Abstract
Sepsis is a devastating complication of infection and injury that, through widespread endothelial dysfunction, can cause perfusion deficits and multi-organ failure. To address the recognised need for therapeutics targetting the endothelial barrier, a topical formulation (CUR; VASCEPTOR™; Vascarta Inc, Summit, NJ) was developed to transdermally deliver bio-active concentrations of curcumin—an anti-inflammatory and nitric oxide promoter. Male, Sprague Dawley rats were treated daily with lipopolysaccharide (LPS, 10 mg/kg, IP) to induce endotoxemia, and topical applications of Vehicle Control (LPS + VC; N = 7) or Curcumin (LPS + CUR; N = 7). A third group received neither LPS nor treatment (No-LPS; N = 8). After 72 h, animals were surgically prepared for measurements of physiology and endothelial dysfunction in the exteriorised spinotrapezius muscle through the extravasation of 67 kDa TRITC-BSA (albumin) and 500 kDa FITC-dextran (dextran). At 72 h, LPS + VC saw weight loss, and increases to pulse pressure, lactate, pCO2, CXCL5 (vs No-LPS) and IL-6 (vs 0 h; p < 0.05). LPS + CUR was similar to No-LPS, but with hypotension. Phenylephrine response was increased in LPS + CUR. Regarding endothelial function, LPS + CUR albumin and dextran extravasation were significantly reduced versus LPS + VC suggesting that Curcumin mitigated endotoxemic endothelial dysfunction. The speculated mechanisms are nitric oxide modulation of the endothelium and/or an indirect anti-inflammatory effect.
Introduction
Sepsis—a driver of systemic inflammatory response syndrome—accounts for 20% of global deaths annually [Citation1]. Despite a complex and multifaceted pathology triggered and perpetuated by widespread innate immune activity, the true “motor of sepsis” is recognised as microvascular dysfunction [Citation2], which stems from endothelial barrier breakdown [Citation3]. While inflammation is a necessary component of homeostatic restoration following infection and injury, the process disrupts normal vascular function [Citation4,Citation5]. Microvessels dilate and their endothelial linings—the endothelial surface layer (ESL/glycocalyx) in contact with the blood—become porous permitting the extravasation of fluids, solutes, neutrophils, and macrophages. Locally, so-called “leaky vessels” have a minor impact on circulatory volume, but can lead to circulatory failure when dysregulated at a systemic level. The damaged endothelium also becomes prothrombotic, increasing red blood cell aggregation to create pockets of microvascular ischaemia [Citation6]. Coupled with hypovolemia, capillary beds become ischaemic causing organ damage, which is proximate to severe morbidity and mortality in sepsis [Citation7].
Sepsis is treated through a combination of antibiotics, fluids, and pressors to eliminate infection and maintain circulatory perfusion, respectively. It is a brute-force approach that is mostly insensitive to localised tissue and organ ischaemia. Growing recognition of endothelial function’s impact on septic outcomes points to the ESL and larger microvasculature as important therapeutic targets [Citation3,Citation8–10]. However, there are presently no FDA-approved drugs that improve survival in sepsis, let alone effectively addressing endothelial dysfunction [Citation11,Citation12].
Curcuminoids have shown potent anti-inflammatory and ESL-protective effects in animal models [Citation13–15] and are protective against microvascular damage in endotoxemia from lipopolysaccharide (LPS) administration [Citation16]. While the term “anti-inflammatory” is often poorly calibrated to describe the impact on in vivo immune activity, evidence suggests the correction of endothelial dysfunction is through enhancement of endothelial-derived nitric oxide (NO) [Citation13,Citation17]. In septic mice, the localised reduction of NO production from endothelial cells via the downregulation of endothelial NO synthase (eNOS) has been shown to increase endothelial dysfunction, organ damage, and mortality [Citation18]. Several studies have shown that exogenously delivered systemic NO can prevent and reverse endothelial dysfunction [Citation19–21], but high levels of NO are also implicated in the pathophysiology of sepsis [Citation22]. Curcuminoids have the desirable, bimodal effect of suppressing the high-output, inducible NOS in immune cells [Citation23] while upregulating eNOS. The net result may meet the need for NO-specific, paracrine signalling to the vasculature without elevation to the systemic NO burden during sepsis.
