 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The main aim of the study, green route to the synthesis of silver nanoparticles (AgNPs) is a new technique that has recently gained popularity due to several advantages over conventional chemical methods. The objective of the study was focused on the green synthesis of AgNPs using Barleria buxifolia leaf extract via a rapid and eco-friendly ultrasonic-assisted technique. The obtained AgNPs were characterized using ultraviolet–visible (UV–Vis) absorption spectrum of the organically reduced silver showed a surface plasmon peak at 435 nm, characteristic for silver colloidal solutions. UV–Vis absorption spectrum, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and energy-dispersive X-ray spectroscopy (EDS) analysis showed that the obtained AgNPs were dispersed spheres with a uniform size of 80 nm. Furthermore, the Fourier-transform infrared spectroscopy (FTIR) and X-ray powder diffraction (XRD) analysis indicated that the surface of the obtained AgNPs was covered with organic molecules in plant extracts. Green synthesized AgNPs showed the highest antioxidant, antibacterial and anti-biofilm activity than a plant extract. In vitro anticancer assay demonstrated half-maximal inhibitory concentration (IC50) values of 31.42, 30.67, 51.07 and 56.26 µg/mL against MCF-7, HeLa and HepG2 cancer cell lines, respectively, which confirms its potent anticancer action. The biocompatibility of green synthesized AgNPs is confirmed by their lack of cytotoxicity against normal human cells. The potent bioactivity exhibited by the green synthesized AgNPs leads towards the multiple use as antioxidant, antibacterial, anti-biofilm and cytotoxic agent.
1. Introduction
Metal nanoparticles (NPs) are fascinating materials in modern research because of their precise features and a vast range of applications in areas such as pharmaceuticals, catalysis, food and agriculture, electronics, chemical units, cosmetics, mechanics and optics, and so on [Citation1–5]. In preferred, the metal nanomaterials synthesized by physical, chemical, electrochemical, microwave-assisted process, hydrothermal, and green chemistry methods [Citation6,Citation7]. However, most physiochemical treatments are expensive, non-degradable, and use hazardous chemicals, which have resulted in several biological dangers [Citation8–10]. Therefore, the green synthesis of NPs has recently received a lot of attention due to its cost-effective requirements such as simplicity, low cost, biocompatibility, non-toxicity, eco-friendliness, and biological characteristics [Citation11]. Previous studies have described the synthesis of various metal nanomaterials using biological systems such as plant extracts [Citation12–14], fungus [Citation15,Citation16], bacteria [Citation17], yeasts [Citation18], and algae [Citation19,Citation20].
Silver nanoparticles (AgNPs) have been explored extensively as a type of metal NP with good physical and chemical properties as well as biocompatibility [Citation21,Citation22]. The green synthesis of AgNPs using environmentally friendly microorganisms and plants has become fashionable in recent years. In comparison to microorganisms, the synthesis of NPs utilizing plant extract is exceedingly straightforward and simple to handle [Citation23,Citation24]. Plant extracts are used as reducing and capping agents in various organic NP synthesis techniques. So far, a variety of plant extracts had been used to efficiently and quickly produce Ag NPs such as Artemisia nilagirica [Citation25], Iresine herbstii [Citation26], Vaccinium macrocarpon [Citation27], Parthenium hysterophorous [Citation28], Urticadioica Linn. [Citation1], Mimusopselengi Linn. [Citation29], Azadirachta indica [Citation30], Lonicera hypoglauca [Citation8], and Duriozibethinus [Citation7]. Furthermore, numerous scientists investigated the antioxidant, antibacterial, antiviral, anticancer, antimalarial, and photocatalytic activities of green manufactured AgNPs produced by plant extracts [Citation31,Citation32].
Barleria buxifolia belongs to the Acanthaceae family and contains a variety of secondary metabolites in its leaf, flower, and root sections. Cough, bronchitis, inflammation, and antibacterial action against human pathogens have all been treated with the roots and leaves as a traditional herbal medication in India [Citation33–35]. However, to the best of our knowledge, there has been no publication on the synthesis of AgNPs utilizing B. buxifolia. In this manner, our research suggested to establish a unique technique for the production of AgNPs using B. buxifolia leaf extract via an ultra-sonication process.
Bacterial infections are recognized as a serious health problem around the world. New bacterial mutations, antibiotic resistance, pathogenic strain outbreaks, and so on [Citation36]. Cancer is a genetic disorder caused by abnormal growth of cells, which is typically treated with chemotherapy, radiation-based therapies, hormone therapy, and surgery. These treatments not only kill cancerous cells but also harm to nearby healthy tissues, resulting in cancer cell eradication that is incomplete [Citation37]. To address these issues, the green synthesized AgNPs provide a variety of drug delivery systems that improve the therapeutic efficacy of existing cancer and bacterial infection treatments.
The current experimental inquiry discusses the green synthesis of AgNPs employing B. buxifolia leaf extract and an ultra-sonication procedure. During the manufacture of AgNPs, we use B. buxifolia leaf extract to act as both a reducing and stabilizing agent. Obtained AgNPs were characterized by using ultraviolet–visible (UV–Vis) spectrum, Fourier-transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (Fe-SEM) and transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), X-ray powder diffraction (XRD), dynamic light scattering (DLS) and checking its antioxidant properties using ABTS assay. Antibacterial, anti-biofilm, and exo poly substances (EPS) reduction activities of AgNPs were also tested against clinical pathogens such as Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, and Shigella spp. Furthermore, the anticancer activity of AgNPs carried out on breast cancer (MCF-7), cervical (HeLa), and liver (HepG2) cell line has also been studied. On the other hand, the silver nanoparticles synthesis and their anti-biofilm and anticancer mechanism were diagrammatically represented in .
