 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Selenium is a non-essential element with beneficial and toxic effects on plants, whose exact role in plant physiology leaves many unanswered questions. Various species of hydroponically grown plants produce defined selenium nano particles (SeNP) with a narrow size distribution and about 2 million selenium atoms by biosynthesis when being exposed to selenite, proving that green synthesis of SeNP is not only possible in plants extracts, but also in living organisms. The detection was performed with single particle inductively coupled plasma mass spectrometry. These results require a new view of the selenium biochemistry in plants and its impact on nutrition, food sciences and medicine. To the best of our knowledge, this is the first report on the synthesis of elemental nanoparticles in general and selenium nanoparticles in particular by living plants.
Introduction
Selenium is an element of the chalcogenide group and one of the most versatile trace elements. In contrast to other species, selenium is not considered essential for plants [Citation1,Citation2]. While among other mammals humans rely on selenocysteine (SeCys) in the catalytic centre of enzymes like glutathione peroxidase (GPx) or thioredoxin reductase (TrxR), the plant homologues contain cysteine (Cys) [Citation3]. Though not considered essential, selenium can still have beneficial impact on plants and increase the activity of said enzymes while also improving the resistance against cold, drought and metallic stress [Citation4]. Still, selenium accumulation bears the danger to impair cell integrity and metabolism. Toxic effects of selenium in plants are mostly caused by unintentional incorporation of SeCys and selenomethionine (SeMet) into proteins [Citation5], but also include oxidative and nitrosative stress [Citation2].
Selenium occurs in multiple oxidative states ranging from -II to + VI just like sulphur and coherently it forms analogous compounds, including selenide (Se2−), selenite (SeO32−) and selenate (SeO42−). Plants are able to take up a variety of selenium compounds, but the most abundant forms of selenium in soil are selenate in alkaline and oxic environments and selenite in anaerobic and acidic environments. Selenate uptake is catalysed by high-affinity sulphate transporters (HASTs) while phosphate transporters such as OsPt2 and aquaporin channels such as OsNIP2;1 catalyse selenite uptake [Citation6]. Generally, the similarities between selenium and sulphur hint towards many functions of selenium in biochemistry. Selenium shows superiority to sulphur in the catalytic centre of enzymes in the form of increased catalytic activity. Due to selenium being a good nucleophile and electrophile, peroxidases containing SeCys instead of Cys can more easily regenerate throughout an oxidoreductive cycle, leading to the hypothesis that one of selenium’s functions is the prevention of irreversible oxidative inactivation [Citation7]. Selenium’s ability to be both rapidly oxidised and reduced, also known as ‘selenium paradox' could explain why non selenium dependent species such as those within the plant kingdom may profit from low doses of selenium, as the unintentional incorporation of SeCys into the active centre of enzymes can benefit their activity.
Due to their similar biochemical characteristics selenium and sulphur share metabolic pathways and are substrates to the same enzymes, which can cause damage. A high concentration of selenium in plant tissues is overall associated with a high concentration of sulphur. In terms of selenium uptake plants can be divided in non-accumulators, secondary accumulators and hyperaccumulators. They are classified by their selenium concentration of either >1000 mg/kg, 100–1000 mg/kg or <100 mg/kg dry weight [Citation2,Citation4]. Selenium hyperaccumulators show a greater ratio of selenium to sulphur concentrations than non-accumulators. Selenate uptake decreases drastically in soil with an excess of sulphate over selenate for non-accumulators, this does not apply for hyperaccumulators [Citation8].
Inorganic selenium compounds can be reductively converted to SeCys [Citation2]. Furthermore, it has been shown that the chloroplastic CpNifS protein that is involved in the formation of iron-sulphur clusters can also target SeCys [Citation9]. For different plants the selenium speciation in different tissues varies due to the selenium compounds the plant is exposed to.
Signs for natural formation of SeNP in plants are so far unknown. The synthesis of selenium nanomaterials has been described using microorganisms like Enterococcus faecalis [Citation10] or yeast [Citation11,Citation12], but it was also found that various plant extracts [Citation13,Citation14] can be used to synthesise SeNP. Sometimes reducing agents such as ascorbic acid were added to the mixture of plant extract and a selenium compound, but mostly it was found that the reductive potential of biomolecules within the plant extract had sufficient reductive potential for the synthesis of SeNP. Those molecules include amino acids, enzymes, flavonoids, phenolic compounds, proteins, saponins, sugars and tannins. While these studies on plant extracts and homogenates give no insight in the natural formation of nano particles in plants, it was our aim to investigate the potential synthesis of SeNP in intact cells of living plants. The cellular synthesis of iron oxide nano particles has already been described, however these are not elemental nanoparticles, but chemical compounds of the iron cations [Citation15]. However, the synthesis of elemental nanoparticles in intact plants has not yet been described.