Despite encouraging preclinical findings, curcuminoids have had no double-blinded clinical successes due to poor aqueous solubility and in vivo bioavailability [Citation24]. A major problem likely stems from the approach of oral administration, which compounds the solubility and uptake issues [Citation25] with chemical modifications by the gut microbiome and rapid first-pass breakdown by the liver [Citation26]. Vascarta Inc. Summit, NJ has produced a highly concentrated curcuminoid (CUR) formulation (∼0.1 M; VASCEPTOR™) that recently underwent a randomised, double-blind, vehicle-controlled 48-person Phase I Clinical Study in India. Along with establishing acceptable safety and tolerability profiles, the study confirmed transdermal delivery in some of the test subjects of free, desmethoxy, and bis desmethoxy curcuminoids via post-dose plasma samples (Study No.: 05850/21-22 Protocol No.: CUR/CR/017/21-22; Vimta Labs LTD; Hyderabad, India). In this vehicle-controlled (VC), proof-of-concept study, we explore the theraputic potential of topical CUR in an endotoxemic rat model of endothelial dysfunction.
Methods and materials
Animals
The LPS Endothelial Dysfunction rat protocol and experimental procedures were conducted at Song Biotechnologies, LLC, Baltimore, MD. Thomas D. Morris, Inc IACUC approved the studies (Protocol # 20-007), which are consistent with the National Institutes of Health guidelines for the humane treatment of laboratory animals, as well as the American Physiological Society’s Guiding Principles in the Care and Use of Animals. Study subjects were twenty-two male Sprague Dawley rats (∼300 g; Charles River Laboratories, Inc., Wilmington, MA) housed in pairs and maintained on a 12/12 light/dark cycle with free access to rat chow and water.
Endotoxemia (LPS) and treatment protocol
Endotoxemia was induced through daily doses of LPS (Lot # 048M4139V; Sigma Aldrich, St. Louis, MO; 10 mg/kg, interperitoneal [IP]) for three consecutive days. LPS arrived lyophilised in 100 mg vials. Multiple vials from the same lot were reconstituted in sterile phosphate buffered saline (PBS) and pooled to ensure consistent dosing of endotoxin. The stock solution of 5 mg/ml was aliquoted and stored at −20 °C until use. Treatments were given concurrently with LPS.
CUR (V3.3, Vascarta Inc, Summit, NJ) is a curcumin-based, transdermal therapeutic. VC was identical to CUR except for the curcumin active ingredient. Topical application involved removal of a 4 × 8 cm region of dorsal fur with hair clippers. Next, a 2.5 cm diameter circle was made using VetBond™ (3M, St. Paul, MN). 0.1 ml of CUR or VC was applied within the circle and the area sealed by overlayment with Tegaderm™ (3M, St. Paul, MN), which prevented the ointment from rubbing off or leaking.
Rat endotoxemia protocol
Group assignment was randomised: (1) LPS + VC (Daily LPS IP + 0.1 ml VC ointment without curcumin; N = 7); (2) LPS + CUR (Daily LPS IP + 0.1 ml CUR ointment with curcumin; N = 7), and (3) No-LPS (No LPS and no ointment, but subjected to same blood sampling, surgical procedures, and extravasation studies; N = 8). Venous blood draws preceded treatment and LPS injections, and were used to measure blood gases and chemistry (0.1 ml; 0, 24, 48, and 72 h; ABL-90 Flex, Radiometer; Copenhagen, Denmark) and spun down for plasma banking (0.7 ml; 0 and 72 h). Inflammatory protein markers specific to innate immunity were assessed through ELISA.
Surgical
At the 72 h mark, animals were inducted to effect with 1–5% isoflurane in air for cannulations. On femoral vein access, anaesthesia was switched to alfaxalone acetate (Alfaxan, Schering-Plough Animal Health, Welwyn Garden City, UK) at a continuous rate of 0.1 mg/kg/min. Alfaxan was chosen for its minimal impact on cardiopulmonary and vascular function per an appropriate anaesthetic depth—heel but not toe pinch response.
One femoral artery and two femoral vein cannulaes in total were installed and kept patent with heparinised PBS (20 IU heparin/mL; not infused; Pfizer, New York, NY). A pressure transducer connected to a multichannel physiological monitoring system (BIOPAC MP-150, BIOPAC Systems, Goleta, CA) was attached to the arterial line. The second femoral vein was for infusion of fluorescently tagged albumin and dextran. A tracheal tube (PE-240) maintained airway patency, but animals inspired room air and were not artificially ventilated. Anaesthesia was maintained until after experimentation when animals were euthanized with a dose of Euthasol (150 mg/kg, pentobarbital component, intravenously; Delmarva, Midlothian, Virginia).
