Abstract
The increasing emergence of bacterial resistance is a challenge for the research community, thus novel antibacterial agents should be developed. Metal nanoparticles are promising antibacterial agents and could solve the problem of antibiotic resistance. Herein, we used Gardenia thailandica methanol extract (GTME) to biogenically synthesise zinc oxide nanoparticles (ZnO-NPs). The characterisation of ZnO-NPs was performed by UV spectroscopy, FTIR, scanning and transmission electron microscopes, dynamic light scattering, and X-ray diffraction. The antibacterial activity of ZnO-NPs was studied both in vitro and in vivo against Pseudomonas aeruginosa clinical isolates. Its minimum inhibitory concentration values ranged from 2 to 64 µg/mL, and it significantly decreased the membrane integrity and resulted in a significant increase in the inner and outer membrane permeability. Also, the ZnO-NPs treated cells possessed a distorted and deformed shape when examined by scanning electron microscope. The in vivo study (biochemical parameters and histological investigation) was conducted and it revealed a protective effect of ZnO-NPs against the deleterious influences of P. aeruginosa bacteria on lung, liver, and kidney tissues. LC-ESI-MS/MS revealed a phytochemical tentative identification of 57 compounds for the first time. We propose that GTME is a useful source for ZnO-NPs which has a promising antibacterial activity.
Graphical Abstract

Introduction
Nanotechnology is termed as it involves the synthesis, characterisation, study, and application of materials with nano-size in the different aspects of science and technology [Citation1]. Currently, nanotechnology can be used in novel and miscellaneous applications ranging from food processing, innovative fabric compounds, agricultural industry, and sophisticated medical applications [Citation2]. The nanoscale of the nanoparticles can confer relatively larger surface areas when compared to the macro-sized particles [Citation3]. Nevertheless, it is reported that the chronic use of nanoparticles could have a deleterious impact as it can cause DNA damage [Citation4].
Metal oxide nanoparticles have many valuable properties and are believed to be a promising tool that has various applications. It can be synthesised by different methods, however, the synthesis process of nanoparticles has many deleterious effects due to the utilisation of toxic chemicals that act as reducing agents [Citation5]. The development of nanomaterials using natural bio-resources as eco-friendly, safe, inexpensive, and viable alternatives to chemical-based synthesis is acquiring an amount of confidence in manufacturing nanomaterials. Mostly, Chemical approaches, result in the adsorption of harmful substances on particle surfaces, which can have negative health consequences [Citation6,Citation7]. Therefore, the usage of natural agents like plant extracts can solve the problem of toxicity that originated when chemical synthesis was applied [Citation8]. The reduction process occurs by the bioactive phytochemicals that are present in the plants [Citation9] and the so-called biogenic method [Citation10,Citation11]. As a result, the biogenic/bio-inspired technique of employing plants to synthesise nanoparticles has been considered as a green route [Citation12].
Gardenia thailandica Triveng. is a flowering plant native to Thailand. Gardenia is a genus of flowering plants in the Rubiaceae family that is native to Africa, Madagascar, Asia, the Pacific Islands, and Australia’s tropical and subtropical areas [Citation13]. Gardenia species have anti-cancer, anti-HIV, antibacterial, antiangiogenic, anti-apoptotic, and thrombolytic properties [Citation14].
Among metal nanoparticles, zinc oxide nanoparticles (ZnO-NPs) is considered to be a promising inorganic substance that possess a relatively small particle size, a property that makes its absorption is easier by the human body. This is in addition to its safety; therefore, ZnO-NPs are commonly used as an anticancer, antimicrobial, antidiabetic, and anti-inflammatory compounds [Citation15].
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogenic bacterium. It may cause a wide range of life-threatening infections either acute or chronic. Its infections are relatively more abundant in patients with immunocompromised defences [Citation16]. It is one of the major nosocomial pathogenic bacteria that affect hospitalised patients. It possesses many variable virulence factors and it is intrinsically resistant to a wide range of currently existing antibiotics [Citation17]. Thus, many efforts are seeking an effective antimicrobial compound against these problematic bacteria.
Antibiotic-resistant bacterial isolates rapidly appear in a quite short period of time when new antibiotics are developed. Metal nanoparticles could be a solution for antibiotic resistance as an overwhelming number of studies have documented and reported their strong antimicrobial activity. In addition, it is assumed that metal nanoparticles have the potential to decrease or eliminate the evolution of resistance among bacteria as they can target multiple bacterial biomolecules at once [Citation18].
This study aimed to examine the in vivo and in vitro antibacterial effect of ZnO-NPs developed by the methanol extract of G. thailandica against P. aeruginosa isolates. Besides, the phytochemical constituents are characterised using LC-MS/MS technique for the first time.