2. Materials and methods
All the analytical reagents used in the study were of analytical grade and were purchased from Merck, India. Nutrient agar for bacterial culture and Mueller–Hinton broth, luriabertani broth methylene blue, sodium hydroxide, methanol, crystal violet and agar for biological were purchased from Hi-Media, Mumbai, India. MTT Assay Kit (ab211091) was also purchased from Sigma Aldrich, Mumbai, India.
The MCF-7, HeLa, and Hep G2 cancer cell line and human normal cell line fibroblast (L929) was obtained from NCCS (National Centre for Cell Sciences), Pune, India. MDR Clinical pathogens were maintained in Biopharmaceutical laboratory, Bharathiar University, Coimbatore, India.
2.1. Preparation of leaf extract
Barleria buxifolia plant leaf obtained from the Marudhamalai hill, Coimbatore, Tamil Nadu, India, and plant taxonomy were identified at Botanical Survey of India, Tamil Nadu agricultural university. The fresh leaves were washed with distilled water, then dried and pulverized to a fine powder with the help of mortar and pestle. This powder (20 g) was dissolved in 150 mL of methanol in a 250 mL Erlenmeyer flask, and the flask were kept in water both at 55 °C for 30 min. The solution (leaf extract) was subsequently filtered by Whatman NO.1 filter paper and held at 4 °C.
2.2. Synthesis of AgNPs via ultrasonic process
The AgNPs were synthesized with slight modification [Citation38]. Approximately 10 mL of crude B. buxifolia leaf extract was mixed with 50 mL aqueous 1MoL NaOH adjusted solution of silver nitrate (0.1 Mol) and the solution pH 9. The solution was then emulsified by subjection to high-power ultrasonic vibration (40 kHz) for different time intervals. Then the solution slowly changed yellow colour to deep brown colour in ionic liquid phase that indicates the formation of AgNPs. After the complete reduction, this solution was centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant was discarded, and the pellet was re-dispersed in dd H2O. Next, the pellet (NPs) was freeze-dried and stored at vial for further use.
2.3. Characterization of AgNPs
The synthesized AgNP solution was characterized by UV–Vis analysis by Shimadzu UV-2500 double-beam spectrophotometer. The dried powder samples were used for X-ray diffraction analysis using Rigaku, X-ray diffractometer to see the phase purity of materials with diffraction angle from 20° to 80°. A Zeiss-EM10C TEM and scanning electron microscopy (SEM) with a Cam scan MV2300 were used to study of size and morphology of powder-formed silver nanomaterial. Moreover, we evaluate its elements and size via DLS. FTIR spectra have been recorded with the help of (Thermo Scientific Nicolet 370. USA) KBr pellets [Citation1,Citation39].
2.4. ABTS radical scavenging activity of Barleria buxifolia and AgNPs
ABTS solution was mixed with potassium persulphate (2.45 mM) in a ratio of 1:0.5 (v/v). The mixture was kept in dark at room temperature for 8 h. In the ABTS solution (190 μL), different concentrations (25–150 μg/mL) of standard, leaf extract, and AgNP (10 μL) solution was added. The reaction mixtures were then incubated for 6 min and the absorbance was taken at 734 nm. The change in absorbance with respective control (containing ABTS solution without antioxidants, expressed as 100% free radicals) was calculated as percentage free radical scavenging. Ascorbic acid was used as positive control [Citation40]:
where A control is the absorbance of the ABTS solution and A sample is the absorbance of the test sample.
2.5. Antibacterial activity
Antagonistic activity of the AgNPs, leaf extract biomass, and amikacin have been assessed using Muller-Hinton agar well diffusion approach towards MDR clinical pathogens, that is, P. aeruginosa, S. enterica, and Shigella spp. as per standard methods. The various concentrations (25–100 µg/mL) of samples were filled in the wells made in the bacterial culture plates. Then the petri dishes consequently prepared were left at room temperature for 10 min for allowing the diffusion of the samples into the agar bacterial lawn. After incubation for 24 h at 37 °C, the plates had been observed. The zone of inhibition was observed and expressed in millimetres [Citation41].
2.6. Biofilm inhibition assay
The ability of AgNPs with different concentrations (25–100 μg/mL) to inhibit the formation of bacterial biofilms was recognized using 24-h-old broth inoculums of E. coli and P. aeruginosa, S. enterica, Shigella spp. using tissue culture plate method. The inoculums have been prepared using 10 mL of trypticase soy broth (TSB) with1% glucose and seeded into 1 cm2 cover slides placed in culture plates. After 24 h, planktonic cells had been removed by way of washing using sterilized distilled water and the glass slides were stained with 0.2% crystal violet stain. Biofilms formation has been visualized by Trinocular Phase Contrast microscope (Kozo Optics) at ×40 magnification. After visualization, the stain was solubilized with 1 mL of 70% ethanol and the stained adherent biofilm was quantified using a micro-ELISA auto reader (model R, Epoch, USA) at a wavelength of 570 nm [Citation42]. The elucidation of biofilm production was finished according to the standard methodology of Stepanovic et al. [Citation43]:
2.6.1. Extraction and quantification of EPS
The biofilms adhered to the walls of test tube were harvested at some stage in the late-log-phase by vigorous shaking and centrifugation at 10,500 RPM for 30 min at 4 °C. The supernatant was filtered via 0.22 μm nitrocellulose membrane filters. Three volumes of chilled 100% ethanol were added to the filtered supernatant and incubated overnight at 4 °C to precipitate EPS. The precipitated EPS was then quantified through the technique of Dubois et al. [Citation44].
