?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Vincamine, a natural chemical, was used as a reducing agent in the synthesis of IgG antibodies mediated biogenic gold nanoparticles (IgGAuNPs). Eventually, the synthesised IgGAuNPs were bioconjugated with the chemotherapeutic drug methotrexate (MTX-IgGAuNPs). The IgG isotype can target cancer cells through polymorphic Fc gamma receptors (FcγRs) and have therapeutic effects. They can restrict cell division by inhibiting different intracellular signal transduction pathways and activating NK cells and macrophages through antibody-dependent cellular cytotoxicity and macrophage-mediated antibody-dependent phagocytosis, respectively. Further, IgGAuNPs and MTX-IgGAuNPs were characterised by physical techniques. Moreover, 3D conformational changes in the structure of IgG were analysed by fluorescence spectroscopy during and after the synthesis of IgGAuNPs. Furthermore, the IgGAuNPs and MTX-IgGAuNPs were effective against lung cancer (A549 cells), while they were found to be non-toxic against normal cells (NRK cells). The effectiveness of IgGAuNPs and MTX-IgGAuNPs was examined by MTT cytotoxicity assay, DCFDA method for the production of ROS, and release of Cyt-c from the mitochondria for caspase-3-mediated apoptosis. Moreover, the confirmation of internalisation of particles into the nucleus was examined under the DAPI assay, and it was found that particles caused nuclear fragmentation, which was also an indication of apoptosis.
Introduction
Lung cancer has been a great challenge since long ago because the mortality rate of this particular cancer worldwide is about 20% among all cancers [Citation1]. The first line of therapies comprises chemotherapy and radiation therapy. These therapies may prolong the life span marginally, but the quality of life does not re-establish itself. Furthermore, these therapies’ manifestations (nausea, vomiting, diarrhoea, hair loss, and alopecia) [Citation2,Citation3] are severe and challenging to manage. It is a fact that chemotherapy is non-specific and has the potential to generate new types of cancer in due course of time [Citation4]. In order to keep the therapy simple and effective, inorganic nanoparticle-mediated drug delivery systems have emerged with immense drug loading [Citation5], major drug release [Citation6], improved bioavailability [Citation7], and enhanced multivalence effects through receptor-ligand interactions [Citation8]. Biogenic nanomaterials are ideal for delivery due to their biocompatibility, high efficiency, and multivalency [Citation9–11]. Targeted antibody-drug conjugates (ADCs) were developed to deliver drugs specifically to the target site. However, it has had limited success due to aggregation, feeble bioavailability, and limited therapeutic efficacy [Citation12]. The new and advanced concept of antibody nanoparticle drug conjugates (ANDCs) successfully achieved directed delivery with enhanced cellular uptake and higher therapeutic efficacy combined with active targeting molecules such as antibodies.
The antibodies are required for site-directed targeting, while nanoparticles take care of cellular internalisation, where unaltered and unmodified drugs are delivered directly into the cytoplasm, organelles, or nucleus. The use and role of monoclonal antibodies (mAbs) are prominent in therapeutics and site-directed delivery. The origin of therapeutic mAbs is mostly from IgG1 isotypes, which can act through multiple mechanisms [Citation13]. Broadly, the antitumor activities of IgG1 mAbs are achieved by blocking growth factor receptors or inducing apoptosis [Citation14]. IgG1 acts through the classical complement pathway activation [Citation15] and recruits innate effects or cells through binding to FcγRs. They also illicit NK and macrophages for efficient potency to kill tumour cells. IgG1 also works via antibody-dependent cellular cytotoxicity (ADCC) [Citation16] by eliciting NK cells to induce apoptosis (through the release of perforins and granzymes) or through the activation of death receptor pathways. The clinical therapeutic success of rituximab (α-CD20) and cetuximab (α-EGFR) in lymphoma and colorectal cancer, respectively [Citation16], has been derived from the binding affinity of IgG against polymorphic FcγRs that established the role of ADCC or ADCP. However, only elicitation of NK and macrophages as effector cells in mAbs therapy could not produce the expected results when Del Re et al. investigated mutated EGFR signalling pathways (e.g. KRAS) [Citation17]. Therefore, the efficacy of mAbs with bioconjugated drugs increases and produces predictable results. In the given investigation, a novel therapeutic bioengineered IgG antibody-mediated synthesised gold nanoparticle (IgGAuNPs) eventually bioconjugated with the chemotherapeutic drug methotrexate (MTX-IgGAuNPs) has been developed to target non-small cell lung cancer. In vitro, experiments were demonstrated to illustrate the potential antitumor efficacy and high selectivity of using a monoclonal antibody over a single chemotherapeutic drug alone. Moreover, it was also demonstrated that MTX-IgGAuNPs have null cytotoxicity against normal cells.
