Abstract
Human papillomavirus (HPV) infection and related diseases are clinical challenges. The efficacy of 5-aminolevulinic acid photodynamic therapy (ALA-PDT) using red laser (630 ± 5 nm) is remarkable and safe. In this study, we aim to investigate the efficacy of ALA-450 nm PDT comparing with ALA-635 nm PDT. We detected cell proliferation and cell apoptosis through MTT assay and flow cytometry assay respectively. Flow cytometry assay determined the intracellular reactive oxygen species (ROS) generation. Western blotting analysis investigated the protein expression. In vivo, immunohistochemical staining assay and TUNEL assay were performer to detect cell apoptosis. ALA-450 nm PDT inhibited the proliferation of End1 and HeLa cells, promoted cell apoptosis more effectively than ALA-635 nm PDT, and induced cell death probably through increasing the intracellular ROS generation and caspase-dependent apoptosis pathway. In vivo, ALA-450 nm PDT significantly inhibited tumour growth and activated cell apoptosis. The ALA-450 nm PDT had an advantage over ALA-635 nm PDT on inhibiting the proliferation of End1 and HeLa cells and inducing cell apoptosis. The ALA-450 nm PDT might be a promising therapeutic strategy for eradicating the HR-HPV infected cells and promoting the integration of diagnosis and treatment of HR-HPV related diseases.
We combined 5-aminolevulinic acid with 450 nm blue laser using as a novel type of photodynamic therapy.
The ALA-450 nm PDT had an advantage over ALA-635 nm PDT on inhibition of the proliferation of End1 and HeLa cells and inducing cell apoptosis in vitro and in vivo.
The ALA-450 nm PDT may provide a novel alternative therapeutic option in patients with persistent HPV infection and promote the integration of diagnosis and treatment.
Highlights
Introduction
Human papillomaviruses (HPV) are two-strand ring DNA viruses that infect human epithelial cells and are associated with various benign and malignant lesions of the mucous membrane and skin [Citation1]. High-risk (HR) HPVs are accounts of several important cancers, including different anogenital and head and neck cancers [Citation2]. Cervical cancer is by far the most common HPV-related disease [Citation3], nearly all of which are due to chronic HPV infection [Citation4]. Cervical cancer is the fourth most common cancer in women worldwide and accounts for almost 570,000 new cases with approximately 85% in less developed areas [Citation5].
In the case of persistent HR-HPV infection, treatment options include antiviral therapies such as cidofovir and intralesional or intramuscular interferon-a, and the application of topical imiquimod in exophytic wart [Citation6]. Additionally, simple or radical vulvectomy and cervicectomy can be used to eliminate vulvar and cervical lesions. Chemotherapy, radiation, and laser therapy can all be utilised to treat more advanced lesions [Citation7]. Unfortunately, side effects have been identified with high rates of recurrence and metastasis, including pain, irritability, superficial ulceration, and hypersensitivity [Citation8].
Since the late 1990s, topical photodynamic therapy (PDT) has been investigated for the treatment of skin and mucosal lesions caused by HPV infection [Citation9]. In heme biosynthesis, 5-aminolevulinic acid (5-ALA) is a naturally occurring metabolite that serves as a precursor to porphyrin. And, as compared to first-generation hematoporphyrin mixes and second-generation porphyrin derivatives, 5-ALA is a third-generation photosensitizer that is more cancer-specific and has lower phototoxicity [Citation10]. In addition to being as successful as standard therapies for condyloma and intraepithelial neoplasia, 5-aminolevulinic acid (ALA)-mediated PDT (ALA-PDT) appears to have superior aesthetic outcomes. PDT is able to eradicate the virus itself and hence lessen recurrence in addition to being able to remove the lesion, cause inflammation, and elicit immunological responses [Citation11–13].
Numerous case studies have shown that PDT-mediated with systemic injection of photosensitizer is helpful in treating primary and recurring gynaecologic malignancies by starting a chain of photocytotoxicity events that kill cancer cells. Based on the absorption spectra of porphyrins, the wavelengths of light were selected: blue because the highest peak is at 400 nm (the Soret band), and red because it has better penetration depth but less absorption at 650 nm (a Q band) [Citation14]. However, nearly all the ALA-PDT so far have used red light semiconductor laser [Citation15–19]. The therapeutic efficacy and mechanism of blue laser-induced PDT have still remained unknown.
In this study, we evaluated the therapeutic efficacy of ALA-PDT induced by blue light semiconductor laser (450 nm) in vitro and in vivo, and further identified the mechanism, which were compared with ALA-PDT mediated by red laser (635 nm). We are trying to find a more effective and convenient way of photodynamic therapy to eradicate the HR-HPV infected cells.