Results
LC-ESI-MS/MS of GTME
The metabolites in G. thailandica leaves methanol extract (GTME) were identified by LC-MS/MS. A total of 57 compounds were tentatively identified in GTME using LC-ESI-MS/MS in a negative ion mode. The major compounds belong to diverse subclasses such as phenolic, flavonoids, iridoids, tannins, coumarins, and organic acids. The complete profiling was presented in and the total ion chromatogram (TIC) of GTME was displayed in showed the major identified compounds in negative ion mode such as hesperidin at retention time 6.264, isoquercitrin at 6.664, kaempferol-3-O-(6-p-coumaroyl)-glucoside at 7.025, chlorogenic acid at 1.444, and E-3,4,5′-Trihydroxy-3′-glucopyranosylstilbene at 9.589. Characterisation of different compounds was presented in Supplementary data.
Figure 1. The total ion chromatogram of GTME showed the major identified compounds by LC-ESI-MS/MS in negative ion mode.
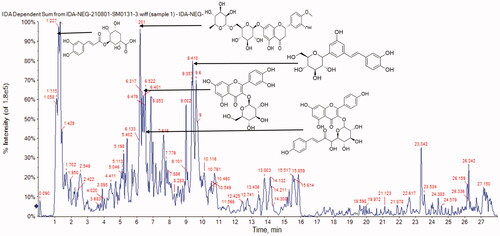
Table 1. Phytochemical profiling of GTME by LC-ESI-MS/MS in negative mode.
Characterisation of ZnO-NPs
UV spectroscopy
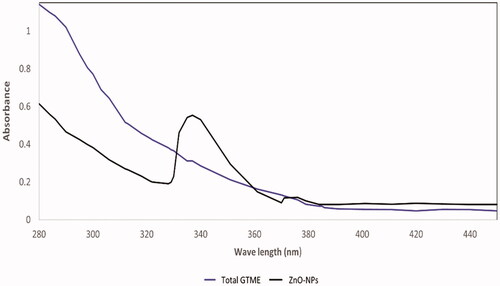
The maximum absorption peak of ZnO-NPs synthesised using GTME was 338.26 nm, demonstrating the synthesis of ZnO in a nano form () [Citation19]. ZnO in a nano form nanoscale has shorter absorption wavelengths than normal ZnO [Citation20]. The UV absorbance bands are an indication of the surface plasmon resonance (SPR) of the formed nanoparticles at the same absorbance with minimum shifts of 2–4 nm according to shape and size of the nanoparticles, This finding is consistent with previous findings, which showed that the absorption peak of zinc oxide nanoparticles was observed around 350 and 360 nm. The colour change of GTME from green solution to yellowish-white transparent solution and a white ppt of ZnO-NPs is another evidence of nano formation [Citation21].
Fourier-transform infra-red (FTIR)
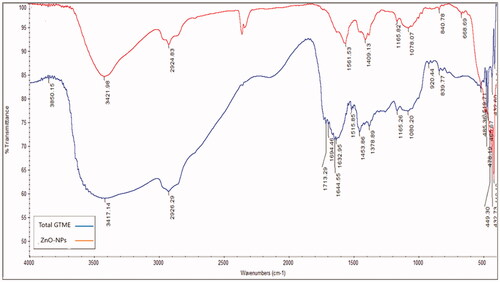
The active reactant of the phytometabolites of GTME which played a role in the formation of ZnO-NPs was studied using infra-red absorption in the range of 400–4000 cm1 (). Results were displayed in Supplementary data.
Zeta potential and dynamic light scattering (DLS)
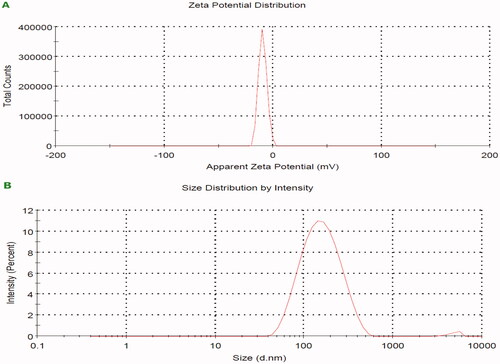
A size-distribution image as dynamic light scattering (DLS) ZnO-NPs intermediated by GTME is represented in . The detected distribution of ZnO-NPs size varied from 146 to 157 nm with a PDI value of 0.218. The ZnO-NPs’ zeta potential was observed to have a peak at −10.6 mV, signifying that the Nano-ZnO particles biosynthesized were negatively charged and moderately distributed in the medium. Generally, the negative sign of zeta potential is considered a good indication of colloidal stability with enhanced repulsion force ensuring optimal nanoparticles dispersibility. The value of zeta potential is highly dependent on individual ingredients composing the core/shell nanoparticles. Low PDI value indicates homogenous nanodispersion with optimum size distribution with no risk for aggregation.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) analysis
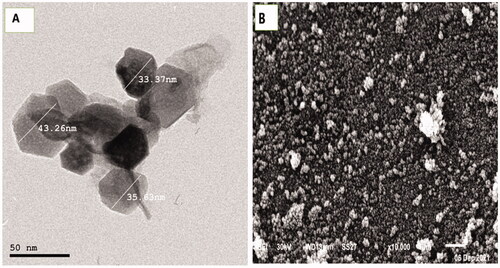
TEM examination using high-resolution power electron microscopy revealed the production of ZnO-NPs with an average particle size of 37.42 nm, as shown in . ZnO-NPs were inspected by SEM to examine their surface morphology. It showed that most of the ZnO-NPs were spherical with an average size of 58 ± 6.88 nm (). SEM analysis illustrated the ZnO-NPs that were dispersed moderately in the medium. The hydrodynamic size and the ligand shell of the produced nanoparticles are assessed by dynamic light scattering, which is distinct from the, which only measures the metallic core.