Material and methods
Materials
IgG antibodies, methotrexate, tetra chloroauric [III] acid (HAuCl4) of purity 99+%, disodium phosphate, and monosodium phosphate were all purchased from Invitrogen, Merck, and Himedia. All solutions are kept in the dark and freshly produced before the experiment to prevent any photochemical reactions.
In-vitro biogenic synthesis of IgG and vincamine enabled AuNPs
IgG antibody-capped AuNPs are formed by green synthesis using vincamine as a reducing agent and IgG antibodies as a capping agent. IgGAuNPs were synthesised in a 1 ml reaction mixture containing 1 mM H[AuCl4], 100 µg/mL IgG antibodies, and 10 µg/mL vincamine in 50 mM phosphate buffer at pH 7.6. The process was carried out at 40 °C for 24 h. As detected by UV-vis spectroscopy, the transformation from transparent to bright red indicated that the reaction had been fully completed. After biosynthesis, unbound antibodies were removed with a 50% v/v ethanol treatment, and the resulting IgGAuNPs were collected by centrifugation at 10,000 rpm for 30 min.
Bioconjugation of methotrexate with IgGAuNPs
Bioconjugation of as-synthesised IgGAuNPs was performed to attach the FDA-approved cancer chemotherapeutic drug MTX. To join the free amino group of MTX with the carboxylate group of the IgG antibodies, the activator and coupling agent 1-Ethyl-3-(3-dimethyl) carbodiimide (EDC) was utilised [Citation18]. The 5 ml reaction mixes included 250 µg of MTX, 3 ml of IgGAuNPs, and 50 mM HEPES buffer (pH 7.6). At the end of the three-hour incubation period at 30 °C, 5 mM of EDC was added in aliquots. A DLS particle size analyser (Model ZEN3600, Malvern Instrument Ltd., Malvern, United Kingdom) was used to estimate the average particle diameter of IgGAuNPs and MTX-IgGAuNPs. The samples were collected in a 1.5 ml limited-volume disposable cuvette for analysis. The sample was diluted to 0.5% (w/v) in de-ionized water. After sonicating the particles for one minute, the average particle size was calculated by measuring a single sample in triplicate. Surface charges of IgGAuNPs and MTX-IgGAuNPs were measured using a Zeta Sizer Nano-ZS, Model ZEN3600 (Malvern Instrument Ltd, Malvern, UK).
Physical characterisation of IgGAuNPs and MTX-IgGAuNPs
The preliminary characterisation of IgGAuNPs and MTX-IgGAuNPs under UV-visible spectroscopy (Shimadzu dual-beam spectrophotometer, model UV-1601 PC) was performed at a 1 nm resolution. The determination of the inorganic core and shape of IgGAuNPs and MTX-IgGAuNPs was done via Transmission Electron Microscopy (TecnaiTMG2Spirit BioTWIN FEI), which is operated at an accelerating voltage of 80 kV. The samples were prepared by putting a drop of nanoemulsion on copper grids, which were further coated with carbon and dried.