2.7. Cell viability assay
The MTT assay was performed according to the method. Briefly, cells per well were seeded in 96 well plate. After seeding the cells, the sample (plant extract, AgNPs) was treated with different concentrations (25, 50, 75, and 100 μg/mL) and the plate was incubated for 24 at 37 °C in a humidified incubator. After incubation, the spent media was discarded and 200 μL of fresh media was added to each well and 10 μL of MTT was added and incubated for another 4 h. After incubation, the media was replaced with 200 μL of DMSO and OD was measured at 570. Half-maximal inhibitory concentration (IC50) values were then calculated [Citation45]:
2.7.1. Morphological assays
The MCF-7 cells that were grown on coverslips (1 × 105 cells/cover slip) were incubated with AgNPs and plant extract with different concentrations (0, IC50, and 100 μg/mL), and they were fixed in an ethanol/acetic acid solution (3:1, v/v). The cover slips were gently mounted on glass slides for morphometric analysis. Three monolayers per experimental group were photo micro graphed. The morphological changes of the MCF-7 cells were analysed using Trinocular Phase Contrast microscope (Kozo Optics) at ×40 magnification.
2.8. Statistical analysis
All the experiments were run in triplicates and data were reported as mean ± standard deviation and the data were analysed by one-way ANOVA Tukey’s HSD analysis of variance, with P = 0.05 being significant, using the (IBM SPSS Statistics 20) statistical software package.
3. Results and discussion
3.1. Characterization of AgNPs
This study integrates an ultra-sound intensified green approach to produce AgNPs using B. buxifolia leaf extract as both a reducing and stabilizing agent. In a typical procedure, an appropriate amount (10 mL) of leaf extract was dissolved in 50 mL of silver nitrate solution. Then, an ultrasonic probe was immersed into the mixture solution for various time intervals (0–240 min), whereas exposing to ultrasound waves on Ag+ ions containing leaf extract changed from light yellow to darkish brown colour, which suggest the formation of AgNPs via reduction process. The Ag+ ions without leaf extract did not show any colour changes even exposing to ultra-sonication for 240 min. From , the UV–Vis results, it is found that green synthesized AgNPs are monodispersed in nature and broad surface plasmon resonance(SPR) peak was also observed at 435 nm with high intensity for the increasing time, which indicates the formation of AgNPs and their stability. Earlier reports recommended that an SPR shift situated from 410 to 450 nm has been detected for AgNPs, which could be attributed to spherical in nature [Citation46] and their size range in 2 nm-100nm [Citation47]. According to these results, we suggest that the process of ultra-sonication makes the AgNPs intrinsically capping with plant molecules and it produced size-controlled spherical shape like non-aggregated monodispersed particles. also these techniques give to a treasured contribution in nano-biotechnology.
Figure 1. (A) UV–Vis spectra of green synthesized Ag NPs. (B) XRD pattern of Ag NPs. (C) SEM image of Ag NPs. (D) TEM image of green synthesized Ag NPs.
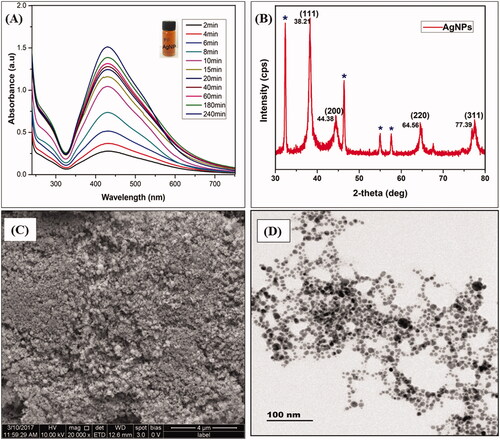
The phase purity and crystalline nature of synthesized AgNPs is studied with the aid of X-ray diffraction analysis as given in . Herein, we observed the crystalline peaks at 2Ө= 38.21°, 44.38, 64.56° and 77.39°, these peaks are attributed to the (111), (200), (220) and (311) crystallographic planes. Those peaks are consistent with the JCPDS card No 65-2871of the AgNPs. In addition, our results strongly agree with previous reports that the sharp diffraction peaks with negligible noise verify the high quality of crystallinity attributed to face-centred cubic (fcc) spinel structure of AgNPs [Citation48,Citation49]. In this manner, the normal grain size of the AgNPs was computed from the solid reflection peak by (111) arrangement utilizing the Scherer formula:
From this equation, the common size of AgNPs became approximately in 80 nm. In addition, the X-ray diffraction results clearly show that the AgNPs formed by the reduction of Ag+ ions by the B. buxifolia leaf extract are crystalline in nature. Further, unassigned peaks at 2θ = 32.35°, 46.31°, 54.56°, and 57.58° denoted by (*)indicate the presence of plant extract (capping agent) with the AgNPs as summarized in ; the similar results were reported by Awwad et al. [Citation50].
Figure 2. (A) Particles size analysis of Ag NPs. (B) EDS spectrum of Ag NPs. (C) FT-IR analysis of Ag NPs and plant extract.