Materials and methods
Cell culture and reagents
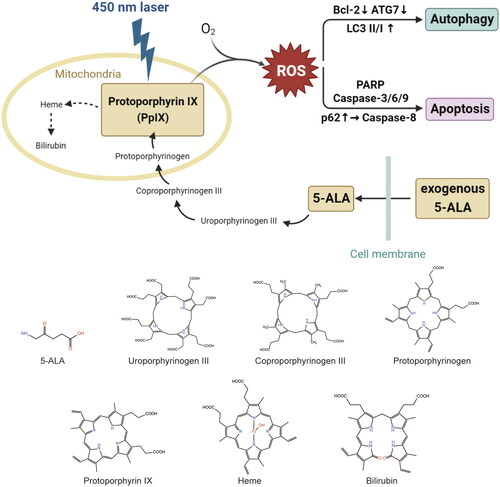
End1/E6E7 cells were supplied by American type culture collection (Manassas, Virginia, United States), and the National Collection of Authenticated Cell Cultures (Shanghai, China) provided HeLa cells as well as HaCaT cells. 1640 (Gibco, Invitrogen, Carlsbad, CA, USA) was used to culture End1/E6E7 cells and DMEM (Gibco, Invitrogen, Carlsbad, CA, USA) was applied to culture HeLa cells and HaCaT cells in a humidified atmosphere of 95% air and 5% CO2 at 37 °C. And we included 10% FBS in the culture medium, as well as 1% penicillin/streptomycin. End1/E6E7 cell line, characterised by normal karyotype and stable phenotype of normal endocervical epithelial cell, is a kind of immortalised standardised reproducible in vitro models of HPV infection and is nontumorigenic in nude mice [Citation20]. HeLa cell line, known as a kind of human cervical cancer cell line, proliferates abnormally rapidly and is usually used in cervical cancer-related studies in vitro and in vivo. 5-aminolevulinic acid (5-ALA), the photosensitizer, was provided by Fudan-Zhangjiang Bio-Pharmaceutical Co., Ltd. (Shanghai, China). In mitochondria, ALA is transformed to a photoactive molecule called protoporphyrin IX (PpIX). In tumour cells, a lot of exogenously supplied ALA is quickly absorbed into the cytoplasm through PEPT-1 (oligopeptide transporter 1, PEPT1) and biosynthesized into PpIX in cell mitochondria. PpIX accumulation in cancer cells is accelerated by overexpression of the ATP-binding cassette (ABC) transporter and certain conversion enzymes. Meanwhile, a decrease in ferrochelatase activity and an increase in transferrin receptor activity cause PpIX accumulation in cancer cells during heme production as well. These distinguishing aspects of heme production are thought to be the common mechanism of diverse cancer cells (Warburg effect) [Citation10]. Therefore, PpIX accumulates in cancer cells but not in healthy cells.
In Vitro ALA-PDT
ALA was dissolved in phosphate-buffered saline (PBS) at 200 mmol/L and stored at −20 °C. When the cells grew to 70%–80% confluence, the culture medium was removed and FBS was added to wipe off the remnant serum. Then the PBS was changed to a serum-free medium with 0.1 mmol/L ALA. After incubated for 4 h, the serum-free medium was replaced by fresh serum-containing medium before PDT. In vitro, we used 450 nm and 635 nm semiconductor laser (Blueray Medical Technologies, Ltd., Shaanxi, China) at 10 mW/cm2 for 10 min, and a light energy density of 6 J/cm2 was performed (The irradiation dose [J/cm2] = fluence rate [mW/cm2] × time [s]).
Cell viability assay
The cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Approximately 20,000 cells were seeded into 24-well plates for 24 h before treated with ALA-PDT, as mentioned above. The serum-containing media was replaced with serum-free media 150 μL and MTT reagent (10×) in each well after 24 h post irradiation, followed by incubation at 37 °C for 3 h. Then the media was replaced with DMSO (200 μL) in each well, and the absorbance was determined at 490 nm using a microplate luminometer (Bio Tek).
Live and dead cell double staining assay
By using a live and dead cell double staining kit, the cytotoxicity was discovered (KeyGEN BioTECH). In 24-well plates, cells were grown for 24 h with or without ALA-PDT therapy. Cells were stained with PBS (500 L) in each well with Calcein AM (2 M) and PI (8 M) for 30 min after removing the medium and washing with PBS. Live and dead cells were observed under a microscope (Calcein AM Ex/Em: 495 nm/520 nm, PI Ex/Em: 530 nm/620 nm) (×40).
Determination of cell apoptosis
The apoptosis was evaluated using FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen). The cells were incubated in 6-well plates at 37 °C in a humidified incubator containing 5% CO2. For ALA-PDT groups, cells were further cultured for 24 h after treatment. Cells were trypsinized, washed twice in PBS, and then resuspended at a concentration of 1 × 106 cells per mL in binding buffer. A 5 ml culture tube was then filled with 100 L of the solution. Cells were incubated for 15 min at RT (25 °C) in the dark after adding 5 L each of FITC Annexin V and PI. And next each tube received 400 L of binding buffer. The apoptosis rate was measured using the flow cytometer.
Intracellular ROS detection
The intracellular ROS was detected by ROS Detection Kit (KeyGEN BioTECH). The cells were incubated in 6-well plates, and 1 ml DCFH-DA diluent was added to each well after wiping off the medium. The cells were incubated for 20 min at 37 °C in a humidified incubator containing 5% CO2 followed by washing with serum-free medium three times. For ALA-PDT groups, PDT treatment was achieved, and then all groups were examined using flow cytometer.