In this study it was our aim to prove the hypothesis that natural formation of SeNP takes place in plants when being objected to selenite. To do so, we grew plants under controlled conditions, to ensure they were not exposed to SeNP. These findings may not only have an impact on the green biosynthesis of nanoparticles (NP), but also raise questions on the role that NP might have in plant physiology and biochemistry. In contrast to previous research that focussed on plant extracts, we investigated the occurrence of so far unmentioned SeNP synthesis in living plant cells and analysed their size distribution using single particle inductively coupled mass spectrometry (sp-ICP-MS). By proving that nanoparticle synthesis is a naturally occurring phenomenon, it is safe to conclude that selenium nano particles are ingested by humans and animals. We see great necessity to investigate the impacts of this matter.
In contrast to plants, selenium is an essential element in the human organism. Selenium impacts thyroid metabolism, the antioxidant system and immune functions. Selenium supplementation has shown beneficial effects on male fertility, various kinds of cancer and then incidence of eclampsia, whereas selenium deficiency can cause cardiovascular and inflammatory diseases [Citation16–21]. Furthermore, anti-infective properties have been described for selenium in general and for selenium nano particles (SeNP) specifically. Anti-viral properties of SeNP have been proven and latest publications even linked selenium levels in patients with the disease progression of Covid-19 and express the desirability to investigate the use of SeNP to fight the viral pandemic [Citation22,Citation23].
On the other hand, the possibility of toxic selenium concentrations and the narrow therapeutic range call for attention. Due to selenium’s chemical similarity with sulphur, unusual seleno-amino acid, mostly SeCys and SeMet, can non-specifically replace their respective sulphuric equivalents, leading to faulty proteins [Citation24]. It is therefore worth noticing that various animal trials state lower toxicity and better bioavailability for SeNP in comparison with both inorganic and organic selenium [Citation25–28]. SeNP are especially promising candidates for cancer treatment since improved efficiency and reduced toxicity can even be enhanced by conjugation with targeting agents on the surface [Citation27]. Different kinds of modified and unmodified SeNP have been proven to be efficient in inducing selective cell death in different kinds of cancer, including cervical carcinoma cells, oestrogen receptor α-positive breast cancer cells and prostate cancer cells [Citation12,Citation27,Citation29]. It has also been shown in mice that, when given the same amount of selenium in form of SeNP of different sizes, the smaller SeNP showed greater increase in the activity of SeCys dependent enzymes like GPx or TrxR. This size effect gives a necessity to synthesise SeNP in a narrow size range, to achieve precise effects in the treated organisms [Citation30]. GIT absorption of SeNP is also size dependent, thus a way to reliably synthesise small SeNP can strongly enhance their pharmacokinetic properties and potential as drugs and food supplements [Citation31].
Materials and methods
Ultrapure water (18.2 MΩ·cm) was produced by Sartorius arium® pro ultrapure water system. For homogenisation of the plants tissues a Qiagen Tissue Ruptor II was used. For plant digestion Macerozyme R-10 enzyme derived from Rhizopus sp. was purchased from bioWorld. Proteinase K was purchased from GeneON. Citric acid and disodium hydrogen citrate for the preparation of a citrate buffer were purchased from Sigma-Aldrich. Gold and selenium reference standard solutions for calibration were purchased from Perkin Elmer. SeNP reference standard suspensions were purchased from Nanocs. Spectra/Por® 3 dialysis membrane MWCO 3.5 kD and Spectra/Por® closures were purchased from Fisher Scientific.
The hydroponic system was purchased from growland. So was the GHE TriPart series of nutritional solutions. Hoagland’s solution was purchased from Biozol. The sodium selenite was purchased from Sigma-Aldrich. Formalin solution 10% was purchased from Sigma-Aldrich. Ethanol 96% was purchased from Merck. All seeds were purchased from local supermarkets.