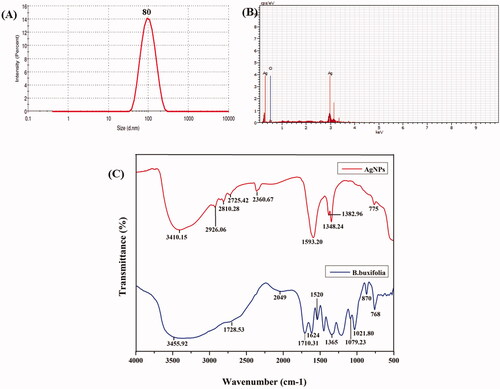
The nanostructures of the AgNPs have been studied through SEM and TEM analysis, which illustrated that AgNPs are mostly in spherical shape without aggregation which is shown in . DLS detector measured the average size of the colloidal AgNPs. shows that the average particles size was found to be 80 nm with poly-dispersed (pdi-0.243) in nature. The purity and composition of the AgNPs, as analysed by EDX, showed high characteristic signals of elemental Ag in AgNPs at approximately 0.15 and 3 keV followed by O (). The O element signals may be attributed to the flavonoid compounds covering the surface of AgNPs.
In addition, shows the FTIR spectra of biogenic AgNPs derived from B. buxifolia leaf extract after reaction with AgNO3 and leaf extract control without AgNO3. The FTIR data indicates the marginal shift in the peak position of spectra as depicted in . FTIR spectra of B. buxifolia leaf extract ( blue line) show the 11 peaks at 3455.92, 2728.53, 2049, 1710.31, 1624, 1520, 1365, 1079.23, 1021.80, 870 and 768 cm−1. Furthermore, the FTIR absorption bands at 1348.20, 1382.96, 1593.20, 2360.67, 2725.42, 2810.28, 2926.06 and 3410.15 cm−1 specifying the presence of plant extract in AgNPs (, red line). The broad shift present at 3410.15 cm−1 in the spectra agrees to -OH stretching vibration showing the presence of poly-phenols and alcohol. Aliphatic -CH stretching appears at 2926.06 cm −1. Carbonyl stretching vibration of the acid groups of distinct fatty acids present in the extract causes a strong peak at 1710 cm−1. In addition, the band at 1593.20 cm−1 corresponds to amide C=O stretching, and some other peaks located at 2926.06, 1348.20, and 1382.96 cm−1, respectively, which are in agreement to C–H stretch (alkanes); C=C stretch (aromatic ring); C–H (aromatics) suggesting, that presence of phytochemicals. FT-IR results revealed that alcoholic, phenols, aromatic, amine, and acid groups might act as a capping and stabilizing agent of Ag+ ions to AgNPs [Citation29,Citation51,Citation52].
3.2. ABTS radical scavenging activity
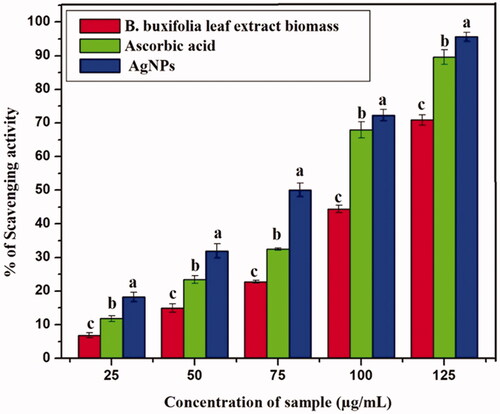
Antioxidants are molecules that prevent cell damage by reacting with the free radicals and have proved critical in infection management of bacterial, fungal, and viral diseases as well as cancer, HIV, and inflammatory diseases in humans. ABTS radical inhibition of leaf extract biomass and AgNPs using different concentrations compared to standard (ascorbic acid) which are shown in (), respectively. Although ascorbic acid was used as a positive control in this manner, it has been shown the highest antiradical action. Thus, AgNPs show tremendous free radical activity significantly with expanding concentration in the range of 25–150 µg/mL when compared with standard and leaf extract biomass. The greatest free radical-scavenger activity represented by the green synthesized AgNPs, standard, and leaf extract biomass estimations of 95.65 ± 1.6%, 89.57 ± 3.1% and 70.85 ± 1.5% separately. Our study strongly agrees with earlier studies that the ability of AgNPs synthesized using leaf extracts prepared from E. scaber and P. granatum as good scavengers of free radicals [Citation53,Citation54].
3.3. Anti-biofilm and EPS inhibition activity of Ag-NPs
Biofilms inhibition of E. coli and P. aeruginosa, S. enterica, and Shigella spp. with different concentrations of AgNPs were imaged by light microscopy. The samples were stained with crystal violet to differentiate between control (without AgNPs) and treated biofilm (with AgNPs). Violet colour indicates the presence of bacterial cells with compromised membranes, which is shown in . From these results here we observed that biofilm inhibition and biofilm disruption or cell dispersion occurred in a dose-dependent manner. The AgNPs showed effective anti-biofilm activity towards the tested biofilm producers. From , it was observed that all the concentrations of AgNPs showed good anti-biofilm activity, even at a minimal concentration of 25 μg/mL. Current results revealed the anti-biofilm activity of AgNPs on Salmonella was significantly higher than other tested bacteria (p < 0.05). In this case, the amount of biofilm formation was sharply decreased by increasing the concentration (BIC) of AgNPs at 100 μg/mL (96.1 ± 1.37% inhibition) and (90.3 ± 1.1% inhibition) (86.7 ± 1.5% inhibition) (76.3 ± 1.2% inhibition) for E. coli, S. enterica, P. aeruginosa and Shigella spp. The toxicity of green synthesized AgNPs against a bacterial pathogen may be due to the small size of NPs, which penetrate into the cell wall, where they interfere with moulting and change the physiological processes [Citation43].