Analysis of 3D confirmational changes
The change in 3D conformation in IgG during and after synthesis of IgGAuNPs was acquired in a 10-mm path length cuvette at room temperature using a Carry Eclipse fluorescence spectrophotometer (MY16040008) and processed with the Carry Eclipse Agilent WinFLR fluorescence software. When measuring the fluorescence of IgGAuNPs, the solutions were excited at a 280 nm wavelength, and the emission spectra were tracked between 320–380 nm and 420–480 nm. Emission and excitation voltages were fixed at 750 V, and emission and excitation spectra were filtered with the Savitzky-Golay filter.
Loading efficacy of MTX on IgGAuNPs by UV-vis spectrophotometer
The loading percentage of MTX was estimated by measuring the change in wavelength intensity at 303 nm before and after the bioconjugation [Citation18]. The absorbance values were calculated to estimate the total percentage loading of MTX on IgGAuNPs. The MTX loading percentage was calculated by plugging the absorbance values of A and B into EquationEquation (1)(1)
(1) [Citation19].
(1)
(1)
The absorbance value A represents the entire amount of MTX drug used in the bioconjugation of IgGAuNPs. In contrast, the MTX in the supernatant of MTX-IgGAuNPs is represented by the absorbance value B. Unbound drug in MTX-IgGAuNPs was determined using the usual MTX graph at 303 nm [Citation18]. The exact amount of bioconjugated MTX could be calculated by deducting the unbound drug from the total drug added for bioconjugation. An equation was used to determine what fraction of MTX was bioconjugated (Equationequation 2(2)
(2) ) [Citation20].
(2)
(2)
Maintenance of animal cell lines
The National Centre for Cell Science (NCCS), Pune, India, was sourced for the human lung cancer cell line (A549 cell line) and standard rat kidney cell line (NRK cell line). The cell lines were nurtured in a humidified incubator at 37 °C and 5% CO2 using DMEM-F12 medium supplemented with 1.5% antibiotic-antimycotic solution consisting of penicillin, streptomycin, and amphotericin B (Himedia, India, Ltd., Mumbai, India).
Determination of cytotoxicity by MTT assay
Cells were grown at a density of 5 × 103 per well in a 96-well plate and incubated in a 5% CO2 humidified incubator at 37 °C for 24 h to determine the cytotoxic efficacy of IgGAuNPs, the pure drug MTX, and MTX-IgGAuNPs against A549 and NRK cells. Following 24 h of incubation, the cells were further incubated with different concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs. Next, 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl-tetrazolium bromide (MTT) (5 mg/ml in PBS) was added to each well, and the plates were incubated for 2 h at 37 °C. Formazan crystals formed during incubation were dissolved in 80 µl of DMSO, and the well media was discarded. The amount of decreased MTT was measured using a microplate reader (BIORAD-680) set to 570 nm and a reference filter of 655 nm. It was determined what percentage of cells were inhibited by utilising EquationEquation (3)(3)
(3) [Citation19].
(3)
(3)
Whereas the absorbance value of the treated sample is the blank absorbance and is the control sample absorbance. The results are demonstrated as percentage inhibition of cells compared to the control.
Cytomorphological analysis
Cytomorphological studies of IgGAuNPs, MTX, and MTX-IgGAuNPs treated lung cancer cells A549 were performed as per our previous work [Citation21]. The healthy adherent cells were grown at a density of 3 × 105 cells in a single well of a 24-well plate for 24 h at 37 °C in a humified chamber with 5% carbon dioxide. Finally, a freshly prepared medium was supplemented with IC50 dose concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs into each well and incubated in a humified environment at 37 °C and 5% carbon dioxide for 24 h. Finally, an inverted phase-contrast microscope was used to examine the morphology of control and treated cells (Nikon ECLIPSE Ti-S, Nikon Corporation, Tokyo, Japan).