X-Ray diffraction (XRD) analysis
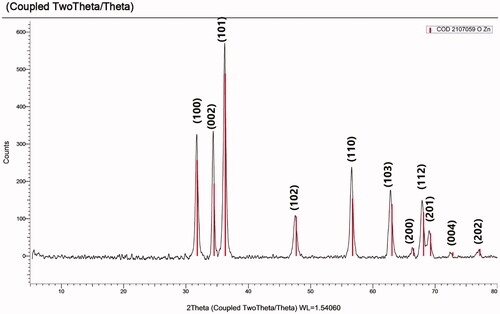
XRD is a tool for assuring the crystallography nature of the formed ZnO-NPS. Herein, the biosynthesized ZnO-NPs exhibited peaks with 2θ values at 31.850, 34.552, 36.358, 47.699, 56.752, 63.094, 66.565, 68.170, 69.290, 72.876, and 77.214 corresponding to (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202), respectively (). Crystal purity confirmation of the biosynthesized ZnO-NPs was proved by the absence of any other peaks in the XRD chart that do not belong to ZnO-NPs [Citation22]. These peaks were observed in a crystal system of hexagonal phase with a space group of P63mc (186) and reference code of (COD 2107059).
In vitro antibacterial activity
Membrane integrity
demonstrates an illustrative example. Results were displayed in Supplementary data.
Membrane permeability
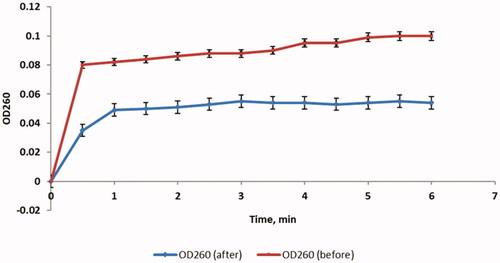
As the inner membrane permeability increases, the entry of O-nitrophenyl-β-galactopyranoside (ONPG) into the bacterial cytoplasm increases. After being in the cytoplasm, the β-galactosidase enzyme converts ONPG into O-nitrophenol (ONP). Consequently, the permeability of the inner membrane was tracked by measuring the absorbance at 420 (OD420) over time as ONP has a yellow colour that can absorb at 420 nm. We observed that there was a significant increase (p < .05) in the inner membrane permeability in 53.33% of P. aeruginosa isolates after treatment with ZnO-NPs and a demonstrative example is shown in .
Figure 8. Charts illustrating the effect of treatment with ZnO-NPs on A) the inner membrane permeability and B) the outer membrane permeability of a representative P. aeruginosa isolate.
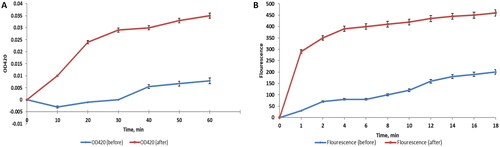
We studied the outer membrane permeability by measuring the fluorescence of 1-N-phenylnaphthylamine (NPN) by a spectrofluorophotometer. The fluorescence of the hydrophobic compound, NPN, can be detected in the hydrophobic region of the cell membrane. We found that there was a substantial increase (p < .05) in the outer membrane permeability in 56.67% of the isolates after treatment with ZnO-NPs and a representative example is exhibited in .
SEM
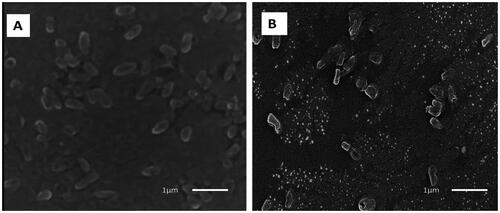
The changes that occurred in the morphology and ultrastructure of P. aeruginosa cells treated with the green synthesised ZnO-NPs were examined by SEM (). The electron micrographs acquired by SEM showed that the untreated cells had rod-shaped with intact smooth outer surfaces. On contrary, the ZnO-NPs treated cells possessed a distorted and deformed shape.