Figure 4. (A) Light microscopic observation (x40) of bacterial adhesion phases on the glass surfaces with different concentration (control, 25 μg/mL, 50 μg/mL, 75 μg/mL, 100 μg/mL) of synthesized Ag NPs against control which is indicative of glass surface with uniformly distributed cells stained with crystal violet. (B) Anti-biofilm activity of synthesized Ag NPs using spectrophotometer assay. (C) Quantification of EPS reduction activity by spectrophotometer assay.
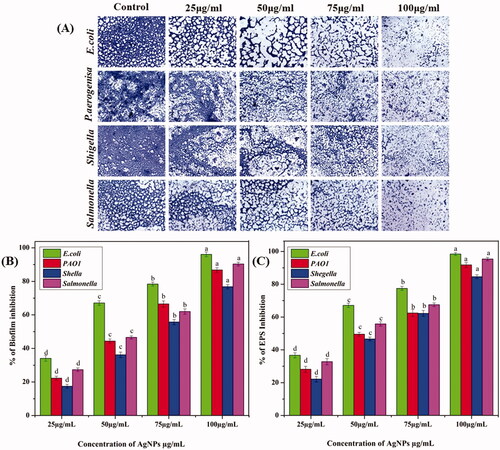
The synthesized AgNPs were able to reduce the EPS of E. coli and P. aeruginosa, S. enterica, and Shigella spp. The concentration of the AgNPs used to assess the EPS inhibition ranged from 25 μg − 100 μg/mL. From , it can be observed that all the concentrations of AgNPs showed a significant reduction of EPS production. The test AgNPs at 100 µg/mL exhibited 98.4 ± 1.4%, 95.3 ± 1.0%, 91.7 ± 1.4 and 84.6% decrease in EPS production of E. coli, S. enterica, P. aeruginosa, and Shigella spp. This result also indicates the disruption of biofilm architecture. Our results correlated with Park et al. [Citation55] reported that AgNPs had more toxic against bacterial cells and their extracellular substance.
3.4. Antibacterial activity of green synthesized AgNPs
The antibacterial property of green synthesized AgNPs, leaf extract biomass and amikacin were tested against MDR strains such as E. coli and P. aeruginosa, S. enterica, and Shigella spp. This was quantified by the minimum inhibitory concentration (MIC) assay, wherein the zone of inhibition was acquired by plating the organisms on a Muller-Hinton agar plate and performing the well diffusion test for various concentrations (25, 50 and 100 µg/mL) for 24 h. The AgNPs, leaf extract biomass and amikacin are exhibited significant (ANOVA, P < 0.05) antibacterial activity against tested pathogens in a dose-dependent manner and the results were summarized in . Overall, our results indicate that AgNPs have better antibacterial properties than the commercial antibiotic and leaf extracts biomass. In addition, here we observed the highest zone of inhibition present at a maximum inhibitory concentration of AgNPs (100 µg/mL) on S. enterica (25.40 ± 0.83) followed by E. coli (21.6 ± 1.10), Shigella spp. (19.4 ± 0.97) and P. aeruginosa (18.6 ± 1.25). Furthermore, the minimum inhibitory concentration (25 µg/mL) also shows better activity, which is displayed in . Generally, shape, size, charges, reactive oxygen species (ROS), metal ion release and other factors influence the antibacterial activity. Comparably, the synthesized AgNPs exhibited more antibacterial activity than other NPs because of their physical and chemical properties [Citation56,Citation57]. As well as some studies reports, that green synthesized AgNPs initiate continuous oxidative stress on the bacterial cell wall, it may lead to bacterial cell death [Citation58]. Therefore, our findings suggest, that the green synthesized AgNPs from B. buxifolia and its antibacterial properties may be useful to food preservation techniques, biomedical, cosmetic and agriculture field.
Table 1. Antimicrobial activity of green synthesized AgNPs and B. buxifolia leaf extract.
3.5. Cytotoxicity/cell viability of AgNPs
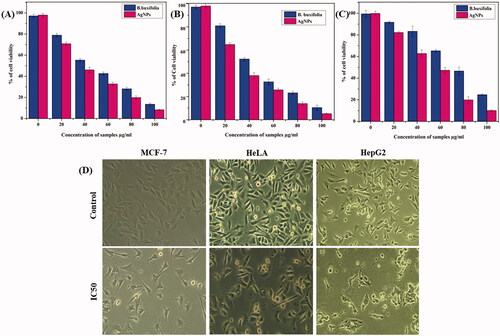
AgNPs broad range biological activities make them promising mediators not only in the fight against infections but also in the fight against serious health problems like cancer, particularly multidrug-resistant cancer cells. The antitumor effect of B. buxifolia mediated AgNPs was investigated by the MTT assay against human breast cancer (MCF-7), cervical (HeLa), and liver (HepG2). The IC50 value of B. buxifolia extract and synthesized AgNPs against MCF-7, HeLa, and HepG2 cell lines were 42.57, 70.12, 76.09 µg/mL and 31.42, 51.07, 56.26 µg/mL, respectively. B. buxifolia did not show much cytotoxicity when compared with AgNPs, which are summarized in . The increased cytotoxicity of the AgNPs in different cell lines may be due to enhanced cellular uptake and retention of the NPs. This is because, due to their small size, the AgNPs can enter cells via endocytosis and are not prone to P-glycoprotein efflux [Citation26]. These results revealed that percentage of cell viability was sharply decreased by increasing the concentration of AgNPs and B. buxifolia. In compared to untreated (control) cells, the morphology of the cancer cells treated with AgNPs: treated cells displayed apoptotic-like signs such as cell shrinkage and separation from surrounding cells, especially in the case of AgNPs treatment. In addition, form irregularity, cytoplasmic blebbing, cellular debris, and nuclear chromatin condensation were all visible . In agreement with our findings, recent research pointed out that AgNPs may be attached to the membrane of cancer cells due to their electrostatic interaction and cause a process of pore formation on cell surface, cell shrinkage, membrane blabbing and deactivation of DNA, Mitochondria, that may ultimately lead to the cell death [Citation59,Citation60].