The left spinotrapezius—a dorsal muscle lining the thoracic vertebrae—was exteriorised as described by S. D. Gray [Citation27] with some adaptation to fit a thermostable animal platform adapted for intravital and fluorescence microscopy [Citation28–30]. The prep was seated on a transparent, heated pedestal that permitted both trans- and epi-illuminative microscopy, and was isolated from the atmospheric by a transparent barrier film (Krehalon; CB-100; Krehalon Limited, Japan). Animals were kept at 37 °C during experimentation, which was monitored by a rectal probe (Part # SS7L, BIOPAC Systems, Goleta, CA).
Microscopy
The core microscopy setup has been described elsewhere [Citation31–33]. Specific to this study: Observation and measurements of the exteriorised spinotrapezius preparation were carried out with a fluorescence microscope (Axioimager2m, Carl Zeiss, Jena, Germany). Arteriolar luminal diameters for arterioles (100 > 30 μm) were imaged by brightfield and measured by converting from pixels to µm using a calibration based on the pixelization of a standard microscope micrometer (Stage Micrometer, Zeiss). Arterioles were imaged before and after administration of the potent vasoconstrictor phenylephrine (PE; 1 mg/kg; West-Ward, Eatontown, NJ) to gauge microcirculatory function (2–3 vessels per experiment).
Fluorescence microscopy was performed using X-Cite 120 LED illumination system (Excelitas Technologies Corp, Walthman, MA), fluorescence camera (Axiocam 702 mono, Zeiss) filter cubes (38HE: FITC/eGFP/Alexa488 and 43HE: CY3/TexasRed/Rhodamine/Alexa568, Zeiss), and Zen II software (Zeiss). Two different sizes of fluorescently tagged molecules—67 kDa tetramethylrhodamine (TRITC) bovine serum albumin (albumin; 1 mg/kg; MilliporeSigma, Burlington, MA) and 500 kDa fluorescein isothiocyanate (FITC) dextran (dextran; 10 mg/kg; MilliporeSigma, Burlington, MA)—were used to assess extravasation rates from the blood vessel lumen to the interstitial space.
Rat endothelial dysfunction protocol
Seventy-two hours after the first LPS dose, animals were prepared for surgery as described above. Once the spinotrapezius was exteriorised and affixed to the animal platform’s thermostatic organ pedestal, the entire assembly was secured to the microscope. Following baseline measurements, fluorescently tagged molecules—albumin (red) and dextran (green)—were infused through a dedicated femoral vein. Images were taken immediately following infusion, at 5 min intervals of 30 min.
Inflammatory protein expression
Plasma samples were measured by ELISA. LIX was analysed by a specific Rat C-X-C motif chemokine 5 (CXCL5) ELISA Kit (Cat. ERCXCL5, Invitrogen, Carlsbad, CA) with a sensitivity of 20 pg/mL and a range of 24.7–6000 pg/mL. The rat-specific Interluekin-6 (IL-6) kit (LEGEND MAX™, Cat. 437107, BioLegend, San Diego, CA) had a range of 18.8 to 1,200 pg/mL.
Statistics
The data are presented as mean ± standard error (SE). Minimum study N values were determined via power analysis of our primary metric (extravasation) after a four animal/group pilot (β = 0.2 and α = 0.05; Origin 2020, OriginLab Corporation, Northhampton, MA). Statistical inter- and intra-comparisons between experimental groups utilised two-way ANOVA (repeated measures or multiple comparisons; Prism 9.4.1, GraphPad Software Inc., San Diego, CA). Intragroup comparisons were to baseline or 0 h, while intergroup were at specific time points. A one-way ANOVA with Tukey’s was used for discrete measurements such as daily weight, change in arteriolar diameters, etc. When a p value below 0.05 was detected, a post-hoc test (Dunnett’s, Tukey’s or Sidak’s) was performed.
Results
Animals and LPS treatment
Rats were age-matched (8–9 weeks old) with similar venous blood chemistry profiles at baseline (BL; 0 h). Two initial differences were detected between No-LPS and LPS + CUR groups for calcium and base deficit, but they were within the normal physiological range (). Starting weights (LPS + VC: 298 ± 15 g; LPS + CUR: 292 ± 11 g; and No-LPS: 271 ± 7 g) for included animals were not different between groups. No-LPS animals gained an additional 15 g over the 72 h dosing time course while LPS-treated groups lost weight: 236 ± 13 g for LPS + VC and 246 ± 13 g for LPS + CUR—which was significantly lower than No-LPS; p = 0.023. Animal behaviour was normal for No-LPS. LPS-treatment produced low activity levels and avoidance. Food intake was not measured but may have been a factor affecting weight.