In vivo antibacterial activity
Biochemical investigation
The biochemical analysis presented that there was a substantial difference (p > .05) between the ZnO-NPs treated infected group, the positive control group, and the gentamicin treated infected group in the white blood cells (WBCs) count, red blood cells (RBCs) count, haemoglobin (Hb), total protein, albumin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea, and serum creatinine as listed in .
Table 2. Impact of ZnO-NPs on different biochemical markers of the infected rats.
Histological assessment
Lung
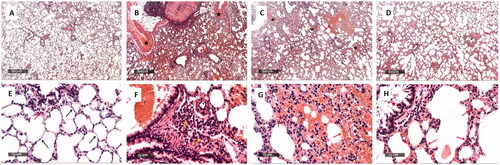
The samples of the positive control exhibited obvious intact histological characteristics of pulmonary parenchyma with almost apparent intact alveolar epithelium. In addition, minimal inflammatory cells infiltrate and normal vasculatures were observed ( and ). On the other hand, the samples of the negative control revealed extremely diffused haemorrhagic pneumonia accompanied by a considerable thickening of the interalveolar wall. Also, there was abundant infiltration of the inflammatory cells in addition to marked congested and dilated pulmonary vasculatures ( and ). The samples of the ZnO-NPs treated infected group showed a reduction of the thickness of the interalveolar walls with minor persistence of the haemorrhagic patches and inflammatory cell infiltrates ( and ). The gentamicin-treated infected samples exhibited a significant amelioration of the infiltration of the inflammatory cells and the haemorrhagic patches ( and ).
Figure 10. Histological examination of the rat lung of: The positive control group (A and E) showed apparent intact histological features of pulmonary parenchyma with almost apparent intact alveolar epithelium with thin interalveolar septa (arrow). Intact bronchiolar structures with few peribronchiolar inflammatory cells infiltrate (red arrow).The negative control group (B and F) showed diffuse haemorrhagic pneumonia with significant thickening of the interalveolar wall and abundant inflammatory cell infiltrates. Marked congested and dilated pulmonary vasculatures (black star) accompanied with severe perivascular mixed inflammatory cells infiltrates (yellow arrow). Most bronchioles have luminal extravasation of blood (red star). The ZnO-NPs treated infected group (C and G) showed a reduction of the thickness of the interalveolar wall with minor persistence of haemorrhagic patches, inflammatory cells infiltrate (red arrow), and congested alveolar capillaries (yellow arrow). Furthermore, mildly congested blood vessels were revealed (black star). The gentamicin-treated infected group (D and H) showed a significant amelioration of the inflammatory cells infiltrates (red arrow) in addition to haemorrhagic patches with mild congested alveolar capillaries and scanty desquamated bronchiolar epithelium (red star). Minimal congested or dilated blood vessels were observed.
Liver
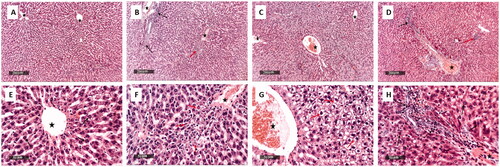
The samples of the positive control exhibited normal histological characteristics of liver parenchyma accompanied with many obviously intact well-organised hepatocytes and intact hepatic vasculatures ( and ). On the other hand, the samples of the negative control revealed multiple precentral focal areas of hepatocellular necrosis with infiltration of inflammatory cells and moderate dilatation and congestion of hepatic blood vessels ( and ). The samples of the ZnO-NPs treated infected group showed mild dilatation of hepatic blood vessels accompanied with few lobular vacuolar degenerative changes and minimal records of inflammatory cells infiltrate ( and ). The gentamicin-treated infected samples exhibited more organised morphological features of the hepatic parenchyma with almost intact hepatocytes and mildly congested blood vessels were shown accompanied with scattered few focal areas of necrosis and few inflammatory cells infiltrate ( and ).
Figure 11. Histological examination of the rat liver of:The positive control group (A and E) showed normal histological features of the liver parenchyma with many apparent intact well-organized hepatocytes, intact subcellular details (arrow), and intact hepatic vasculatures (star).The negative control group (B and F) showed multiple pericentral focal areas of hepatocellular necrosis with mononuclear cells infiltrates (red arrow) with severe periportal mixed inflammatory cell infiltrates. In addition, there are abundant neutrophils (black arrow) and moderate dilatation and congestion of hepatic blood vessels (star).The ZnO-NPs treated infected group (C and G) showed mild dilatation of the hepatic blood vessels (star) with few lobular vacuolar degenerative changes (red arrow) and minimal records of inflammatory cells infiltrate.The gentamicin-treated infected group (D and H) showed well-organized morphological features of the hepatic parenchyma with almost intact hepatocytes (black arrow). Nevertheless, mildly congested blood vessels were observed (star) with scattered few focal areas of necrosis (red arrow) and mild inflammatory cell infiltrates (dashed arrow).