The sp-ICP-MS analysis was performed with a Perkin Elmer NexION 350 D equipped with a quartz cyclonic spray chamber (Perkin Elmer, Waltham MA, USA) and a glass nebuliser (Ar 1.0 SLPM @ 43 psi, Golden CO, USA). Peristaltic pump tubing (polyvinyl chloride) was obtained from Perkin Elmer, with an inner diameter of 0.38 mm and flared ends. Samples were prepared in 50 ml polypropylene tubes (Sarstedt AG and Co.KG, Nümbrecht, Germany) or 15 ml sterile polypropylene tubes (CELLSTAR® TUBES, Germany).
Plant treatment
Brown mustard (Brassica juncea), barley (Hordeum vulgare), flax (Linum usitatissimum), lentil (Lens culinaris), bell pepper (capsicum annuum), butter lettuce (Lactuca sativa), carrot (Daucus carota subsp. sativus) and cucumber (Cucumis sativus) were used in our research.
The seeds were surface sterilised with 10% formalin solution for 10 min and rinsed thoroughly with ultrapure water. For up to 7 days the seeds were germinated on filter paper, moisturised with ultrapure water, and subsequently transferred into a hydroponic system, containing a growth solution with all essential nutrients that was spiked with sodium selenite. The concentration of sodium selenite in the nutritional solution was 5 mg/l, equivalent to a selenium concentration of 28.91 μM/l. For barley and brown mustard the GHE TriPart Series was used as nutritional solution, for the other plants Hoagland Solution at quarter strength was used. Water was added every other day to keep the water level roughly constant. Light was distributed with two 55 W neon tubes and a reflector. The neon tubes emitted a colour temperature of 6500 K. The days where split into a 16-h light and an 8-h darkness period, in which no artificial light was emitted. The light source was placed about 30 cm above the seeds and was powered from 6 am to 10 pm. The plants grew 28 to 42 days. Following the harvesting, shoot and root were separated and rinsed with the root crown being disposed to avoid intermixing.
The root tissues were cleaned with DI water, roughly cut, and stirred in 0.1 M citrate buffer (pH 6) for 48 h to clean the surface from the nutritional solution, and afterwards again rinsed with DI water. After cleaning 100 mg of root tissue were transferred in 8 ml of 0.1 M citrate buffer and homogenised with a tissue ruptor for 2 min. The shoot tissue was just rinsed with DI water without being washed and stirred for 48 h before being homogenised. 2 ml of Macerozyme R-10 solution (50 mg/ml) and 50 µl of Proteinase K (20 mg/ml) were added to the homogenate and shaken for 24 h at 37 °C. 500 µl of the supernatant were spiked with 500 µl of ethanol, and diluted to 5 ml with 0.1 M citrate buffer (pH 6) and transferred into a piece of dialysis tubing made from regenerated cellulose with a MWCO of 3500 Da. The closed tubing was stirred in a mixture of 50 ml ethanol and 450 ml of aforementioned citrate buffer for 24 h. Subsequently the content of the tubing was diluted back to a volume of 5 ml. 500 µl of dialysis product were diluted to 5 ml with ultrapure water and measured directly. The study was performed with two individual growth cycles for every plant. Each time, three plants per species were grown and their roots and shoots were analysed, making for a total of six specimens per plant that were included in the study. If the analysis resulted in a concentration of dissolved selenium greater than 1 ppb, which was found to be prone to false positive results, 500 µl of dialysis product were instead spiked with 50 µl of ethanol and diluted to 10 ml, resulting in an analysis at half concentration compared to the rest. This was done for the following samples: Carrot 2 and 3 root in the first replicate. Butter lettuce 2 root and shoot in the second replicate. Capsicum 2 shoot in the second replicate. Lens 1, 2 and 3 root in the second replicate.
ICP-MS method and parameters
A method was developed using the Syngistix software with nano application measuring the 80Se isotope with a relative abundance of 49.61%. Argon-dimer interference was removed using hydrogen in a dynamic reaction cell (DRC). The dwell time was set to 50 µs and measuring time to 120 s, while see settling time was eliminated completely. The sample flow rate was determined daily. Further instrumental parameters included:
Results
All plants were healthy. Shape and colour were unremarkable and primary and secondary roots developed normally.