Table 1. Venous blood chemistry (whole blood).
Systemic variables at 72 h
Effects of endotoxemia included hypotension and a trend towards tachycardia. LPS + CUR’s mean arterial pressure was significantly lower than No-LPS by 20 mmHg (p < 0.05) whereas LPS + VC’s drop of 12 mmHg was numeric (p = 0.26). Heart rate was elevated in LPS + VC non-significantly, while LPS + CUR remained approximate to No-LPS. Pulse pressure was highest for LPS + VC (p < 0.05 vs No-LPS; ).
Table 2. Cardiovascular variables.
Venous blood gases and chemistry
LPS treatments were evident in oximetry and some metabolic makers. At 48 h, the percentage of venous oxyhaemoglobin (SO2) was significantly depressed in both LPS + VC and LPS + CUR compared to No-LPS. At 72 h, LPS + VC remained depressed from its BL while LPS + CUR had recovered, but differences were not detected between groups. Venous blood oxygen tension (PVO2) was normal for LPS + CUR at 72 h, but LPS + VC and No-LPS were lower than their baselines. LPS + VC showed low PVCO2 compared to BL and No-LPS group for all time points whereas LPS + CUR was only depressed compared to No-LPS at 48 h and recovered by 72 h. The base deficit was lower for LPS + VC and LPS + CUR at 24 and 48 h compared to No-LPS. At 72 h, only LPS + VC remained below BL (). Lactate was elevated for both LPS-treated groups compared with No-LPS, but LPS + VC also showed a longitudinal rise with respect to 0 h (p < 0.05; ).
Extravasation
LPS + CUR and No-LPS showed similar rates of extravasation while LPS + VC was markedly increased. Interstitial accumulation of both tagged molecules was ∼ two-fold higher for LPS + VC after thirty minutes of observation. Both 67 kDa albumin and 500 kDa dextran plotted similarly in terms of interstitial accumulation between groups ( and ). Comparisons of LPS + VC to No-LPS were not illustrated, but a difference was detected at 10 min for albumin.
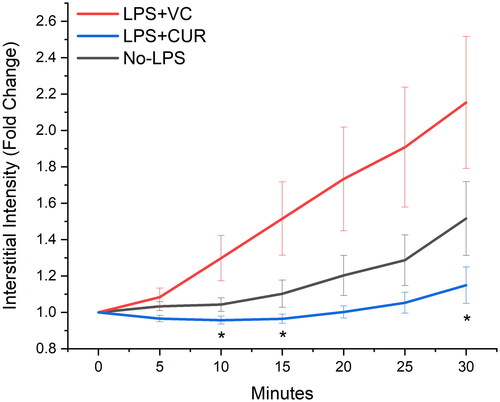
Figure 1. 67 kDa TRITC-BSA extravasation. Tagged albumin was administered IV and monitored with fluorescence microscopy of the spinotrapezius microvasculature. Data are perivascular (interstitial) accumulation over 30 min, with intensity data normalised to BL and expressed as fold change. Exposure times varied slightly between experiments (1500–2000 ms) to account for differing tissue thicknesses but were not changed within each time course. Data are mean SEM. * indicates p < 0.05 versus LPS + VC.
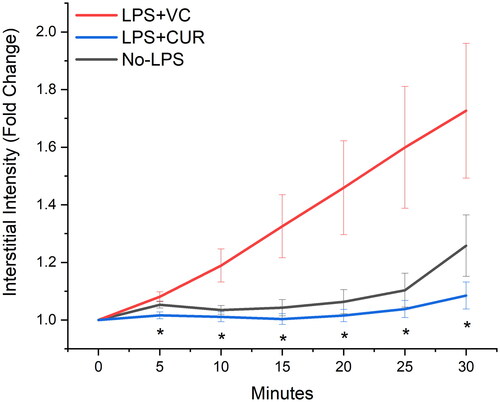
Figure 2. 500 kDa FITC-dextran extravasation. Tagged dextran was administered IV and monitored with fluorescence microscopy of the spinotrapezius microvasculature. Data are perivascular (interstitial) accumulation over 30 min, with intensity data normalised to BL and expressed as fold change. Exposure times varied slightly between experiments (1500–2000 ms) to account for differing tissue thicknesses but were not changed within each time course. Data are mean SEM. * indicates p < 0.05 versus LPS + VC
Protein size difference
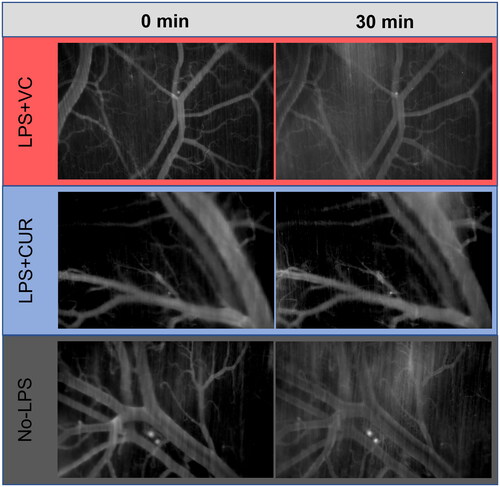
When overlaid, extravasation profiles for 67 kDa albumin and 500 kDa dextran were in good agreement. Two-way ANOVA did not detect differences between them, but for the LPS + VC group, the dextran curve was right-shifted compared to albumin. The most consistent extravasation profiles between albumin and dextran were with the LPS + CUR group, which also had the lowest overall interstitial fluorescent accumulation. contains representative images of 0 and 30 min for albumin.