Figure 5. In vitro cytotoxicity of B.buxifolia aqueous extract and synthesized Ag NPs in different cancer cell lines after 2 h of incubation by MTT assay. (A) Cytotoxicity studies of Ag NPs against breast cancer cell lines (MCF-7). (B) Cytotoxicity studies of Ag NPs against cervical cancer cell lines (HeLa). (C) Cytotoxicity studies of Ag NPs against liver cancer cell lines (HepG2). (D) Morphological changes of cancer cells treated with Ag NPs visualized by inverted phase-contrast micrographs magnification 200×.
3.5.1. Biocompatibility studies of AgNPs against human fibroblast cell lines
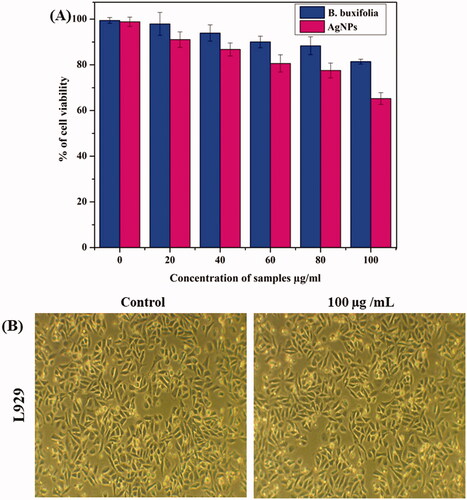
In addition, the cyto-compatibility of AgNPs against human fibroblasts (L929) cell lines was investigated in this work utilizing a colorimetric MTT assay. After 24 h, cell viability was assessed in human fibroblast (L929) cell lines treated with SNPs at various doses of 0, 20, 40, 60, 80, and 100 g/mL. Our findings revealed that AgNPs had a 90–95% of cell viability present at all doses, which are shown in . As well as no morphological changes were observed in . From these results, we understood that the B. buxifolia and green synthesized AgNPs were nontoxic toward normal cells, as well as higher toxicity against various tumour cells. These results are strictly dealing with the earlier investigations [Citation60–62]. The fact that B. buxifolia stabilized AgNPs are safe even at higher concentrations validates their harmless nature and offers up new paths for their use in diverse biomedical fields.
4. Conclusion
In conclusion, the formation of AgNPs starts quickly after the addition of AgNO3 with B. Buxifolia leaf extract with the aid of ultrasonic vibration. To the best of our knowledge, this is the first time for the synthesis of AgNPs using B. buxifolia via the ultra-sonication process. UV, XRD, SEM, TEM, DLS and FTIR analysis has accurately characterized the AgNPs. Characterization fact showed that the AgNPs had been crystalline in nature and physical identification exposes the spherical shape with size range at 80 nm. The green synthesized AgNPs exhibit strong antioxidant properties and enhanced antibacterial, anti-biofilm and EPS inhibition activity against MDR strains. Additionally good anticancer impact towards breast cancer cell lines MCF-7, HeLa, and HepG2. In addition, the biocompatibility of green synthesized AgNPs is confirmed by their lack of cytotoxicity against normal human cells (L929). From our studies, we mentioned some promising points of the ultrasonic-assisted green process, is a simple, unhazardous, cost-less, Eco-friendliness and this process reduce the time to making the nanocrystals without agglomeration. Therefore, we think our findings provide a precious contribution to the various fields like, food preservation, pharmaceutical industry, and agriculture, textile industry for wastewater treatment.
Author contributions
Sekar Vanaraj and Chellasamy Panneerselvam conceived and designed the experiments. Balakrishnan Cindhu, Kathirvel Preethi, and Swamiappan Sathiskumar performed the laboratory experiments. Mohammed Ali Alshehri and Samy Sayed contributed materials like reagents and analysis tools. Sekar Vanaraj and Chellasamy Panneerselvam analysed the data, prepared the figures, and wrote the original draft. Chellasamy Panneerselvam, Balakrishnan Cindhu, Kathirvel Preethi, Swamiappan Sathiskumar, Mohammed Ali Alshehri, Samy Sayed and Sekar Vanaraj contributed to formal analysis and data curation and reviewed/edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgement
The authors gratefully acknowledge the laboratory support of Microbial Biotechnology department, Bharathiar University, Coimbatore, Tamil Nadu, India and the authors appreciate Taif University Researchers Supporting Project number (TURSP2020/92), Taif University, Taif, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data are included in the manuscript file.
Additional information
Funding
References
- Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016;9(3):217–227.