Kidney
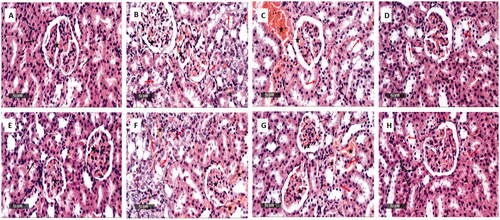
The samples of the positive control exhibited normal histological characteristics of renal parenchyma with obvious intact renal corpuscles, renal tubular segments, and renal vasculatures ( and ). On the other hand, the samples of the negative control revealed marked degeneration and necrosis in the tubular segments with many intraluminal cellulars depress accompanied by many congested blood vessels ( and ). The samples of the ZnO-NPs treated infected group revealed mild degenerated tubular epithelium as well as mild dilatation and congestion of the blood vessels ( and ). The gentamicin-treated infected samples exhibited scattered few tubular epithelial degenerative changes and apparent intact tubular segments with minimal congested blood vessels ( and ).
Figure 12. Histological examination of the rat kidney of: The positive control group (A and E) showed normal histological features of the renal parenchyma with apparent intact renal corpuscles (star), renal tubular segments with almost intact tubular epithelium (arrow).The negative control group (B and F) showed noticeable degenerated and necrotic tubular segments with many intraluminal cellular depress (red arrow) with many congested intertubular blood vessels as well as glomerular tufts (star). The ZnO-NPs treated infected group (C and G) showed mild degenerated tubular epithelium (red arrow) and mild dilatation and congestion of the intertubular blood vessels (star). The gentamicin-treated infected group (D and H) showed scattered few tubular epithelial degenerative changes (red arrow) with apparent intact tubular segments (black arrow) and minimal congested intertubular blood vessels.
Discussion
The vast spread of P. aeruginosa as a human and environmental pathogen is attributed to its ability to resist a wide range of antibiotics, its capability to withstand different environmental conditions, and its various virulence factors. Therefore, there is a high demand to find out new antimicrobials which can kill or inhibit the growth of this pathogenic microorganism without being affected by the resistance mechanisms. The scientific community has paid much attention to natural compounds, like plants, as a promising source for antimicrobials due to their high antimicrobial activity, low cost, high bioavailability, and low toxicity [Citation23]. In the present study, we investigated the antibacterial activity of the green synthesised ZnO-NPs against P. aeruginosa clinical isolates in vitro and in vivo.
LC/MS/MS chromatographic technique was used to identify the major GTME phytometabolites involved in the biological effects on the living human systems. A total of 57 compounds were tentatively recognised based on the base beak spectra and fragmentation patterns in the negative ion mode. The chemical classes of the identified phytometabolites are flavonoids and flavonoid glycosides such as hesperetin, apigenin, acacetin, luteolin, baicalein, naringenin, quercetin, kaempferol, isorhamnetin, and daidzein derivatives; phenolic acids, and their glycoside forms mainly as chlorogenic, caffeic, quinic, and rosmarinic acids; anthocyanidin glycoside such as cyanidin-3-glucoside, delphinidin-3-O-β-glucopyranoside, and delphinidin-3-O-(6′’-O-α-rhamnopyranosyl-β-glucopyranoside); and iridoids mainly geniposide, geniposide rhamnoside, 10-deoxygeniposide, coumaroyl geniposide, and 10-deoxygeniposidic acid.
In the current study, GTME was used for the biological synthesis of ZnO-NPs as it has reducing and capping factors involved in the formation of the ZnO-NPs. The formed ZnO-NPS were characterised using UV maximum absorption at 409.16 nm that proved the formation of the nanometal zinc oxide, FTIR was used to identify the functional groups of the GTME that contribute to the ZnO-NPs formation, other characterisation techniques were performed; TEM of ZnO-NPs revealed the production of hexagonal ZnO-NPs with an average particle size of 37.42 nm, while SEM was employed to inform the uniformity of surface structure of the biosynthesized ZnO-NPs. Moreover, the crystallography structure and the charge of ZnO-NPs were measured successfully by XRD and zeta potential, respectively. ZnO-NPs were successfully formed due to the role of the active phytoconstituents of GTME especially flavonoid glycosides and phenolic acids that reduced the zinc acetate to ZnO-NPs and inhibited the degradation, agglutination, or deformation of the formed nanoparticles. Our findings were also in line with earlier research on the antibacterial properties of various flavonoids or flavonoid glycosides [Citation24], iridoids [Citation25], phenolic acids [Citation26], and anthocyanins derivatives [Citation27].