Figure 3. TRITC albumin in the microvasculature. Images (5× magnification) were captured by a monochromatic digital camera using identical flash intensity and exposure times for each series. Contrast and brightness were adjusted for presentation using the Zen II software and standardised for all images. Intensity data were collected from the interstitial space (dark areas at 0 min not populated by brighter microvasculature) and converted to fold change at 30 min as an indicator of TRITC-Albumin extravasation. Images are representative of the 0 and 30 min time points for each group.
Inflammatory protein expression
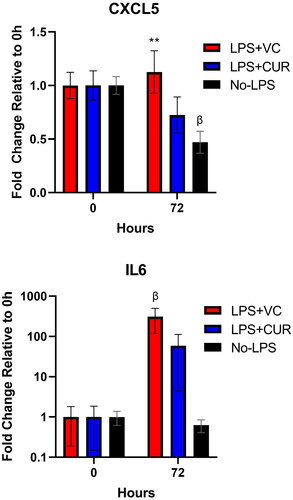
Neutrophil chemokine CXCL5 (LIX) and IL-6 protein expression were quantified from plasma using specific rat ELISA protocols. For CXCL5, expression levels at 0 h were similar between groups at 1840 ± 227, 2149 ± 294, and 1839 ± 154 pg/ml for LPS + VC, LPS + CUR, and No-LPS, respectively. The impact of LPS appeared poorly captured at 72 h, but LPS + VC was noted to be significantly higher than No-LPS group (p = 0.005), but not 0 h. No-LPS, however, decreased from 0 h (p = 0.016). Raw, 0 h values for IL-6 were 406, 411, and 520 pg/ml for LPS + VC, LPS + CUR, and No-LPS, respectively. IL-6 showed a significant, 309 fold-change increase for LPS-VC (p = 0.49). LPS + CUR also rose, numerically, by 59 fold, but was not significant vs 0 h (p = 0.95; ).
Figure 4. Plasma expression of neutrophil chemokine CXCL5 (LIX) and IL-6. Plasma samples were quantified with ELISA. Values for both CXCL5 and IL-6 proteins (pg/ml) were normalised to baseline (before initial LPS treatment) at 0 h for a comparison of relative change within groups. For CXCL5, differences between LPS + VC and LPS + CUR were trending at 72 h (p = 0.14), but not resolved at N values of 7. The Y-axis for IL-6 was set to Log10 and a significant rise was seen for LPS + VC compared to 0 h. For LPS + VC vs. LPS + CUR, p = 0.25. β indicates p < 0.05 versus 0 h. ** indicates p < 0.01 versus No LPS.
Pressor effect on arteriolar diameters
A PE challenge assessed arteriolar contractility as an indicator of microvascular function. Before dosing, arterioles were similarly sized at, 58 ± 2.9, 71 ± 5.4, and 61 ± 5.7 µm for LPS + VC, LPS + CUR, and No-LPS, respectively. Contractile response was highest for LPS + CUR (p = 0.01 vs LPS + VC; 0.04 vs No-LPS) while No-LPS and LPS + VC were not different from each other ().