Analysis and quantification of reactive oxygen species (ROS) generation
ROS generation in viable A549 cells was detected by labelling with 5- and 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFHDA) dye following treatment with IgGAuNPs, MTX, and MTX-IgGAuNPs. In oxidative stress, cells generate reactive oxygen species (ROS), deacetylating the non-fluorescent DCFHDA dye and causing it to glow brilliant green [Citation22]. The A549 cells were seeded at a density of 3 × 105 cells/well into 24 well plates and then incubated for 24 h at 37 °C in a 5% CO2 incubator. After that, they were treated with the IC50 concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs and then incubated for another 24 h. Subsequently, 200 µl (10 µM) of DCFHDA dye was added to each well, and the plates were incubated for 30 min at 37 °C. After incubation, the reaction solutions are removed, and 200 µl of PBS is added to each well. The analysis of intracellular fluorescence was performed using an inverted fluorescent microscope (Nikon ECLIPSE Ti-S, Japan).
Detection of internalisation of particles by DAPI staining
Examining the apoptotic effect of IgGAuNPs, MTX, and MTX-IgGAuNPs on A549 cells is performed with the nuclear fluorescent dye DAPI. The A549 cells were seeded at a density of 3 × 105 cells/well into 24 well plates and then incubated for 24 h at 37 °C in a 5% CO2 incubator. After that, they were treated with the IC50 concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs and then incubated for another 24 h. Following incubation, the cells are fixed with 4% paraformaldehyde for 5 min before being permeabilized with a solution containing 3% paraformaldehyde and 0.5% Triton X-100. Following permeabilization, cells are stained with 200 µl (300 nM) DAPI dye for 15 min, and then fluorescence microscopy photos are taken (Nikon ECLIPSE Ti-S, Japan).
Determination of mitochondrial membrane potential (ΔΨ m)
Mito Tracker Red CMX Ros labelling dye is used to measure the potential of the mitochondrial membrane in A549 cells that have been treated with IgGAuNPs, MTX, and MTX-IgGAuNPs. Seeding 24 well plates with 3 × 105 A549 cells per well allows for optimal adhesion before treating with IC50 concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs. After 24 h of incubation, the cells are stained with 200 l of Mito Tracker Red (300 nM) and left to incubate in the dark for 30 min. The stained cell photos were taken with an inverted fluorescence microscope (Nikon ECLIPSE Ti-S, Japan).
Statistical analysis
All statistical analyses were performed using the Origin 6.0 software (USA), as described previously [Citation20].
Results
The given investigation revealed that vincamine and IgG-mediated synthesised GNPs were proposed to bioconjugate with methotrexate, an anticancer drug [Citation23]. The as-produced nanomedicine (MTX-IgGAuNPs) was tested against non-small cell lung cancer (A549 cell line), and a strong effect was achieved due to IgG-mediated targeting and the synergistic effect of IgG, vincamine (a known anti-tumour natural product), and methotrexate. The anionic nanomedicine was observed to internalise directly into the nucleus, where methotrexate acted on DNA. This delivery system successfully delivered methotrexate into the nucleus, unaltered or undegraded.
Synthesis and characterisation of IgGAuNPs
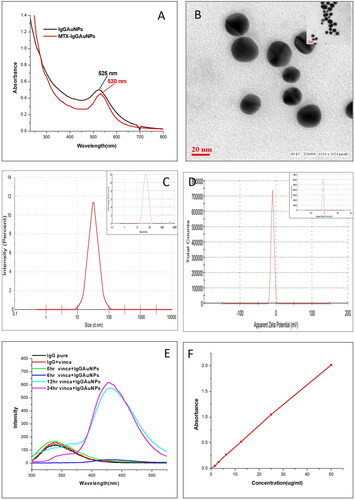
The reduction of HAuCl4 to produce gold nanoparticles can be seen as a clear in vitro production pathway. The quality and size of the gold nanoparticles synthesised via IgG antibody mediation are sensitive to temperature and pH. The reaction conditions that determine the final size, shape, stability, and complete synthesis of IgGAuNPs are the concentrations of gold chloride and vincamine and the solution’s pH. The synthesis of IgGAuNPs initiated by reducing Au+3 to Au0 by vincamine and IgG antibodies will function as a capping agent. The formation of IgGAuNPs was declared complete after UV-visible spectroscopy and TEM confirmation. After the complete synthesis of IgGAuNPs, UV-visible spectroscopy showed a maximum (λmax) at 525 nm (). High-resolution measurements of the inorganic core of IgGAuNPs (determined to be 18.2 nm) were made using TEM. Round particles were uniform in size and shape (monodispersed) (, inset image).