Western blotting analysis
Following different management, cells in each group were incubated for 24 h and extracted total protein. The BCA Protein Assay Kit was used to determine the protein content, and the protein samples were boiled to encourage protein denaturation. SDS-PAGE was used to separate the proteins (10 g), and polyvinylidene difluoride membranes were used to transfer them (Millipore Corporation). PVDF membranes were treated with the primary antibody for an overnight period at 4 °C after being blocked with 5% bovine serum albumin for 2 h at 37 °C. PVDF membranes were incubated with a secondary antibody for an hour at room temperature after being rinsed with TBST buffer three times (10 min each time). The chemiluminescence detection device (Millipore Corporation) is used to detect the response after three TBST washes (10 min each). β-Actin is used as the internal parameter.
ALA-PDT in vivo
Healthy BALB-C female nude mice (n = 20; 4–5 weeks; 18–20 g weight; Xi’an Huishi Biotechnology Company, China; Certificate No. 2017-1132) were subcutaneously implanted with HeLa cells (5 × 106/mL) in the right axilla. At the medical laboratory animal centre, feeding was done using a specific pathogen-free (SPF) protocol. We kept track of the tumour volume and weight changes throughout the day. PDT was used when the transplanted tumour’s volume was between 100–150 mm3. In accordance with China’s national regulations for the care and use of animals, the Institutional Animal Care and Treatment Committee of Xi’an Jiaotong University approved the tests.
Tumour-bearing mice were randomly divided into four groups, including the control group (PBS), PDT (450 nm), ALA-PDT (200 mg/kg; 635 nm), ALA-PDT (200 mg/kg; 450 nm). Three hours after the injection of ALA, mice in ALA-PDT groups would receive irradiation. In vitro, the protocol for light power density was 100 mW/cm2, with an irradiation time for 10 min. Nude mice were euthanized after 14 days of observation, tumour samples were obtained, and Bcl-2 protein expression was assessed by immunohistochemistry (IHC). A TdT-mediated deoxyuridine triphosphate (dUTP) nick-end labelling (TUNEL) apoptosis test kit was also used to measure the level of cellular death.
Immunohistochemical staining assay
Sections of the 10% formalin-fixed, paraffin-embedded tissues were cut into 5 µm-thick pieces. The samples were also deparaffinized with xylene, rehydrated with a gradient alcohol series, and treated twice for five minutes each with 500 W microwave energy in 10 mM citrate buffer (pH 6.0). To stop endogenous peroxidase activity, the sections were submerged in H2O2 (3%) solution after being rinsed in Tris-buffered saline (pH 7.6). The material was then combined with a primary antibody (Bcl-2; Servicebio, China), 5% foetal bovine serum (FBS; 1:20), and incubated overnight at 4 °C. The first antibody was tested using an adequate secondary antibody at 37 °C and incubated for 30 min the next day after washing the sections. To create colour, diaminobenzidine (DAB) was utilised. Next, haematoxylin reverse staining was carried out. Final steps included sealing, drying, and seeing the plates under an inverted microscope (Nikon, Tokyo, Japan). The claybank or brown staining of the cytoplasm served as the indicator of Bcl-2 protein expression. Bcl-2 positive cells were counted under a microscope (×400) in each section for 5 fields, and the mean number of positive cells in the 5 fields was determined as the number of positive cells in the section.
TUNEL assay
The TUNEL reaction mixture was applied to dewaxed, dehydrated, washed, and then incubated paraffin sections for 60 min at 37 °C before a second wash and fluorescence microscopy examination. The conversion agent POD (Roche, Switzerland) was also added to the mixture before being incubated at 37 °C for 30 min before another round of washing. Following the addition of the DAB substrate solution, the samples were incubated at room temperature for 5–20 min before being examined using an optical microscope (×100). The number of cells that underwent apoptosis was counted in five randomly chosen fields.
Statistical analysis
Data analysis was carried out using GraphPad Prism 9.0 (La Jolla) statistical software. The mean and standard deviation of the data were displayed. A value of p < 0.05 was regarded as statistically significant when determining the statistical significance between means using the Student’s t test.
Results
ALA-450 nm treatment promoted cell death in End1 and HeLa cells
To evaluate the effect of ALA-450 nm on cell proliferation, HaCaT, End1, and HeLa cells were exposed to 450 nm laser with 6 J/cm2 after being treated with different concentrations of ALA (0, 0.05, 0.1, and 0.2 mmol/L), and the cell viabilities were determined after irradiation via MTT assay. Compared with the normal epithelial cells, HaCaT cells, ALA-450 nm treatment significantly inhibited cell proliferation of End1 and HeLa cells (). In addition, the cell viability inhibition induced by ALA-450 nm PDT was indeed regulated according to the energy density of blue laser. With the increase of irradiation dose, the cell viabilities of End1 and HeLa cells observably decreased, compared with the group without irradiation ().
Figure 1. ALA-450 nm inhibited cell viability of End1 and HeLa cells and caused no damage in HaCaT cells. (A) Cells were treated with various concentrations of ALA (0, 0.05, 0.1, and 0.2 mmol/L), (B) and various intensities of irradiation (0, 3, 6, 12, and 24 J/cm2). (C and D) The ALA-PDT (450 nm; 0.1 mmol/L, 6 J/cm2) induced the cell death of End1 and HeLa cells detected by fluorescence microscope (40×). (E) The cell viability of HaCaT, End1 and HeLa cells treated with 5-ALA (0.1 mmol/L) or 450 nm (10 mW/cm2) only. (F) Live and dead cell double staining of HaCaT cells treated with ALA-450 nm (40×). (G) The apoptosis of HaCaT cells measured by flow cytometry followed by ALA-635 nm and ALA-450 nm. Data represent the mean ± SD (N = 4). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not statistically significant.