Discussion
Endotoxemia was induced in rats to model systemic inflammatory conditions (SIRS, Sepsis, etc) that feature endothelial dysfunction. LPS—notably in LPS + VC—caused a moderate increase in plasma lactate, which speaks to the severity of the daily, 10 mg/kg IP dosing. To put that in context, elevated lactate often occurs in shock—as a general disorder—indicating ischaemic or hypoxic tissues. LPS also produced weight loss, lethargy, and “hunkering down” behaviour during otherwise active hours. Cardiovascular profiles were stressed with a trend towards tachycardia in LPS + VC and hypotension for LPS + CUR compared with the No-LPS group. The 30-min extravasation profiles showed the largest differences between groups with albumin and dextran leaving the LPS + VC at 2X the rate of LPS + CUR and No-LPS, which had virtually identical profiles. Overall, LPS + CUR had greater similarity to No-LPS treated animals than LPS + VC.
Degradation of the vascular endothelial barrier—ESL—is an early indicator of inflammation from sterile damage or pathogenic presence [Citation34]. Moreover, it is the driver of cardiovascular collapse as systemic inflammation worsens. LPS + CUR led to visual () and quantitative ( and ) reductions in both albumin and dextran accumulation in the interstitium, which suggests a potent and perhaps specific activity in maintaining circulatory volume during systemic inflammation. However, the data were potentially confounded by noted, but non-threatening, hypotension in LPS + CUR. A possible explanation lies with reports of curcumin lowering blood pressure by raising endothelial NO bioavailability and dilating peripheral arterioles [Citation13,Citation35]. Since reduced eNOS function exacerbates sepsis [Citation18], the effect may have been concomitant. Additional studies are needed to quantify changes to ESL thickness and shedding, but solute retention is compelling data for barrier function. The hypotension noted for LPS + CUR prevents exclusion of a reduced hydrostatic effect, however, as microvascular dimensions and flow conditions were similar between groups, hypotension was an unlikely primary effector of reduced extravasation.
The No-LPS group was included to establish an experimental control for systemic variables and an extravasation rate without LPS. Oximetry metrics (SO2, PVO2, etc) were important for assessing circulatory status over the 72 h blood draw and treatment phase. Although differences were noted with LPS at earlier time points, No-LPS showed the same decline in SO2 and PVO2 as the LPS groups. Since blood draws were the only injurious commonality, we propose the reduction in blood volume had a greater impact on oximetry variables than LPS treatments. Regarding extravasation and in line with expectations, there was a steady-state rate of tagged 67 kDa albumin accumulation in the interstitial fluid of No-LPS. A low level of albumin leak is typical in healthy vasculature [Citation36], however the same was noted for the larger 500 kDa dextran particles. Whether it was a limitation of exteriorising the spinotrapezius muscle, a concentration effect (10 mg/kg dextran versus 1 mg/kg albumin), or a true characterisation of No-LPS vascular permeability is unclear. But relative comparisons to treatment groups remain valid in the context of the model system.
The endotoxemic ESL injury did not significantly differentiate between the two particle sizes. The intention was to compare solute size to extravasation rate, but barrier porosity was more substantial than expected, which proved equally informative. Differential extravasation rates have been noted for albumin and smaller particle sizes for milder or non-inflammatory insults to the endothelium like platelet depletion [Citation37]. Those authors also noted that greater endothelial damage can permit larger particles and whole cells to extravasation at a diffusion-limited rate. Here, extravasation profiles were similar, suggesting sufficient porosity to keep even the 500 kDa particle diffusion-limited. Curcumin was effective in reducing overall extravasation but still did not separate the particles by size.
An intriguing finding that requires further study was the enhanced vasoconstrictive response to PE. Arterioles are the major resistance vessels and improved contractility offers a finer level of control over blood pressure. Clinically, hypotension in sepsis is treated with pressers to maintain viable blood pressure. Endotoxemia from LPS reduces contractile responses from arterioles [Citation38], and was demonstrated—but insufficiently powered—in this study by a numeric decrease in LPS + VC compared to No-LPS. It is noteworthy that the No-LPS PE response was numerically lower than historical data (arteriolar delta of 40 ± 8 µm after 1 mg/kg PE) from the exteriorised spinotrapezius preparation in healthy rats [Citation33], which suggests an impact from the daily blood draws. LPS + CUR’s relative improvement of PE response for this study was clear, however, and given that PE bypasses the endothelium to directly affect vascular smooth muscle contractility, we can only speculate on the mechanism. Numerous studies have shown that curcumin has the potential to address vascular dysfunction triggered by excess ROS and the activation of multiple inflammatory pathways, but this would be the first report, to our knowledge, describing a curcuminoid effect on arteriolar response to PE. Further research is encouraged since there is an urgent unmet medical need for therapeutics that can reduce or eliminate the need for pressers. Current dosing schedules are tailored to meet the needs of critical organ perfusion at the eventual cost of destructive ischaemia in the peripheral circulation. A topical/transdermal therapeutic agent would be well-poised to supplement existing clinical protocols.