Figure 1. Characterisation of synthesised IgGAuNPs and MTX-IgGAuNPs by (A) UV-Visible spectroscopy, (B) Transmission electron microscope of IgGAuNPs (Inset image) and MTX-IgGAuNPs, (C) Dynamic light scattering of IgGAuNPs (Inset image) and MTX-IgGAuNPs (D) Zeta potential of IgGAuNPs (Inset image) and MTX-IgGAuNPs (E) Changes in fluorescence intensity in IgGAuNPs during synthesis (excitation λ = 280nm emission λ = 320–380nm and 420–480nm) in the reaction mixture at different incubation time (0 h, 6 h, 12 h, 24 h) as well as IgG pure and IgG + vinca, (F) UV-Visible spectroscopy graph of pure methotrexate at different concentration for drug loading efficiency.
Bioconjugation of methotrexate drug with IgGAuNPs and its characterisation
Bioconjugation of MTX to the as-synthesised IgGAuNPs was achieved by exposing the amino group of IgG antibodies on the surface of the NPs and linking it to the carboxylate group of the MTX drug. Without using a spacer, binding was accomplished via EDC/NHS chemistry, which formed a covalent bond (i.e. a peptide bond) [Citation21]. The UV-visible spectra representing surface plasmon resonance of IgGAuNPs and MTX-IgGAuNPs have been shown in . It was found that λmax shifted for bioconjugated MTX drugs (MTX-IgGAuNPs) from 525 nm to 530 nm. After each cycle, the resulting spectra were found to have become less concentrated and broader. MTX drug coupling to IgGAuNPs was confirmed by a red shift of the absorbance spectra by 5 nm, along with widening and decreased intensity () [Citation24]. Finally, high-resolution pictures were captured using TEM, and typical diameters of MTX-IgGAuNPs were determined to be 21.4 nm (). The binding of medication was further confirmed by the blurred, less clear TEM images of MTX-IgGAuNPs. Under TEM, the spherical particles were finally determined to be monodispersed [Citation19].
In addition, DLS analyses were also performed to investigate the increase in hydrodynamic radius of MTX-IgGAuNPs in comparison to IgGAuNPs. When nanoparticles are suspended in an aqueous solvent, a double layer of the solvent (or water) will cover their surfaces. DLS measurements provide information on the inorganic core and capping material, as well as the solvent deposit that forms on the nanoparticle’s surface [Citation25]. DLS of synthesised IgGAuNPs revealed a particle size of 46.3 nm (), whereas the particle size after bioconjugation with MTX was 56 nm (). In addition, the zeta potential is used to calculate ionisation, distribution, concentration, exposure/shielding of charged fractions, and nanoparticle adsorption [Citation26]. The zeta-potentials of IgGAuNPs and MTX- IgGAuNPs were estimated to be −9.67 mV and −10.1 mV, respectively ().
Investigation of conformational changes of IgG antibodies during synthesis
The 3D structural changes in IgG antibodies during and after the synthesis of IgGAuNPs were also studied by fluorescence spectroscopy (). The tertiary structure of IgG antibodies was found to lead to a shift in the chemical environment adjacent to the tryptophan fluorophore. The experiment showed few changes in the 3D structure of IgG antibodies after interacting with GNPs. When HAuCl4 was incubated with the IgG antibodies and vincamine, the fluorescence intensity of the IgG antibodies was observed at 150 a.u. at 0 h between λ = 320–380.