The MIC values of ZnO-NPs against the tested P. aeruginosa isolates ranged from 2 to 64 µg/mL. To study the mechanism of this antimicrobial activity, we assessed the impact of ZnO-NPs on the membrane properties. The bacterial membrane is a major target for many antimicrobials as it is a selectively permeable powerful barrier. If this barrier is affected or disturbed, this will cause deleterious consequences on the bacterial cell and may lead to its death [Citation28]. So, we explored the integrity of the bacterial membrane before and after treatment with ZnO-NPs by tracking the leakage of the substances, that possess absorbance at 260 nm, over time. Interestingly, we observed that treatment with ZnO-NPs has led to a considerable reduction (p < .05) in the integrity of the bacterial membrane in 60% of the tested isolates.
Various techniques could be utilised for assessing the permeability of the bacterial inner and outer membranes. Herein, the ONPG method was utilised to study the impact of the green synthesised ZnO-NPs on the inner membrane permeability. This technique relies on the fact that the penetration of this compound into the bacterial cell increases when the bacteria start to lose control of their inner membrane permeability [Citation29]. In addition, we used the NPN technique for exploring the effect of ZnO-NPs on the outer membrane permeability. NPN is a fluorescent hydrophobic probe that usually exhibits a weak fluorescence in the case of an intact bacterial outer membrane. On the other hand, if the bacterial outer membrane is compromised, its penetration into the bacterial cells increases producing an increase in the quantum yield and fluorescence [Citation30]. Our results revealed that ZnO-NPs resulted in a considerable rise (p < .05) in the inner and outer membrane permeability of the tested bacterial cells in 53.33% and 56.67% of the isolates, respectively.
Taken together, there could be a synergism between the two mechanisms, the increase in the inner and outer membrane permeability of the tested P. aeruginosa isolates after ZnO-NPs treatment and the net result of these two mechanisms is the death of the bacterial cells. In general, it is assumed that nanoparticles have a relatively high surface to volume ratio than the bulk metallic counterparts, a property that enables them to interact with the bacterial cell membrane easily. It is documented that metallic nanoparticles have multiple targets to counteract bacterial activity. Metal oxide nanoparticles can exert their antibacterial activity mainly via interaction with the bacterial cell membrane causing its damage [Citation31]. Besides their ability to disturb the different metabolic processes of bacteria. Moreover, some studies have reported that the antibacterial activity of nanoparticles differs between Gram-positive and Gram-negative bacteria. This is due to the presence of a thick peptidoglycan layer in Gram-positive bacteria which serves as an additional barrier for penetration of nanoparticles [Citation32].
SEM analysis was performed to study the alterations in the bacterial cell morphology and ultrastructure, upon ZnO-NPs treatment. Therefore, we examined the tested bacteria by SEM to get benefit from its high magnification power and resolution in studying the morphological changes. Noteworthy, we observed that the bacterial isolates appeared with a distorted and deformed shape after treatment with ZnO-NPs when compared to the non-treated ones. A finding that confirms our results concerning the deleterious effects of ZnO-NPs on the membrane integrity as well as the inner and outer cell membrane permeability. ZnO-NPs exhibited a significant improvement in the biochemical markers as well as histological characters of lungs, liver, and kidney in the infected rats. These results are comparable to the findings of Hamouda et al. [Citation33].
Materials and methods
Plant materials and extract preparation
Gardenia thailandica Tirveng. Leaves were collected from a private garden on Egypt Alexandria desert road. The plant’s identity was confirmed by Dr/ Esraa Ammar, Plant Ecology, Botany Department, Faculty of Science, Tanta University. A voucher specimen (PGA-GT-128-W) was preserved in the herbarium of the Department of Pharmacognosy, Tanta Pharmacy. The powdered plant (600 g) was extracted with methanol using a cold maceration method (3 × 5 L). The extract was concentrated at reduced pressure to obtain a residue (7.89 g).
Drugs and chemicals
All of the chemicals and solvents used in this work were obtained from Sigma-Aldrich (St. Louis, MO, USA) and were of high analytical quality.
LC-Ms/MS profiling of GTME
GTME was reconstituted in a 50:25:25 mixture of distilled water, methanol, and acetonitrile, then it was analysed using an ExionLCTM AD UPLC and a TripleTOF 5600+ Time-of-Flight Tandem Mass Spectrometer (AB SCIEX). Adopting the criteria described previously [Citation34–36]. To identify compounds, PeakViewTM software was used to compare retention time and m/z values obtained by MS and MS2. The XIC Manager in PeakViewTM software was used to calculate the peak area values. Extracted ion chromatograms (XICs) for each targeted analyte were automatically created and compared to a user-defined threshold.