While much of the discussion has focussed on the microvasculature, the curcuminoids in CUR appeared to possess some independent function as an anti-inflammatory. Mild, but comparative decreases in CXCL5—otherwise known as LPS Induced CXC (LIX)—and IL-6 suggest an incomplete effect. The true impact on CXCL5 was likely missed by the 72 h time point since it is an early indicator of innate immune activity. Also, neutrophils are not the dominant innate immune cell in rodents as they are in humans, which may have also suppressed activity. IL-6’s large fold increases aligned better with expectations of endotoxemia, but statistical resolution between groups was hampered by the small sample size—fine for our primary in vivo metrics, but insufficient for biomarkers. Still, the cytokine trends align with the PE response data and suggest it to be inflammatory-mediated. Additionally, IL-6 was positively associated with extravasation, which is consistent with inflammation as a well-documented driver of “leaky” blood vessels.
Limitations
The goal of this study was to treat endothelial dysfunction as a complication of endotoxemia, not to treat any specific pathology. We cannot confirm how the curcuminoids in CUR may interfere with necessary immune processes during an active infection. We speculate it serves as a necessary regulator against an excessive and life-threatening response, but additional, longitudinal studies are needed using active disease to confirm. We infer ESL damage as changes to extravasation rates but did not measure direct markers such as Syndecan 1 due to a preference to put our volume-limited blood samples into inflammatory biomarker quantification. However, the exciting extravasation data necessitates a more mechanistic look at ESL function in terms of biomarkers and microscopy.
Conclusion
The systemic inflammation seen in sepsis can produce dangerous hypotension leading to tissue ischaemia, hypoxia, organ damage, and death. The catalyst of this progression into shock is the breakdown of the endothelial barrier and resultant circulatory hypovolemia. CUR, a transdermal curcuminoid formulation utilising a proprietary solvent system, mitigated LPS-induced endothelial dysfunction and improved cardiovascular and metabolic indicators of endotoxemia. The suspected mechanism involves preservation or restoration of the ESL through either a direct NO or indirect anti-inflammatory effect. As septic morbidity and mortality are inextricably linked to endothelial dysfunction, effectively delivered curcuminoids with specific endothelial-preservative effects may offer an important therapeutic benefit.
Authors’ contributions
William Nugent: Study design, data analysis, writing. Danuel Carr: Data collection. Joel Friedman: Study funding, test article development, study design, writing. Bjorn Song: Study funding, study design, data analysis, data collection, writing.
Disclosure statement
Bjorn Song and Joel Friedman are co-founders of Vascarta Inc., producer of CUR/VASCEPTOR. Song Biotechnologies was provided study funds by Vascarta Inc. and William Nugent and Danuel Carr are employees of Song Biotechnologies. No other potential conflicts are declared.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211.
- Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9 Suppl 4(Suppl 4):S13–S9.
- Goldenberg NM, Steinberg BE, Slutsky AS, et al. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med. 2011;3(88):88ps25.
- Chignalia AZ, Yetimakman F, Christiaans SC, et al. The glycocalyx and trauma: a review. Shock. 2016;45(4):338–348.
- Wiesinger A, Peters W, Chappell D, et al. Nanomechanics of the endothelial glycocalyx in experimental sepsis. PLoS One. 2013;8(11):e80905.
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36(1):296–327. 2008
- Park JS, Kim SJ, Lee SW, et al. Initial low oxygen extraction ratio is related to severe organ dysfunction and high in-hospital mortality in severe sepsis and septic shock patients. J Emerg Med. 2015;49(3):261–267.
- Chalkias A, Laou E, Mermiri M, et al. Microcirculation-guided treatment improves tissue perfusion and hemodynamic coherence in surgical patients with septic shock. Eur J Trauma Emerg Surg. 2022;48(6):4699–4711.
- Ince C, Mayeux PR, Nguyen T, et al. The endothelium in sepsis. Shock. 2016;45(3):259–270.
- Langston JC, Rossi MT, Yang Q, et al. Omics of endothelial cell dysfunction in sepsis. Vasc Biol. 2022;4(1):R15–R34.
- Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203.
- DeMerle KM, Angus DC, Baillie JK, et al. Sepsis subclasses: a framework for development and interpretation. Crit Care Med. 2021;49(5):748–759.
- Boonla O, Kukongviriyapan U, Pakdeechote P, et al. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53.