The sp-ICP-MS analysis was performed on a NexION 350 D system. We utilised the system’s ability to reduce the dwell time to 50 µs to decrease noise and detection limit. The detection of 80Se, the isotope of interest, is highly interfered by 40Ar dimer cations, we used the direct reaction cell (DRC) technology and introduced hydrogen gas to eliminate the disturbance. This may have been affecting the selenium signal strength. The slope, indicating the ratio between selenium concentration and signal strength is not large enough to allow for precise size differentiation in the lower nm area, therefore steps of 5 nm are used in the following figures, which is intended to represent a more realistic distribution of particles within the samples.
While the majority of particles is in the size range between 30 and 65 nm, few particles were found with diameters up to 400 nm. For the sake of clarity these outliers have not been included in the histograms in , please consult the supplementary information for the full list.
Figure 1. Size distribution of SeNP in root and shoot tissues of barley and brown mustard plants. Histograms for the sp-ICP-MS analysis of the first duplicate of (a) mustard root, (c) mustard shoot, (e) barley root and (g) barley shoot and the second duplicate of (b) mustard root, (d) mustard shoot, (f) barley root and (h) barley shoot. The error indicators represent the standard deviation for the three replicates that were measured from every sample. While in some measurements, nano particles with a size of up 400 nm where detected, these histograms are cropped to show the main distribution of SeNP. A full list of the results and histograms showing individual data points for every run can be found in the supplementary information.
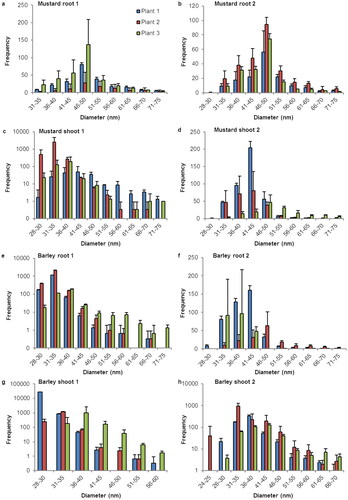
Figure 2. Size distribution of SeNP in root and shoot tissues of linum and lens plants. Histograms for the sp-ICP-MS analysis of the first duplicate of (a) linum root, (c) linum shoot, (e) lens root and (g) lens shoot and the second duplicate of (b) linum root, (d) linum shoot, (f) lens root and (h) lens shoot. The error indicators represent the standard deviation for the three replicates that were measured from every sample. While in some measurements, nano particles with a size of up 400 nm where detected, these histograms are cropped to show the main distribution of SeNP. A full list of the results and histograms showing individual data points for every run can be found in the supplementary information.
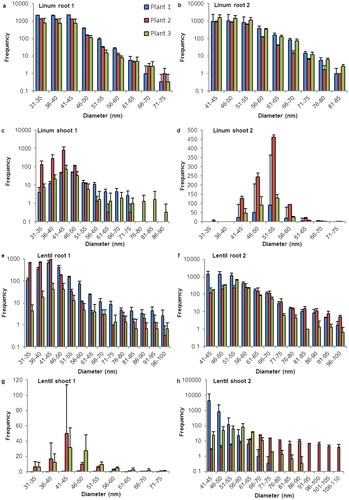
Figure 3. Size distribution of SeNP in root and shoot tissues of capsicum and butter lettuce plants. Histograms for the sp-ICP-MS analysis of the first duplicate of (a) capsicum root, (c) capsicum shoot, (e) butter lettuce root and (g) butter lettuce shoot and the second duplicate of (b) capsicum root, (d) capsicum shoot, (f) butter lettuce root and (h) butter lettuce shoot. The error indicators represent the standard deviation for the three replicates that were measured from every sample. While in some measurements, nano particles with a size of up 400 nm where detected, these histograms are cropped to show the main distribution of SeNP. A full list of the results and histograms showing individual data points for every run can be found in the supplementary information.
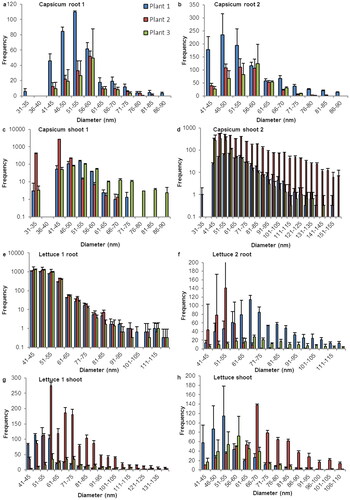
Figure 4. Size distribution of SeNP in root and shoot tissues of cucumber and carrot plants. Histograms for the sp-ICP-MS analysis of the first duplicate of (a) cucumber root, (c) cucumber shoot, (e) carrot root and (g) carrot shoot and the second duplicate of (b) cucumber root, (d) cucumber shoot, (f) carrot root and (h) carrot shoot. The error indicators represent the standard deviation for the three replicates that were measured from every sample. While in some measurements, nano particles with a size of up 400 nm where detected, these histograms are cropped to show the main distribution of SeNP. A full list of the results and histograms showing individual data points for every run can be found in the supplementary information.