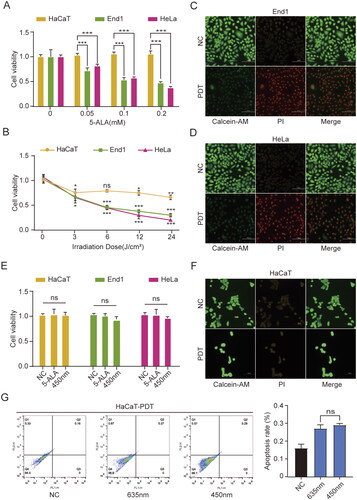
The cytotoxicity of ALA-450 nm PDT group was further evaluated by Live and dead cell double staining. Compared with the non-treatment groups, a majority of End1 and HeLa cells were observed to be dead emitting red fluorescence 24 h after ALA-450 nm (6 J/cm2) treatment.
The physiological security of 450 nm laser was evaluated next. We found no significant difference in cell viability among the control group, 5-ALA-treated group, and 450 nm-treated group (). Furthermore, live and dead cell double staining of HaCaT cells indicated that after treated with ALA-450 nm PDT, HaCaT cells remained at the similar rate of live cells (). And HaCaT cells showed semblable apoptosis rate among non-treatment group, 635 nm-treated group and 450 nm-treated group ().
These findings suggested that low-dose ALA combined 450 nm inhibited cell proliferation and promoted cell death in HPV-infected cells, End1 and HeLa cells, but not in normal epithelial cells. And the inhibition effect was dependent on both the concentration of ALA and the energy density of blue laser. In addition, 450 nm laser (6 J/cm2) had no negative effects on cells, which verified the physiology security of 450 nm laser.
The effect of ALA-450 nm on cytotoxicity, cell apoptosis, and ROS generation is greater than ALA-635 nm
To compare the capacity of ALA-450 nm and ALA-635 nm on cell proliferation inhibition and explore the underlying mechanism, we evaluated the cell viability, cell apoptosis, and intracellular ROS generation with blue and red laser treatment respectively. Obviously, 450 nm laser showed more evident lethality than 635 nm treatment towards End1 cells and HeLa cells (). As shown in , 450 nm laser could induce more cell apoptosis than 635 nm. Under low-dose irradiations, no significant difference was observed between 450 nm and 635 nm treatment in HaCaT cells (). However, in End1 cells (), ALA-450 nm represented a more effective cell death than 635 nm following the raising dose of laser. ALA-PDT induced oxidative stress by increasing ROS production, resulting in disturbance of the apoptotic pathway [Citation21]. In order to compare the intracellular ROS production of 450 nm and 635 nm treatments, we evaluated the ROS after ALA-450 nm and ALA-635 nm () PDT with different irradiation doses. Apparently, ALA-450 nm PDT generated more ROS than ALA-635 nm PDT at the same irradiation in HeLa cells.
Figure 2. ALA-450 nm had a greater influence than ALA-635 nm on cytotoxicity, cell apoptosis, and ROS generation. (A) End1 cells were respectively treated with ALA-450 nm and ALA-635 nm on different concentrations of ALA (0, 0.05, 0.1, and 0.2 mmol/L). (B) ALA-635 nm and ALA-450 nm caused apoptosis in HeLa cells as determined using flow cytometric analysis with annexin V/PI staining. (C and D) HaCaT and End1 cells were treated with ALA-635 nm and ALA-450 nm respectively under different irradiation intensities (0, 3, 6, 12, and 24 J/cm2). (E) The ROS generation induced by ALA-635 nm and ALA-450 nm in HeLa cells. Data represent the mean ± SD (N = 4). ***p < 0.001, **p < 0.01, *p < 0.05, ns, not statistically significant.
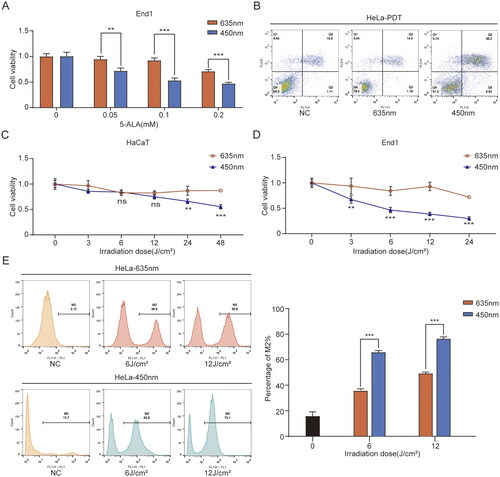
These results indicated that ALA-450 nm showed a stronger effect than 635 nm on cell proliferation inhibition, induced cell apoptosis, and promoted ROS generation in End1 and HeLa cells.