- Sankrityayan H, Majumdar AS. Curcumin and folic acid abrogated methotrexate induced vascular endothelial dysfunction. Can J Physiol Pharmacol. 2016;94(1):89–96.
- Tubsakul A, Sangartit W, Pakdeechote P, et al. Curcumin mitigates hypertension, endothelial dysfunction and oxidative stress in rats with chronic exposure to lead and cadmium. Tohoku J Exp Med. 2021;253(1):69–76.
- Lukita-Atmadja W, Ito Y, Baker GL, et al. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock. 2002;17(5):399–403.
- Santos-Parker JR, Strahler TR, Bassett CJ, et al. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging. 2017;9(1):187–208.
- Coletta C, Modis K, Olah G, et al. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18(5):511.
- Gundersen Y, Corso CO, Leiderer R, et al. The nitric oxide donor sodium nitroprusside protects against hepatic microcirculatory dysfunction in early endotoxaemia. Intensive Care Med. 1998;24(12):1257–1263.
- Tian R, Peng R, Yang Z, et al. Supplementation of dietary nitrate attenuated oxidative stress and endothelial dysfunction in diabetic vasculature through inhibition of NADPH oxidase. Nitric Oxide. 2020;96:54–63.
- Williams AT, Muller CR, Govender K, et al. Control of systemic inflammation through early nitric oxide supplementation with nitric oxide releasing nanoparticles. Free Radic Biol Med. 2020;161:15–22.
- Spiller F, Oliveira Formiga R, Fernandes da Silva Coimbra J, et al. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide. 2019;89:32–40.
- Jung KK, Lee HS, Cho JY, et al. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006;79(21):2022–2031.
- Nelson KM, Dahlin JL, Bisson J, et al. The essential medicinal chemistry of curcumin. J Med Chem. 2017;60(5):1620–1637.
- Anand P, Sundaram C, Jhurani S, et al. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267(1):133–164.
- Hassanzadeh K, Buccarello L, Dragotto J, et al. Obstacles against the marketing of curcumin as a drug. Int J Mol Sci. 2020;21(18):6619.
- Gray SD. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res. 1973;5(3):395–400.
- Golub AS, Pittman RN. Thermostatic animal platform for intravital microscopy of thin tissues. Microvasc Res. 2003;66(3):213–217.
- Nugent WH, Song BK, Pittman RN, et al. Simultaneous sampling of tissue oxygenation and oxygen consumption in skeletal muscle. Microvasc Res. 2016;105:15–22.
- Song BK, Nugent WH, Moon-Massat PF, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 2014;95:124–130.
- Nugent WH, Carr DA, MacBryde R, et al. Gavage approach to oxygen supplementation with oxygen therapeutic Ox66 in a hypoventilation rodent model of respiratory distress. Artif Cells Nanomed Biotechnol. 2021;49(1):709–716.
- Nugent WH, Cestero RF, Ward K, et al. Effects of sanguinate(R) on systemic and microcirculatory variables in a model of prolonged hemorrhagic shock. Shock. 2017;52(1S Suppl 1):108–115.
- Song BK, Light WR, Vandegriff KD, et al. Systemic and microvascular comparison of lactated ringer’s solution, VIR-HBOC, and alpha-alpha crosslinked haemoglobin-based oxygen carrier in a rat 10% topload model. Artif Cells Nanomed Biotechnol. 2020;48(1):1079–1088.
- Czabanka M, Peter C, Martin E, et al. Microcirculatory endothelial dysfunction during endotoxemia–insights into pathophysiology, pathologic mechanisms and clinical relevance. Curr Vasc Pharmacol. 2007;5(4):266–275.
- Czyzynska-Cichon I, Janik-Hazuka M, Szafraniec-Szczesny J, et al. Low dose curcumin administered in hyaluronic acid-based nanocapsules induces hypotensive effect in hypertensive rats. Int J Nanomedicine. 2021;16:1377–1390.
- Renkin EM, Joyner WL, Gustafson-Sgro M, et al. Albumin extravasation rates in tissues of anesthetized and unanesthetized rats. J Appl Physiol. 1989;66(5):2056–2060.
- Gupta S, Konradt C, Corken A, et al. Hemostasis vs. homeostasis: platelets are essential for preserving vascular barrier function in the absence of injury or inflammation. Proc Natl Acad Sci U S A. 2020;117(39):24316–24325.
- Hall E, Brookes ZL. Angiopoietin-1 increases arteriolar vasoconstriction to phenylephrine during sepsis. Regul Pept. 2005;131(1-3):34–37.