- Rahman AU, Khan AU, Yuan Q, et al. Tuber extract of Arisaema flavum eco-benignly and effectively synthesize silver nanoparticles: Photocatalytic and antibacterial response against multidrug resistant engineered E. coli QH4. J Photochem Photobiol B. 2019;193:31–38.
- Yang B, Yang Z, Wang R, et al. Silver nanoparticle deposited layered double hydroxide nanosheets as a novel and high-performing anode material for enhanced Ni–Zn secondary batteries. J. Mater. Chem A. 2014;2(3):785–791.
- Bastus NG, Merkoci F, Piella J, et al. Synthesis of highly Mono disperse citrate-stabilized silver nanoparticles of up to 200 nm: kinetic control and catalytic properties. Chem Mater. 2014;26(9):2836–2846.
- Boca SC, Potara M, Gabudean AM, et al. Chitosan-coated triangular silver nanoparticles as a novel class of biocompatible, highly effective photothermal transducers for in vitro cancer cell therapy. Cancer Lett. 2011;311(2):131–140.
- Zhang D, Gokce B, Barcikowski S. Laser synthesis and processing of colloids: fundamentals and applications. Chem Rev. 2017;117(5):3990–4103.
- Sengan M, Veeramuthu D, Veerappan A. Photosynthesis of silver nanoparticles using Durio zibethinus aqueous extract and its application in catalytic reduction of nitro aromatics, degradation of hazardous dyes and selective colorimetric sensing of mercury ions. Mater. Res. Bull. 2018;100:386–393.
- Jang SJ, Yang IJ, Tettey CO, et al. In-vitro anticancer activity of green synthesized silver nanoparticles on MCF-7 human breast cancer cells. Mater Sci Eng C Mater Biol Appl. 2016;68:430–435.
- Cheng F, Betts JW, Kelly SM, et al. Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using amino cellulose as a combined reducing and capping reagent. Green Chem. 2013;15(4):989–998.
- He J, Kunitake T, Nakao A. Facile in situ synthesis of noble metal nanoparticles in porous cellulose fibers. Chem Mater. 2003;15(23):4401–4406.
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583.
- Mohapatra B, Kuriakose S, Mohapatra S. Rapid green synthesis of silver nanoparticles and nanorods using Piper nigrum extract. J. Alloys Compd. J ALLOY COMPD. 2015;637:119–126.
- Alomar TS, AlMasoud N, Awad MA, et al. An eco-friendly plant-mediated synthesis of silver nanoparticles: Characterization, pharmaceutical and biomedical applications. Mater Chem Phys. 2020;249:123007.
- Veisi H, Hemmati S, Shirvani H, et al. Veisi, H, green synthesis and characterization of monodispersed silver nanoparticles obtained using oak fruit bark extract and their antibacterial activity. Appl Organometal Chem. 2016a;30(6):387–391.
- Feroze N, Arshad B, Younas M, et al. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc Res Tech. 2020;83(1):72–80.
- Guilger-Casagrande M, Germano-Costa T, Pasquoto-Stigliani T, et al. Biosynthesis of silver nanoparticles employing Trichoderma harzianum with enzymatic stimulation for the control of Sclerotinia sclerotiorum. Sci Rep. 2019;9:14351 https://doi.org/10.1038/s41598-019-50871-0.
- Ghiuta I, Croitoru C, Kost J, et al. Bacteria-Mediated synthesis of silver and silver chloride nanoparticles and their antimicrobial activity. Appl Sci. 2021;11(7):3134.
- Korbekandi H, Mohseni S, Mardani Jouneghani R, et al. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol. 2016;44(1):235–239.
- Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM, et al. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J Biol Sci. 2019;26(6):1207–1215.
- Prasad TNVKV, Elumalai EK. Marine algae mediated synthesis of silver nanoparticles using Scaberia agardhü Greville. J Biol Sci. 2013;13(6):566–569.
- Shaham G, Veisi H, Hekmati M. Silver nanoparticle‐decorated multi walled carbon nanotube/pramipexole nano composite: synthesis, characterization and application as an antibacterial agent, appl. Organomet Chem. 2017;31:37–35.
- Azizi Z, Pourseyedi S, Khatami M, et al. Stachys lavandulifolia and lathyrus sp. mediated for green synthesis of silver nanoparticles and evaluation its antifungal activity against Dothiorella sarmentorum. J Clust Sci. 2016;27(5):1613–1628.
- Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, et al. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003;19(4):1357–1361.
- Das J, Velusamy P. Biogenic synthesis of antifungal silver nanoparticles using aqueous stem extract of banana. Nano Biomed Eng. 2013;5:34–38.
- Vijayakumar M, Priya K, Nancy FT, et al. Biosynthesis, characterisation and anti-bacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crops Prod. 2013;41:235–240.
- Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf B Biointerfaces. 2012;98:112–119.
- Khodadadi B, Bordbar M, Yeganeh-Faal A, et al. Green synthesis of Ag nanoparticles/clinoptilolite using Vaccinium macrocarpon fruit extract and its excellent catalytic activity for reduction of organic dyes. J Alloys Compd. 2017;719:82–88.
- Adur AJ, Nandini N, Mayachar KS, et al. Bio-synthesis and antimicrobial activity of silver nanoparticles using anaerobically digested Parthenium slurry. J Photochem Photobiol B. 2018;183:30–34.
- Prakash P, Gnanaprakasam P, Emmanuel R, et al. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf B Biointerfaces. 2013;108:255–259.
- Ahmed S, Saifullah , Ahmad M, Swami BL, et al. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci. 2016;9:1–7.