Green synthesis of ZnO-NPs by GTME and characterisation of the formed ZnO-NPs
ZnO-NPs were produced utilising GTME, as described by Attia et al with minor modifications [Citation37]. The GTME (1 g) dissolved in ethyl alcohol (100 ml) was allowed to react with zinc acetate (10 g), mixed in doubly distilled water (D.W.) (1 L), and heated in a boiling water bath for 30 min to produce ZnO-NPs, the reaction mixture temp. was adjusted at 70°C. The pH was then raised to 11 by adding 0.1 M sodium hydroxide (only a few drops), resulting in the formation of a white precipitate of ZnO-NPs. For complete reduction of zinc acetate to ZnO-NPs, the mixture was set for an hour at room temp. The produced ZnO-NPs were centrifuged at 4000 rpm for 10 min, then washed three times with D.W. and three times with ethanol to yield a 900 mg white powder of ZnO-NPs after freeze-drying. Characterisation of the formed ZnO-NPs was displayed in Supplementary data.
In vitro antibacterial activity was displayed in supplementary data
In vivo antibacterial activity
Animals
Twenty-eight white male albino rats (130–150 g and 8 weeks old) were obtained from the animal house at the faculty of Veterinary Medicine, Cairo University (Cairo, Egypt). They were permitted to adapt for one week before the start of the in vivo experiment under standard conditions of room temperature (25 ± 2 °C) and 12-h light/dark cycle. In addition, they were permitted access to standard food and filtered water.
Experimental design
Before the induction of the systemic infection in rats, they were weighted, then they were infected via subcutaneous injection of P. aeruginosa suspension (0.2 ml, 2.0 × 107 CFU/mL) for 2 days. The rats were randomly distributed into 4 groups (each containing 7 rats). They were grouped as follows: normal non-infected group (positive control), the untreated infected group (negative control), gentamicin treated infected group (2 mg/kg as standard drug), and ZnO-NPs treated infected group (5 mg/kg). All these groups were intra-peritoneally injected with the different treatments 24 h after the second subcutaneous bacterial injection for 20 days. At the end of the in vivo experiment, all rats were weighed and not permitted to eat overnight. They were then anaesthetised using diethyl ether and euthanized by cervical dislocation and blood samples were instantly collected [Citation33]. Biochemical investigation and histological assessment were displayed in Supplementary data.
Statistical analysis
All the performed tests were performed in triplicate and the acquired results are expressed as mean ± standard deviation (SD). We used GraphPad Prism 8 software (USA) for assessing the statistical significance at p < .05.
Conclusion
The green synthesised ZnO-NPs from Gardenia thailandica leaves extract demonstrated a promising in vivo and in vitro antibacterial activity against P. aeruginosa clinical isolates. Thus, the green synthesised ZnO-NPs by GTME could be regarded as a future antimicrobial compound to treat systemic infections caused by P. aeruginosa. Future clinical investigations should be performed to assure the therapeutic value of ZnO-NPs in humans.
Supplemental Material
Download MS Word (33.4 KB)Acknowledgments
This work was supported by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University (Riyadh, Saudi Arabia), through the Research Groups Program Grant no. (RGP-1440-0022)(2). The authors, therefore, gratefully acknowledge the DSR technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jan H, Shah M, Usman H, et al. Biogenic synthesis and characterization of antimicrobial and antiparasitic zinc oxide (ZnO) nanoparticles using aqueous extracts of the Himalayan Columbine (aquilegia pubiflora). Front Mater. 2020;7:249.
- Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7(3):219–242.
- Jeevanandam J, Barhoum A, Chan YS, et al. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–1074.
- Bahadar H, Maqbool F, Niaz K, et al. Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J. 2016;20(1):1.
- Chavali MS, Nikolova MP. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl Sci. 2019;1(6):1–30.
- Ahmed B, Ameen F, Rizvi A, et al. Destruction of cell topography, morphology, membrane, inhibition of respiration, biofilm formation, and bioactive molecule production by nanoparticles of Ag, ZnO, CuO, TiO2, and Al2O3 toward beneficial soil bacteria. ACS Omega. 2020;5(14):7861–7876.
- Ali K, Saquib Q, Ahmed B, et al. Bio-functionalized CuO nanoparticles induced apoptotic activities in human breast carcinoma cells and toxicity against Aspergillus flavus: an in vitro approach. Process Biochem. 2020;91:387–397.
- Naveed Ul Haq A, Nadhman A, Ullah I, et al. Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J Nanomater . 2017;2017:1–14.
- Castillo-Henríquez L, Alfaro-Aguilar K, Ugalde-Álvarez J, et al. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials. 2020;10(9):1763.
- Begum NA, Mondal S, Basu S, et al. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of black tea leaf extracts. Colloids Surf B Biointerfaces. 2009;71(1):113–118.
- Das J, Velusamy P. Biogenic synthesis of antifungal silver nanoparticles using aqueous stem extract of banana. Nano BioMed Eng. 2013;5(1):34–38.
- Ali K, Ahmed B, Ansari SM, et al. Comparative in situ ROS mediated killing of bacteria with bulk analogue, eucalyptus leaf extract (ELE)-capped and bare surface copper oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2019;100:747–758.