In general, no clear tendency can be observed for the difference in root and shoot tissues regarding number or size distribution of SeNP. A broader size distribution and a larger number of SeNP appears to manifest in both root and shoot tissue of the respective plant.
In mustard there is a slight shift for the most abundant particle size. While in the root tissue of all plants the maximum was observed between 46 and 50 nm, the most frequently detected particle sizes in the shoot tissue were between 30 and 45 nm.
Barley root and shoot tissue show very similar SeNP distribution pattern, with just few slightly larger particles in the root tissues.
Linum roots show a broader distribution and a higher number of SeNP than linum shoots. The large number of detected particles and the shapes of the histograms of linum root tissue also suggest a considerable amount of SeNP with a diameter of 30 nm or less, that were not detected due to the size detection limit.
The results for lentils in both root and show tissues a very inconsistent. While all plants clearly show occurrence of SeNP the number of particles, the size range and size distribution vary strongly among all samples.
Unlike the other species capsicum shows consistently more SeNP in the shoot samples in comparison the root tissues. While all root samples show strong selenium nano signals mostly ranging from 40 to 90 nm with a maximum between 46 and 60 nm, the corresponding shoot tissues contain larger amounts of nano particles in a broader distribution ranging from 30 to 160 nm.
The lettuce samples showed overall broad distribution of SeNP. While there was a lot of variation among the different plants of this species and maximum particle numbers lying between 40 and 70 nm, particles were detected with diameters of up to 110–140 nm in all root and shoot samples.
Cucumbers have a rather narrow size distribution of SeNP compared to the large number of particles that can be found in root and shoot tissue. With no clear differences between the two plant parts, a quite sharp cut of can be seen around 75–90 nm. However, the shape of the histograms suggests the existence of particle with a size of less than 30 nm that fell below the size detection limit, especially in the roots.
Carrots show many SeNP in root and shoot samples, yet clear differences can be observed. Generally, there are more particles in the root tissue with a broader distribution from 36 to 120 nm, whereas the observed carrot shoots contain particles mostly in the area between 36 and 65 nm.
Discussion
With a flow rate of about 0.25 ml/min, a measuring time of 2 min, and a concentration of either 100 µg/ml or 50 µg/ml, each histogram in represents the amount of SeNP that are detected in a sample that represents 50 µg or 25 µg of root or shoot tissue. With the developed method we detected SeNP in root and shoot tissue of all observed species. We observed variation within the results in terms of number, size, and distribution of nano particles but nonetheless noticed some patterns and consistency. For most species the most detected particle size was located between 30 and 65 nm. Samples with a narrow size distribution usually showed a symmetrical size distribution on both sides of the maximum. Samples with a wider size distribution often lacked symmetry in size distribution with a large quantity of SeNP bigger than the mean. The fairly bell-shaped histograms for barley, brown mustard and lens raise the assumption, that the actual size distribution for other plants might be more symmetrical than the one portrayed here, which is restricted by the size detection limit.
It should be kept in mind, that not only sp-ICP-MS is quite a new method, but even more the analysis of SeNP with an sp-ICP-MS method has rarely ever been done. Furthermore, given that no naturally derived SeNP in plants have ever been analysed before, the newly developed digestion and dialysis methods create many unknown variables and therefore the quantitative aspect of the given results should not be overestimated. However, several hundred to several thousand particles were found in every sample and the results clearly indicate the occurrence of SeNP in both plant shoots and roots, for all the included plants. The consistent detection of NP in a specific range suggests the existence of a system within the plant physiology that leads to the synthesis of SeNP. Samples were taken from the nutrient solutions of different growth cycles and tested for SeNP. None were found, from which we concluded, that the nano particle synthesis was not caused by microorganisms within the solution but took place within the plants.
Given, that the main share of NP found in this study is no larger than 50 nm in diameter and the earlier described size effect stating that smaller NP can have a stronger impact on the activity of selenium dependent enzymes, the here described NP or plants containing those NP are very promising candidates for the treatment and prevention of diseases that are linked to a low selenium status.