Further, after 6 h of incubation at 40 °C, the peak is shifted to λ = 420-480 with low peak intensity. At 12 h of incubation, the peak intensity increases to 560 a.u.; at 24 h, the peak increases to 620 a.u.; indeed, prolonged incubation does not further increase or decrease the fluorescence of IgG antibodies (). The experiment shows 3D structural changes between native IgG antibodies and encapsulated IgG antibodies upon encapsulation over GNPs [Citation1].
Quantification of drug loading efficiency
Using EquationEquation (1)(1)
(1) , the percentage of MTX loaded onto IgGAuNPs was calculated by replacing the original values of A (=2.016) and B (=0.41). Furthermore, MTX bioconjugation to IgGAuNPs was measured to be 79.66%, indicating a successful coupling of MTX. UV-Visible spectroscopy also quantified the bioconjugated MTX to IgGAuNPs (). The standard curve was calibrated as per the method described somewhere else, using the absorbance of pure MTX (at 303 nm) [Citation18] at 5–50 µg/ml. Bioconjugation of MTX with IgGAuNPs was shown to be significantly high, with 79.14% efficiency.
In vitro anticancer study of MTX-IgGAuNPs, methotrexate and IgGAuNPs
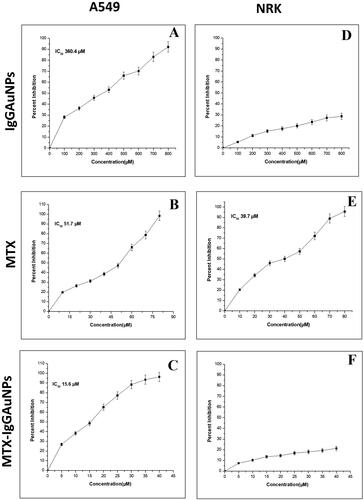
The potential of MTX-IgGAuNPs was observed to be significantly greater than pure MTX against A549 cells, while it did not show any cytotoxic effect against NRK cells (). The inhibition increased with an increase in the concentration of IgGAuNPs, MTX, and MTX-IgGAuNPs. The IC50 values of IgGAuNPs, MTX, and MTX-IgGAuNPs against A549 cells were found to be 360.4 µM, 51.7 µM, and 15.6 µM, respectively (). However, MTX-IgGAuNPs and IgGAuNPs showed no significant cytotoxicity against NRK cells ( and ). On the contrary, MTX was found to show a cytotoxic effect against NRK cells with IC50 values of 39.7 µM respectively ().
3.6. Analysis of cytomorphological changes in the A549 cells
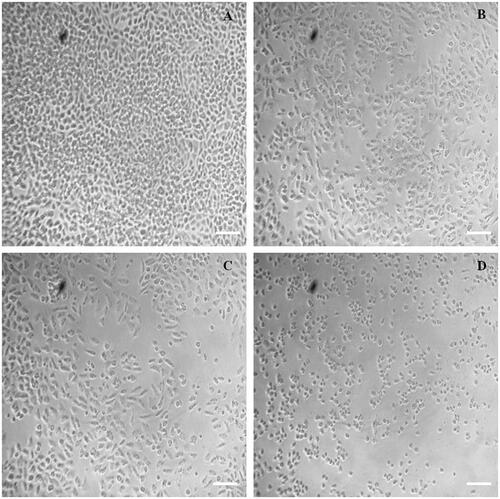
After 24 h of treatment with IgGAuNPs, MTX, or MTX-IgG-AuNPs at their IC50 concentrations, the morphologies of A549 cells were examined using a phase contrast microscope to reveal the differences between the three treatments (). The surface adhesion and morphology of untreated (control) cells have not changed noticeably (). After 24 h of treatment, the inhibited cells become detached from the surface of the wells, and some cells have perfect plasma membranes, indicating the onset of apoptosis. Shrinkage and apoptosis were seen in MTX-treated cells, but only at concentrations orders of magnitude higher than those needed to trigger apoptosis by MTX-IgGAuNPs in lung cancer cells.