ALA-450 nm treatment might cause cell death through apoptotic pathway and intracellular ROS generation
We evaluated the cell death pathway through blue laser PDT, mainly concerning apoptosis and autophagy. ALA-450 nm increased the rate of cell apoptosis along with the fluence rise in HeLa cells (). ALA-450 nm PDT activated cell apoptosis in End1 and HeLa cells, which was not happen in HaCaT cells (). We found that the expression of PARP, Caspase-3, in End1 cells after ALA-450 nm PDT was suppressed, and concurrent with the increase of Cleaved PARP and Cleaved Caspase-3 (Additional file: Figure S1A). Similarly, several apoptosis indicators such as Caspase-3, Caspase-6, Caspase-9, PARP were downregulated while the inhibitor of apoptosis proteinsin, Bcl-2, was upregulated in both End1 and HeLa cells after blue laser induced PDT (Additional file: Figure S1A, B). These results suggested that caspase-dependent pathway might work in the ALA-450 nm PDT-induced cell apoptosis. Meanwhile, cell autophagy was observed after treatment (). The expression of ATG7, Caspase-8 and Bcl-2 decreased. And the expression of LC3 II/I, and p62 increased significantly (Additional file: Figure S1(B,C)). Therefore, we speculated that the therapeutic effect of ALA-450 nm mainly worked by inducing cell apoptosis and autophagy.
Figure 3. ALA-450 nm activated cell apoptosis in HeLa and End1 cells. (A) Apoptosis induced by ALA-450 nm under different irradiation doses (0, 3, 6, 12 J/cm2) using flow cytometry. (B) The effects of ALA-450 nm on the protein levels of PARP; Cleaved PARP; Caspase-3; Cleaved Caspase-3; Bcl-2 in HaCaT and End1 cells, and the protein level of Caspase-3; Caspase-6; Caspase-9; PARP; Bcl-2 in HaCaT and HeLa cells. (C) The influence of ALA-450 nm on the protein level of Caspase-8, ATG7, LC3 I, LC3 II, p62 in HaCaT and HeLa cells. (D and E) The ROS generation in HeLa and End1 cells activated by diverse light intensity according to ALA-450 nm treatment. (F) Apoptosis induced by ALA-450 nm in End1 cells. Data represent the mean ± SD (N = 3). ***p < 0.001; **p < 0.01.
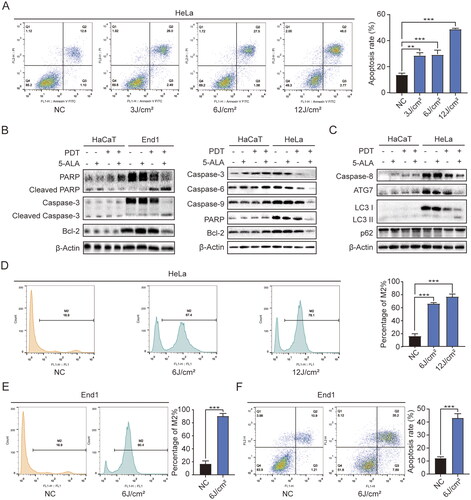
To verify the relationship between ALA-450 nm-induced cell apoptosis and intracellular ROS, the ROS generation was detected through flow cytometry. It was proved that ALA-450 nm stimulated ROS generation () in HeLa cells, as well as in End1 cells (), and the change was regulated by irradiation dose of blue laser. In our study, we could tell that in , along with the rise of irradiation dose, the decrease of cell viability of End1 and HeLa cells was less and less, which was not linearity. Meanwhile, according to , with the rise of irradiation dose, the increase of cellular ROS was not as much as the beginning. Therefore, we believed the decrease in cell viability of End1 and HeLa cells was correlated to the increase in ROS [Citation21]. It had been verified that ROS generation would cause depolarisation of the mitochondrial membrane [Citation22], which is one of the most representative events of early apoptosis. These results indicated that the ALA-450 nm PDT had a strong effect on ROS generation and therefore, might promote apoptosis.
ALA-450 nm PDT caused the inhibition of tumour growth and apoptosis
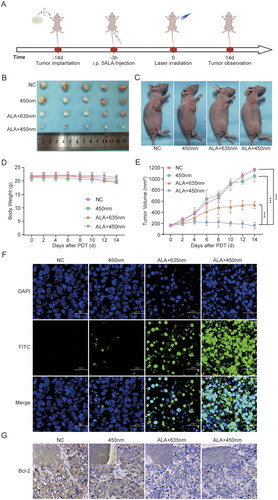
ALA-450 nm therapeutic efficacy was further tested in HeLa cell xenograft-bearing nude mice. To establish tumour models, HeLa cells were subcutaneously inoculated in the right axilla of the mice (). Photodynamic therapy was performed at 3 h postinjection of 5-ALA. And the body weight of four groups tumour-bearing mice had no significant difference (). The in vivo cancer therapeutic efficacy was evaluated by continuously monitoring the growth of the tumours every 2 days after different treatments. After the therapy, the tumour growth of ALA-635 nm and ALA-450 nm treated mice were inhibited, and ALA-450 nm group showed a more significant suppression than ALA-635 nm group. Meanwhile, the group treated with 450 nm only had similar tumour growth relative to the untreated control group () which confirmed that it was 5-ALA combined 450 nm that inhibited the tumour growth, but not the blue laser only. The TUNEL staining and immunofluorescence Bcl-2 staining of tumours verified that severer cell apoptosis and necrosis occurred in the tumours of ALA-450 nm PDT group (). These data suggested that ALA-450 nm PDT induced the strongest tumour cell death and inhibited the tumour growth.