Previous research focussed on the synthesis of SeNP using plant extracts. The materials used include leaves, fruits, peels and flowers. Different temperatures, times and the use of a microwave oven or use of additional reductive agents like ascorbic acid are mentioned [Citation16]. This form of green synthesis is described to produce stable nano particles and is reliable, eco-friendly, and cost effective [Citation13].
The particles synthesised with plant extracts show similar size distributions as the naturally grown ones in our study [Citation13,Citation32]. Phytochemicals with reductive properties including polyphenols, flavonoids and saponins are also discussed as stabilising agents within the particles. Following the assumptions that the SeNP formed in living plants are constituted in the same way, their actual size might be larger than described in this study, since the sp-ICP-MS size calculation is based solely on the detected selenium isotopes. However, given that the nanoparticles derived from plant extracts are formed in a few hours, sometimes under the influence of heat, it is safe to assume that they do not contain the same components in the same ratio.
While the role of SeNP in plants and their commonness or even ubiquitousness in the plant kingdom call for further research, the developed method was able to proof the biosynthesis of SeNP in living plants. All species that were analysed belong to very different orders of Angiospermae. In addition to that, plants like brown mustard are known to be selenium accumulators unlike other like barley, which is a non-accumulator. Still, all observed plants form SeNP, which is a strong hint that the formation of SeNP is not an isolated event, but one that occurs in plants with very different selenium tolerances and is part of a so far undiscovered metabolic pathway. These findings indicate that NP might be considerably more common in plants than expected and could be part of a physiological system that is to be studied.
Concerning human health and nutrition it can be stated that SeNP act superior to other selenium sources in terms of toxicity and effectiveness. Due to their small size and narrow size distribution, plant based SeNP are promising candidates for food supplements and drugs as they can also be expected to show great bioavailability.
Aside from the medical potential, we see cause for future research on three main botanical questions that we aim to investigate in the future: Is the biosynthesis of SeNP ubiquitous within the plant kingdom? How common are SeNP in edible parts of plants, such as fruits and vegetables? Is there a physiological system that involves the synthesis and metabolism of NP of further elements?
The formation of selenium nanoparticles in the metabolism of intact plants has not yet been described. By means of a combination method developed and optimised by us using enzymes, dialysis and single-particle mass spectrometry with inductively coupled plasma, selenium nanoparticles were reliably detected in both shoots and roots of eight different plant species. The existence of selenium nanoparticles thus appears to be a newly discovered natural biological principle commonly found in the plant kingdom. The very common selenium nanoparticles found in most plants have a mean size diameter of about 48 nm (30–65 nm).
According to the formulas
the nanoparticles consist of 0.52, 2.1, and 5.2 million selenium atoms with a size of 30 nm, 48 nm, and 65 nm, respectively. It would be interesting to clarify the internal structure and the coating of the surface, and of course the plant physiological function.
Author contributions
J.V. and K.G. designed the experiments. J.V. performed the experiments and analysed the data with supervision by K.G. J.V. and K.G. wrote the manuscript.
Supplemental Material
Download PDF (5.7 MB)Acknowledgments
We thank the German Federal Institute for Drugs and Medical Devices for providing the laboratory equipment that was used for this study and Dr. Norwig for his work and support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data for are shown in the supplementary information.
Additional information
Funding
References
- Schiavon M, Pilon-Smits EAH. The fascinating facets of plant selenium accumulation – biochemistry, physiology, evolution and ecology. New Phytol. 2017;213(4):1582–1596.
- White PJ. Selenium metabolism in plants. Biochim Biophys Acta Gen Subj. 2018;1862(11):2333–2342.
- Bela K, Horváth E, Gallé À, et al. Plant glutathione peroxidases: emerging role of the antioxidant enzymes in plant development and stress responses. J Plant Physiol. 2015;176:192–201.
- Gupta M, Gupta S. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci. 2016;7:2074.
- Kolbert Z, Molnár Á, Feigl G, et al. Plant selenium toxicity: proteome in the crosshairs. J Plant Physiol. 2019;232:291–300.
- White PJ. Selenium accumulation by plants. Ann Bot. 2016;117(2):217–235.
- Reich HJ, Hondal RJ. Why nature chose selenium. ACS Chem Biol. 2016;11(4):821–841.