Estimation of reactive oxygen species (ROS) generation A549 cells
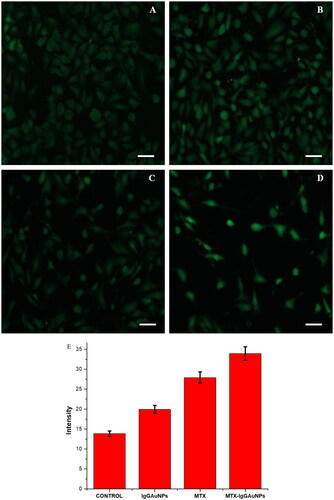
Using 5-(and6)- carboxy-2′,7′- dichlorodihydrofluorescein diacetate (DCFHDA) (Sigma-Aldrich) as an oxidation-sensitive fluorogenic marker of ROS in the viable cells, we estimated the amount of intracellular ROS generation in A549 cells upon interaction with IgGAuNPs, MTX, and MTX-IgGAuNPs at IC-50 concentrations (). We found that the level of fluorescence was proportional to the amount of reactive oxygen species (ROS) produced by the cells. Analysis showed that MTX-IgGAuNPs-treated A549 cells produced higher fluorescence intensities compared to controls (). showed that MTX-treated A549 cells generated remarkable fluorescence, and showed that IgGAuNPs-treated A549 cells emit brilliant fluorescence with defaced morphological structures due to an elevated disrupting impact on the compactness of the plasma membrane caused by ROS production. It’s likely that the overproduction of reactive oxygen species contributes to the detrimental effects by inducing oxidative stress and apoptosis (ROS). Image J was used to quantify the fluorescence, and it was observed that at their respective IC-50 concentrations, IgGAuNPs, MTX, and untreated cells all created less fluorescence than did MTX-IgGAuNPs ().
Figure 4. Images showing DCFDA staining under phase contrast microscope after 24 h of treatment on A549 cells at their respective IC50 concentrations at Scale bar = 50 µM; 20X magnification. (A) Control of DCFDA, (B) IgGAuNPs treated cells, (C) MTX treated cells, (D) MTX-IgGAuNPs treated cells, and (E) Graph showing change in intensity of DCFDA stained control, IgGAuNPs, MTX, and MTX-IgGAuNPs treated cells.
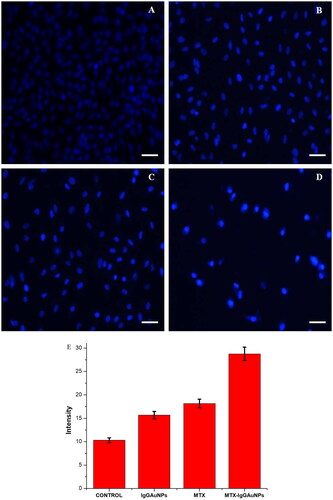
Analysis of changes in nuclear morphology
Using 5-(and6)- carboxy-2′,7′- dichlorodihydrofluorescein diacetate (DCFHDA) (Sigma-Aldrich) as an oxidation-sensitive fluorogenic marker of ROS in the viable cells, we estimated the amount of intracellular ROS generation in A549 cells upon interaction with IgGAuNPs, MTX, and MTX-IgGAuNPs at IC-50 concentrations (). We found that the level of fluorescence was proportional to the amount of reactive oxygen species (ROS) produced by the cells. Analysis showed that MTX-IgGAuNPs-treated A549 cells produced higher fluorescence intensities than controls (). showed that MTX-treated A549 cells generated remarkable fluorescence, and showed that IgGAuNPs-treated A549 cells emit brilliant fluorescence with defaced morphological structures due to an elevated disrupting impact on the compactness of the plasma membrane caused by ROS production. The overproduction of reactive oxygen species likely contributes to the detrimental effects by inducing oxidative stress and apoptosis (ROS). Image J was used to quantify the fluorescence, and it was observed that at their respective IC-50 concentrations, IgGAuNPs, MTX, and untreated cells all created less fluorescence than MTX-IgGAuNPs ( and ).
