et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4(8):4539–4550.
- Mooney CM, Jimenez-Mateos EM, Engel T, et al. RNA sequencing of synaptic and cytoplasmic Upf1-bound transcripts supports contribution of nonsense-mediated decay to epileptogenesis. Sci Rep. 2017;7:41517.
- Tarn D, Ashley CE, Xue M, et al. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc Chem Res. 2013;46(3):792–801.
- Pedraza D, Díez J, Colilla M, et al. Amine-functionalized MSNs: a new nanoantibiotic for bone infection treatment. Biomed Glass. 2018;3:1–12.
- Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63(3):170–183.
- Lavrič PK, Warmoeskerken MM, Jocic D. Functionalization of cotton with poly-NiPAAm/chitosan microgel. Part I. Stimuli-responsive moisture management properties. Cellulose. 2012;19(1):257–271.
- Hartono SB, Qiao SZ, Liu J, et al. Functionalized mesoporous silica with very large pores for cellulase immobilization. J Phys Chem C. 2010;114(18):8353–8362.
- Lei C, Shin Y, Magnuson JK, et al. Characterization of functionalized nanoporous supports for protein confinement. Nanotechnology. 2006;17(22):5531–5538.
- Kuruba R, Wilson A, Gao X, et al. Targeted delivery of nucleic-acid-based therapeutics to the pulmonary circulation. AAPS J. 2009;11(1):23–30.
- Briggs L, Russell W. 107. Sophora alkaloids. Part IV. The alkaloids from the seeds of the Chatham Islands species. J Chem Soc. 1942;555–556.
- Monteiro-Riviere NA, Samberg ME, Oldenburg SJ, et al. Protein binding modulates the cellular uptake of silver nanoparticles into human cells: implications for in vitro to in vivo extrapolations? Toxicol Lett. 2013;220(3):286–293.
- Choi K, Riviere JE, Monteiro-Riviere NA. Protein corona modulation of hepatocyte uptake and molecular mechanisms of gold nanoparticle toxicity. Nanotoxicology. 2017;11(1):64–75.
- Shahabi S, Treccani L, Dringen R, et al. Utilizing the protein corona around silica nanoparticles for dual drug loading and release. Nanoscale. 2015;7(39):16251–16265.
- Clemments AM, Muniesa C, Landry CC, et al. Effect of surface properties in protein corona development on mesoporous silica nanoparticles. RSC Adv. 2014;4(55):29134–29138.
- Ritz S, Schöttler S, Kotman N, et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16(4):1311–1321.
- Shen J, Kim HC, Su H, et al. Cyclodextrin and polyethylenimine functionalized mesoporous silica nanoparticles for delivery of siRNA cancer therapeutics. Theranostics. 2014;4(5):487–497.
- Zhang W, Fang Y, Shi X, et al. Effect of bisphenol a on the EGFR-STAT3 pathway in MCF-7 breast cancer cells. Mol Med Rep. 2012;5(1):41–47.
- Uchihara Y, Komori R, Tago K, et al. Methotrexate significantly induces apoptosis by inhibiting STAT3 activation in NPM-ALK-positive ALCL cells. Biochem Pharmacol. 2019;170:113666.
- Gremese E, Alivernini S, Tolusso B, et al. JAK inhibition by methotrexate (and csDMARDs) may explain clinical efficacy as monotherapy and combination therapy. J Leukoc Biol. 2019;106(5):1063–1068.