- Murugan K, Venus JSE, Panneerselvam C, et al. Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus? Environ Sci Pollut Res Int. 2015;22(21):17053–17064.
- Chung IM, Park I, Seung-Hyun K, et al. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res Lett. 2016;11(1):40.
- Chander PA, Sri HY, Sravanthi NB, et al. In vitro anthelmintic activity of Barleria buxifolia on Indian adult earthworms and estimation of total flavonoid content, Asian Pac. J. Trop. Dis 2014;4:S233–S235.
- Chetty KM, Sivaji K, Rao KT. Flowering plants of Chittoor district, Andhra Pradesh, India. Tirupati: Student Offset Printers; 2008; p. 34–35.
- Tamil Selvi S, Jamuna S, Thekan S, Department of Botany, Kongunadu Arts and Science College, Coimbatore, Tamil Nadu-641029, India, et al. Profiling of bioactive chemical entities in Barleria buxifolia L. using GC-MS analysis–a significant ethno medicinal plant. J Ayu Her Med. 2017;3(2):63–77.
- Siddiqi KS, Rahman AU, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):1–13.
- Behera A, Awasthi S. Anticancer, antimicrobial and hemolytic assessment of zinc oxide nanoparticles synthesized from Lagerstroemia indica. Bio Nano Sci. 2021;11(4):1030–1048.
- Deshmukh AR, Gupta A, Kim BS. Ultrasound assisted green synthesis of silver and iron oxide nanoparticles using fenugreek seed extract and their enhanced antibacterial and antioxidant activities. Biomed Res Int. 2019;2019:1714358. ) 2019.
- Roni M, Murugan K, Panneerselvam C, et al. Evaluation of leaf aqueous extract and synthesized silver nanoparticles usingNerium oleander against Anopheles stephensi (Diptera: Culicidae). Parasitol Res. 2013;112(3):981–990.
- Al-Shmgani HS, Mohammed WH, Sulaiman GM, et al. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif Cells Nanomed Biotechnol. 2017;45(6):1–1240.
- Vanaraj S, Jabastin J, Sathiskumar S, et al. Production and characterization of bio-AuNPs to induce synergistic effect against multidrug resistant bacterial biofilm. J Clust Sci. 2017a;28(1):227–244.
- Vanaraj S, Keerthana BB, Preethi K. Biosynthesis, characterization of silver nanoparticles using quercetin from Clitoria ternatea L to enhance toxicity against bacterial biofilm. J Inorg Organomet Polym. 2017b;27(5):1412–1422.
- Stepanovic S, Vukovic D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007;115(8):891–899.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–356.
- Vivek R, Thangam R, Muthuchelian K, et al. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012;47(12):2405–2410.
- Zaheer Z, Rafiuddin . Silver nanoparticles to self-assembled films: green synthesis and characterization. Colloids Surf B. 2012;90:48–52.
- Pradhan N, Pal A, Pal T. Silver nanoparticle catalysed reduction of aromatic nitro compounds. Colloids Surf A Physicochem Eng. 2002;196(2-3):247–257.
- Vincent S, Kovendan K, Chandramohan B, et al. Swift Fabrication of Silver Nanoparticles Using Bougainvillea glabra: Potential Against the Japanese Encephalitis Vector, Culex tritaeniorhynchus Giles (Diptera: Culicidae). J Clust Sci. 2017;28:37–58.
- Markowska K, Grudniak AM, Wolska KI. Silver nanoparticles as an alternative strategy against bacterial biofilms. Acta Biochim Pol. 2013;60(4):523–530.
- Awwad AM, Salem NM, Abdeen AO. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int J Ind Chem. 2013;4(1):29.
- Baghbani-Arani F, Movagharnia R, Sharifian A, et al. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J Photochem Photobiol B. 2017;173:640–649.
- Balaji DS, Basavaraja S, Deshpande R, et al. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces. 2009;68(1):88–92.
- Kharat SN, Mendhulkar VD. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater Sci Eng C. 2016;62:719–724.
- Saratale RG, Shin HS, Kumar G, et al. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif Cells Nanomed Biotechnol. 2018;46(1):211–222.
- Park H-J, Kim H, Cha S, et al. Removal characteristics of engineered nanoparticles by activated sludge. Chemosphere. 2013;92(5):524–528.
- Ghosh S, Patil S, Ahire M, et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomed. 2012;7:483–496.
- Dakhil AS. Biosynthesis of silver nanoparticle (AgNPs) using lactobacillus and their effects on oxidative stress biomarkers in rats. J King Saud Univ Sci. 2017;29(4):462–467.
- Satpathy S, Patra A, Ahirwar B, et al. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S71–S85.
- Kanipandian N, Kannan S, Ramesh R, et al. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull. 2014;49:494–502.
- Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–13619.
- Kummara S, Patil MB, Uriah T. Synthesis, characterization, biocompatible and anticancer activity of green and chemically synthesized silver nanoparticles: a comparative study. Biomed Pharmacother. 2016;84:10–21.
- Jadhav K, Deore S, Dhamecha D, et al. Phytosynthesis of silver nanoparticles: characterization, biocompatibility studies, and anticancer activity. ACS Biomater Sci Eng. 2018;4(3):892–899.

![Schematic diagram 1. Schematic depiction of green synthesis of silver nanoparticles and their antibacterial, antibiofilm, anticancer, and biocompatibility activity [42]](/cms/asset/7625be36-09ff-4b22-a7fb-e7f9627e5a8a/ianb_a_2084100_sch0001_c.jpg)