- Lima LW, Pilon-Smits EAH, Schiavon M. Mechanisms of selenium hyperaccumulation in plants: a survey of molecular, biochemical and ecological cues. Biochim Biophys Acta Gen Subj. 2018;1862(11):2343–2353.
- Van Hoewyk D, Garifullina GF, Ackley AR, et al. Overexpression of AtCpNifS enhances selenium tolerance and accumulation in arabidopsis. Plant Physiol. 2005;139(3):1518–1528.
- Shoeibi S, Mashreghi M. Biosynthesis of selenium nanoparticles using Enterococcus faecalis and evaluation of their antibacterial activities. J Trace Elem Med Biol. 2017;39:135–139.
- Jiménez-Lamana J, Abad-Álvaro I, Bierla K, et al. Detection and characterization of biogenic selenium nanoparticles in selenium-rich yeast by single particle ICPMS. J Anal At Spectrom. 2018;33(3):452–460.
- Nayak V, Singh KRB, Singh AK, et al. Potentialities of selenium nanoparticles in biomedical science. New J Chem. 2021;45(6):2849–2878.
- Anu K, Singaravelu G, Murugan K, et al. Green-Synthesis of selenium nanoparticles using garlic cloves (Allium sativum): biophysical characterization and cytotoxicity on vero cells. J Clust Sci. 2017;28(1):551–563.
- Cittrarasu V, Kaliannan D, Dharman K, et al. Green synthesis of selenium nanoparticles mediated from ceropegia bulbosa roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci Rep. 2021;11(1):1032.
- Janthima R, Siri S. Cellular biogenesis of metal nanoparticles by water velvet (Azolla pinnata): different fates of the uptake Fe3+ and Ni2+ to transform into nanoparticles. Artif Cells Nanomed Biotechnol. 2021;49(1):471–482.
- Kryukov GV, Castellano S, Novoselov SV, et al. Characterization of mammalian selenoproteomes. Science. 2003;300(5624):1439–1443.
- Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66(15):2457–2478.
- Rayman PM. Selenium and human health. Lancet. 2012;379(9822):1256–1268.
- Carlisle AE, Lee N, Matthew-Onabanjo AN, et al. Selenium detoxification is required for cancer-cell survival. Nat Metab. 2020;2(7):603–611.
- Kang D, Lee J, Wu C, et al. The role of selenium metabolism and selenoproteins in cartilage homeostasis and arthropathies. Exp Mol Med. 2020;52(8):1198–1208.
- Pyrzynska K, Sentkowska A. Biosynthesis of selenium nanoparticles using plant extracts. J Nanostruct Chem. 2022;12(4):467–480.
- Lin Z, Li Y, Guo M, et al. Inhibition of H1N1 influenza virus by seleniumnanoparticles loaded with zanamivir through p38and JNK signaling pathways. RSC Adv. 2017;7(56):35290–35296.
- He L, Zhao J, Wang L, et al. Using nano-selenium to combat coronavirus disease 2019 (COVID-19)? Nano Today. 2021;36:101037.
- Hoffman KS, Vargas-Rodriguez O, Bak DW, et al. A cysteinyl-tRNA synthetase variant confers resistance against selenite toxicity and decreases selenocysteine misincorporation. J Biol Chem. 2019;294(34):12855–12865.
- Jia X, Li N, Chen J. A subchronic toxicity study of elemental nano-Se in Sprague-Dawley rats. Life Sci. 2005;76(17):1989–2003.
- Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42(10):1524–1533.
- Menon S, Devi KS, Santhiya R, et al. Selenium nanoparticles: a potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf B Biointerfaces. 2018;170:280–292.
- Bhattacharjee A, Basu A, Bhattacharya S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus. 2019;62(3):259–268.
- Liao G, Tang J, Wang D, et al. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J Surg Oncol. 2020;18(1):81.
- Peng D, Zhang J, Liu Q, et al. Size effect of elemental selenium nanoparticles (nano-Se) at supranutritional levels on selenium accumulation and glutathione S-transferase activity. J Inorg Biochem. 2007;101(10):1457–1463.
- Skalickova S, Milosavljevic V, Cihalova K, et al. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90.
- Ghaderi RS, Adibian F, Sabouri Z, et al. Green synthesis of selenium nanoparticle by abelmoschus esculentus extract and assessment of its antibacterial activity. Mater Technol. 2022;37:1289–1297.