Figure 4. In vivo therapeutic efficacy of ALA-450 nm PDT. (A) Schematic illustration of the schedule for tumour implantation and ALA-450 nm PDT. (B) Images of tumours collected after observation for 14 days. (C) Images of selective sacrificed mice at the end of experiment. (D) The body weight of mice after treatment. (E) Growth curves of tumours in HeLa tumour-bearing mice after different treatments. (F) TUNEL staining (100×) and (G) immunohistochemistry staining (400×) of tumour sections from HeLa tumour-bearing mice 14 days after different treatments. Values are the mean ± SD (N = 5), ***p < 0.001 compared to controls.
Discussion
Numerous disorders, including cutaneous carcinoma, assiduous cervical HR-HPV infection, CIN, and cervical cancer, are largely caused by persistent HR-HPV infection [Citation23]. Although there are many different types of treatments, more significant and obvious lesions are frequently treated with traditional therapies including physical therapy and surgery [Citation24]. There is still a lack of effective treatment for asymptomatic chronic cervical HR-HPV infection, CIN, and early cervical cancer (). Additionally, traditional therapies like physical procedures are invasive and have a high recurrence rate since unseen lesions cannot be entirely eradicated. These adverse effects include cervical morphological alterations and cervical insufficiency. Therefore, it is necessary to search a new treatment method.
Figure 5. Diagram of ALA-450 nm PDT induced cell death. After the PpIX accumulated in the HPV-infected cervical cells, with the exposure of blue laser and oxygen, the cellular ROS production leads to changes of diversified proteins. The latter further causes cell apoptosis through caspase dependent pathway. Meanwhile, it is verified that multiple autophagy related proteins, such as Bcl-2, ATG7, LC3, are involved in the cell autophagy regulation.
High-grade cervical intraepithelial neoplasia (CIN) and early cervical cancer have both showed promising result when treated with photodynamic therapy (PDT), an organ-saving therapeutic strategy [Citation25]. Previous research has demonstrated that PDT has better tissue selectivity, higher safety, and less tissue damage around the lesion than conventional therapies [Citation26–28]. PDT specifically avoids cervical deformity and cancer recurrence, as well as urethral perforation and anal cavity stenosis [Citation29,Citation30]. Additionally, the majority of pre-clinical and clinical researches have shown that PDT is especially beneficial to women in terms of future childbearing [Citation31,Citation32].
5-ALA-PDT has been used clinically to treat cancer in a number of organs, including the brain [Citation33–36], skin [Citation37–39], pharynx [Citation40], blood and lymph [Citation41], oesophageal cancer [Citation42], urethra and prostate [Citation10], and uterus [Citation43]. However, nearly all the studies about 5-ALA-PDT are using orange-red light (635 nm) as excitation wavelength, considering the violet light may cause damage to cells and tissues. In our study, low-dose violet light (450 nm) made almost no damage on cells. At the same time, low-dose blue laser-induced 5-ALA-PDT brought about a more significant effect than red laser with the same dose. And this was mainly manifested in severe cell damage and death, the more generation of intracellular ROS, and the more significant efficacy of the treatment in vitro. The results verified that violet light (450 nm) induced a more obvious effect on 5-ALA photodynamic therapy than orange-red light (635 nm), mainly through generating ROS and mediating cell apoptosis and necrosis. Additionally, since blue laser causes less thermal effect and shallower penetration depth in tissue than red laser, blue laser is able to avoid extra trauma.
To be more specific, according to our results, after ALA-450 nm PDT application, a large increase of ROS was detected, which might cause lots of changes in cellular protein levels. We found that some cell apoptosis-related proteins were activated, such as caspase-3, caspase-6, caspase-9, PARP (, S1(A,B)), which suggests that ALA-450 nm PDT induced cell apoptosis through caspase-dependent pathway. And the increase of p62 would activate caspase-8 and subsequent apoptosis processing (, S1C) [Citation44]. Additionally, it has been reported that the deletion of autophagy-related genes or the use of an autophagy inhibitor increased the infectivity of virions [Citation45,Citation46]. HPV employs numerous ways to impede host autophagy in order to create a successful infection. By regulating the Akt/mTOR pathway, E6 and E7 may suppress autophagy [Citation47,Citation48]. In a cervical carcinoma model, however, E6 and E7 deficiency stimulates autophagy. This suggests that autophagy and HPV have an inverse relationship [Citation49]. And it was observed that the expression of p62 and LC3 II/I was up-regulated and the expression of Bcl-2 was decreased (, S1(B,C)), which indicate that the ALA-450 nm PDT might promote autophagy in cervical cancer cells by activating the ROS-JNK-Bcl-2 pathway and promoting the release of BECN1 from Bcl-2 [49]. The findings above might suggest the cell death induced by ALA-450 nm PDT was related to cell apoptosis and autophagy.
Furthermore, it has been reported that End1/E6E7 cell line can be used as a standardised reproducible model in vitro of HPV-infected endometrial cells. According to our studies, ALA-450 nm PDT has significant efficacy on it, which inspires the application in the early treatment of cervical high-risk HPV infection targeting the (pre-)cancerous lesions and cancerisation avoidance. Meanwhile, having a therapeutic effect both in early HPV-infected cells and cervical cancer cells allows ALA-450 nm PDT to improve treatment efficiency and broader applications.