- Attallah NG, Elekhnawy E, Negm WA, et al. In vivo and in vitro antimicrobial activity of biogenic silver nanoparticles against Staphylococcus aureus clinical isolates. Pharmaceuticals. 2022;15(2):194.
- Zongram O, Ruangrungsi N, Rungsihirunrat K. RAPD fingerprinting and genetic relationship of gardenia species in Thailand. Songklanakarin J Sci Technol. 2017;39(4):471–477.
- Rasha E, Alkhulaifi MM, AlOthman M, et al. Effects of zinc oxide nanoparticles synthesized using Aspergillus niger on Carbapenem-Resistant Klebsiella pneumonia in vitro and in vivo. Front Cell Infect Microbiol. 2021;11:1077.
- Ahmed B, Solanki B, Zaidi A, et al. Bacterial toxicity of biomimetic green zinc oxide nanoantibiotic: insights into ZnONP uptake and nanocolloid-bacteria interface. Toxicol Res. 2019;8(2):246–261.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Slavin YN, Asnis J, Häfeli UO, et al. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15(1):1–20.
- Srinivasan N, Rangasami C, Kannan J. Synthesis structure and optical properties of zinc oxide nanoparticles. Int J Appl Eng Res. 2015;10:343–345.
- Abomuti MA, Danish EY, Firoz A, et al. Green synthesis of zinc oxide nanoparticles using salvia officinalis leaf extract and their photocatalytic and antifungal activities. Biology. 2021;10(11):1075.
- Fawzy H, Ae MM. Production of zinc and copper as nanoparticles by green synthesis using Pseudomonas fluorescens. Pakistan J Biol Sci. 2021;24(4):445–453.
- Bigdeli F, Morsali A, Retailleau P. Syntheses and characterization of different zinc (II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron. 2010;29(2):801–806.
- Saleem S, Ahmed B, Khan MS, et al. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from eucalyptus globulus plants. Microb Pathog. 2017;111:375–387.
- Farhadi F, Khameneh B, Iranshahi M, et al. Antibacterial activity of flavonoids and their structure-activity relationship: an update review. Phytother Res. 2019;33(1):13–40.
- Reddy YM, Kumar S, Saritha K, et al. Phytochemical profiling of methanolic fruit extract of gardenia latifolia ait. by LC-MS/MS analysis and evaluation of its antioxidant and antimicrobial activity. Plants. 2021;10(3):545.
- Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. JCM. 2019;9(1):109.
- Ma Y, Ding S, Fei Y, et al. Antimicrobial activity of anthocyanins and catechins against foodborne pathogens Escherichia coli and Salmonella. Food Control. 2019;106:106712.
- Baptista PV, McCusker MP, Carvalho A, et al. Nano-strategies to fight multidrug resistant Bacteria-"A Battle of the Titans"—“. Front Microbiol. 2018;9:1441.
- Bouyahya A, Abrini J, Dakka N, et al. Essential oils of origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J Pharm Anal. 2019;9(5):301–311.
- He Q, Liu D, Ashokkumar M, et al. Antibacterial mechanism of ultrasound against Escherichia coli: alterations in membrane microstructures and properties. Ultrason Sonochem. 2021;73:105509.
- Ahmed B, Hashmi A, Khan MS, et al. ROS mediated destruction of cell membrane, growth and biofilms of human bacterial pathogens by stable metallic AgNPs functionalized from bell pepper extract and quercetin. Adv Powder Technol. 2018;29(7):1601–1616.
- Patil S, Chandrasekaran R. Biogenic nanoparticles: a comprehensive perspective in synthesis, characterization, application and its challenges. J Genet Eng Biotechnol. 2020;18(1):1–23.
- Hamouda RA, Yousuf WE, Mohammed AA, et al. Comparative study between zinc oxide nanoparticles synthesis by biogenic and wet chemical methods in vivo and in vitro against Staphylococcus aureus. Microbial Pathogenesis. 2020;147:104384.
- Attallah NGM, Negm WA, Elekhnawy E, et al. Elucidation of phytochemical content of Cupressus macrocarpa leaves: in vitro and in vivo antibacterial effect against methicillin-resistant Staphylococcus aureus clinical isolates. Antibiotics. 2021;10(8):890.
- Alotaibi B, Mokhtar FA, El-Masry TA, et al. Antimicrobial activity of brassica rapa L. Flowers extract on gastrointestinal tract infections and antiulcer potential against Indomethacin-Induced gastric ulcer in rats supported by metabolomics profiling. J Inflamm Res. 2021;14:7411–7430.
- Elmongy EI, Negm WA, Elekhnawy E, et al. Antidiarrheal and antibacterial activities of Monterey cypress phytochemicals: in vivo and in vitro approach. Molecules. 2022;27(2):346.
- Attia GH, Alyami HS, Orabi MA, et al. Antimicrobial activity of silver and zinc nanoparticles mediated by eggplant green calyx. Int J Pharmacol. 2020;16(3):236–243.