It is well known that violet laser has shown great ability on cutting and vaporising [Citation50,Citation51], as well as photodynamic diagnosis. Therefore, it is promising to create an apparatus equipped both the function of diagnosis and removal, finally, to realise the integration of diagnosis and treatment. And 450 nm will play an irreplaceable role in combining 5-ALA-PDD (Photodynamic diagnosis) and 5-ALA-PDT, which will greatly improve the medical efficiency of a number of diseases, especially cancer and precancerous lesions. Simultaneously reducing recurrence rate and negative side effects, blue laser will play a significant part in promoting the integration of diagnosis and treatment in the future.
However, we have not studied the specific molecular mechanism of ALA-450 nm PDT such as the gene expression and regulation after ALA-450 nm PDT, and changes of HPV structure and function after treatment. These matters will expect further theoretical and empirical researches and experimental studies.
Conclusion
Our study demonstrated that ALA-450 nm PDT had more significant therapeutic efficacy than ALA-635 nm in HeLa and End1 cells, which induces intracellular ROS generation and cell apoptosis both in vitro and in vivo. It is promising to develop and apply ALA-450 nm for future cervical cancer diagnosis and treatment.
Author contributions
Yuqing Chen, Yibo Mei, Lijiang Gu, Xing Li, Peng Guo, Dalin He and Lihong Chen were involved in the conception and design, and the analysis and interpretation of the data. All the authors drafted the paper and revised it critically for intellectual content and the final approval of the version to be published; and that all authors agree to be accountable for all aspects of the work.
Supplemental Material
Download TIFF Image (1.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are openly available in “figshare” at https://doi.org/10.6084/m9.figshare.21067765.v1.
Additional information
Funding
References
- Pešut E, Đukić A, Lulić L, et al. Human Papillomaviruses-associated cancers: an update of current knowledge. Viruses. 2021;13(11):2234.
- Miao F, Lv T, Zhang Y, et al. Induction of apoptosis in HPV16 E7 transfected human keratinocyte by ALA-mediated photodynamic therapy. Photodiagnosis Photodyn Ther. 2016;13:205–210.
- Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet. 2013;382(9895):889–899.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93(4):284–292.
- Okunade KS. Human papillomavirus and cervical cancer. J Obstet Gynaecol. 2020;40(5):602–608.
- Rivera A, Tyring SK. Therapy of cutaneous human papillomavirus infections. Dermatol Ther. 2004;17(6):441–448.
- Nelson EL, Stockdale CK. Vulvar and vaginal HPV disease. Obstet Gynecol Clin North Am. 2013;40(2):359–376.
- Lacey CJ. Therapy for genital human papillomavirus-related disease. J Clin Virol. 2005;32(Suppl 1): s82–90.
- Wierrani F, Kubin A, Jindra R, et al. 5-aminolevulinic acid-mediated photodynamic therapy of intraepithelial neoplasia and human papillomavirus of the uterine cervix–a new experimental approach. Cancer Detect Prev. 1999;23(4):351–355.
- Fukuhara H, Yamamoto S, Karashima T, et al. Photodynamic diagnosis and therapy for urothelial carcinoma and prostate cancer: new imaging technology and therapy. Int J Clin Oncol. 2021;26(1):18–25.
- Giomi B, Mastrolorenzo A, Tiradritti L, et al. Off label treatments of genital warts: the role of photodynamic therapy. G Ital Dermatol Venereol. 2012;147(5):467–474.
- Wang HW, Zhang LL, Miao F, et al. Treatment of HPV infection-associated cervical condylomata acuminata with 5-aminolevulinic acid-mediated photodynamic therapy. Photochem Photobiol. 2012;88(3):565–569.
- Nkune NW, Simelane NWN, Montaseri H, et al. Photodynamic Therapy-Mediated immune responses in Three-Dimensional tumor models. IJMS. 2021;22(23):12618.
- Alexiades-Armenakas M. Laser-mediated photodynamic therapy. Clin Dermatol. 2006;24(1):16–25.
- Shi H, Li J, Peng C, et al. The inhibitory activity of 5-aminolevulinic acid photodynamic therapy (ALA-PDT) on Candida albicans biofilms. Photodiagnosis Photodyn Ther. 2021;34:102271.
- Radakovic S, Silic K, Tanew A. [Not available]. J Dtsch Dermatol Ges. 2020;18(5):492–494.
- Borgia F, Giuffrida R, Coppola M, et al. Efficacy and safety of conventional versus daylight photodynamic therapy in children affected by multiple facial flat warts. Photodiagnosis Photodyn Ther. 2020;31:101819.
- Xie F, Yu HS, Wang R, et al. Photodynamic therapy for genital warts causes activation of local immunity. J Cutan Med Surg. 2019;23(4):370–379.
- Jin Y, Guan Z, Wang X, et al. ALA-PDT promotes HPV-positive cervical cancer cells apoptosis and DCs maturation via miR-34a regulated HMGB1 exosomes secretion. Photodiagnosis Photodyn Ther. 2018;24:27–35.
- Fichorova RN, Rheinwald JG, Anderson DJ. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod. 1997;57(4):847–855.
- Wiehe A, O'Brien JM, Senge MO. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. 2019;18(11):2565–2612.
- Wang J, Wang Q, Chen P, et al. Podophyllotoxin-combined 5-aminolevulinic acid photodynamic therapy significantly promotes HR-HPV-infected cell death. Photodermatol Photoimmunol Photomed. 2022;38(4):343–353.
- Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123(12):2219–2229.
- Hu Z, Li J, Liu H, et al. Treatment of latent or subclinical genital HPV infection with 5-aminolevulinic acid-based photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;23:362–364.
- Unanyan A, Pivazyan L, Davydova J, et al. Efficacy of photodynamic therapy in women with HSIL, LSIL and early stage squamous cervical cancer: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2021;36:102530.
- Shan X, Wang N, Li Z, et al. An open uncontrolled trial of topical 5-aminolevulinic acid photodynamic therapy for the treatment of urethral condylomata acuminata in male patients. Indian J Dermatol Venereol Leprol. 2016;82(1):65–67.
- Nguyen K, Khachemoune A. An update on topical photodynamic therapy for clinical dermatologists. J Dermatolog Treat. 2019;30(8):732–744.
- Adnane F, El-Zayat E, Fahmy HM. The combinational application of photodynamic therapy and nanotechnology in skin cancer treatment: a review. Tissue Cell. 2022;77:101856.
- Xie J, Wang S, Li Z, et al. 5-aminolevulinic acid photodynamic therapy reduces HPV viral load via autophagy and apoptosis by modulating ras/raf/MEK/ERK and PI3K/AKT pathways in HeLa cells. J Photochem Photobiol B. 2019;194:46–55.
- Li Z, Teng M, Wang Y, et al. The mechanism of 5-aminolevulinic acid photodynamic therapy in promoting endoplasmic reticulum stress in the treatment of HR-HPV-infected HeLa cells. Photodermatol Photoimmunol Photomed. 2021;37(4):348–359.
- Ahn TG, Lee BR, Kim JK, et al. Successful full term pregnancy and delivery after concurrent chemo-photodynamic therapy (CCPDT) for the uterine cervical cancer staged 1B1 and 1B2: preserving fertility in young women. Gynecol Oncol Case Rep. 2012;2(2):54–57.
- Choi MC, Jung SG, Park H, et al. Fertility preservation by photodynamic therapy combined with conization in young patients with early stage cervical cancer: a pilot study. Photodiagnosis Photodyn Ther. 2014;11(3):420–425.
- Mahmoudi K, Garvey KL, Bouras A, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neurooncol. 2019;141(3):595–607.
- Cramer SW, Chen CC. Photodynamic therapy for the treatment of glioblastoma. Front. Surg. 2020;6:81.
- Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50(5):399–419.
- Nordmann NJ, Michael AP. 5-Aminolevulinic acid radiodynamic therapy for treatment of high-grade gliomas: a systematic review. Clin Neurol Neurosurg. 2021;201:106430.
- Champeau M, Vignoud S, Mortier L, et al. Photodynamic therapy for skin cancer: how to enhance drug penetration? J Photochem Photobiol B. 2019;197:111544.
- Naidoo C, Kruger CA, Abrahamse H. Simultaneous photodiagnosis and photodynamic treatment of metastatic melanoma. Molecules (Basel, Switzerland. 2019;24(17):3153. )
- Zou Y, Zhao Y, Yu J, et al. Photodynamic therapy versus surgical excision to basal cell carcinoma: meta-analysis. J Cosmet Dermatol. 2016;15(4):374–382.
- Jin X, Xu H, Deng J, et al. Photodynamic therapy for oral potentially malignant disorders. Photodiagnosis Photodyn Ther. 2019;28:146–152.
- Oka T, Matsuoka KI, Utsunomiya A. Sensitive photodynamic detection of adult T-cell leukemia/lymphoma and specific leukemic cell death induced by photodynamic therapy: current status in hematopoietic malignancies. Cancers. 2020;12(2):335.
- Wu H, Minamide T, Yano T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig Endosc. 2019;31(5):508–516.
- Matoba Y, Banno K, Kisu I, et al. Clinical application of photodynamic diagnosis and photodynamic therapy for gynecologic malignant diseases: a review. Photodiagnosis Photodyn Ther. 2018;24:52–57.
- Lee SH, Cho WJ, Najy AJ, et al. p62/SQSTM1-induced caspase-8 aggresomes are essential for ionizing radiation-mediated apoptosis. Cell Death Dis. 2021;12(11):997.
- Griffin LM, Cicchini L, Pyeon D. Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes. Virology. 2013;437(1):12–19.
- Ishii Y. Electron microscopic visualization of autophagosomes induced by infection of human papillomavirus pseudovirions. Biochem Biophys Res Commun. 2013;433(4):385–389.
- Spangle JM, Münger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J Virol. 2010;84(18):9398–9407.
- Menges CW, Baglia LA, Lapoint R, et al. Human papillomavirus type 16 E7 up-regulates AKT activity through the retinoblastoma protein. Cancer Res. 2006;66(11):5555–5559.
- Xie X, Hu L, Liu L, et al. Punicalagin promotes autophagic degradation of human papillomavirus E6 and E7 proteins in cervical cancer through the ROS-JNK-BCL2 pathway. Transl Oncol. 2022;19:101388.
- Fornaini C, Merigo E, Rocca JP, et al. 450 nm blue laser and oral surgery: preliminary ex vivo study. J Contemp Dent Pract. 2016;17(10):795–800.
- Hess M, Fleischer S. Photoangiolytic lasers in laryngology. Laryngorhinootologie. 2020;99(9):607–612.