Figure 5. Images showing DAPI staining under phase contrast microscope after 24 h of treatment on A549 cells at their respective IC50 concentrations at Scale bar = 50 µM; 20X magnification. (A) Control of DAPI, (B) IgGAuNPs treated cells, (C) MTX treated cells, (D) MTX-IgGAuNPs treated cells, and (E) Graph showing change in intensity of DAPI stained control IgGAuNPs, MTX, and MTX-IgGAuNPs treated cells.
Analysis of disruption of the mitochondrial membrane potential (Ψm) in A549 cells
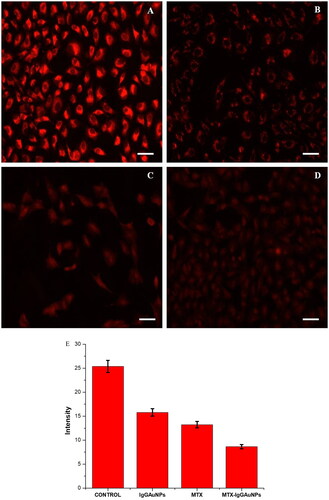
The release of cyt-c results in the degradation of mitochondrial membrane potential, which causes apoptosis. Mito Tracker Red CMX Ros was used to measure the mitochondrial membrane potential (ΔΨm) after A549 cells were treated with IC-50 concentrations of IgGAuNPs, MTX, and MTX-IgGAuNPs. After treatment, drop-in fluorescence intensity was estimated, corresponding to a decrease in ΔΨm, as indicated in . The effect of MTX-IgGAuNPs on ΔΨm was found to be effective, and a decrease in ΔΨm in the A549 cell line at IC-50 concentrations of IgGAuNPs, MTX and MTX-IgGAuNPs () was observed as compared to the control (). The intensities of all the treated cells, along with the untreated control, were determined through Image J software, and it was found that the intensities of treated cells were diminished compared to the untreated control (). Hence, the high decrease level of ΔΨm in MTX-IgGAuNPs treated A549 cells compared to native MTX was due to the increased efficacy of MTX when bioconjugated to IgGAuNPs.
Figure 6. Mitochondrial depolarisation by disrupting mitochondrial membrane potential (DWm) in A549 cells and images observed by staining with Mitotracker Red CMXROS at Scale bar = 50 µM; 20X magnification. (A) Untreated control cells, (B) IgGAuNPs treated cells, (C) MTX treated cells, (D) MTX-IgGAuNPs treated cells, at their respective IC50 concentrations, and (E) Graph showing change in intensity of Mitotracker Red CMXROS stained control, IgGAuNPs, MTX, and MTX-IgGAuNPs treated cells.
Discussion
IgG, the most preferably used antibody [Citation27], targets and acts against cancer via complement activation (by inserting the lytic membrane attack complex in the tumour cell membrane or initiating NK/macrophages), inhibition of critical intracellular signal transduction pathway(s), or antibody-dependent toxicity. The unaltered 3D structure of IgG antibodies on IgGAuGNPs was found to recognise and annihilate lung cancer cells via Fcγ receptors [Citation27,Citation28]. The role of the most abundant antibody, IgG, in current antibody drugs is predominant due to their promising potency, better pharmacokinetics, pharmacodynamics, long half-life, and restricted off-target toxicity. However, there are limitations to their use against cancer. Limitations such as poor penetration and bystander activation of the immune system can be overcome using nanoparticles. Therefore, bioconjugation of IgG with NPs enhances their role against cancer. An intact, highly polar IgG antibody spans enough time in the blood to reach its target because the liver clears it, whereas smaller antibodies (<60 kDa) are cleared by the kidneys [Citation29]. Vincamine, an alkaloid with distinct pharmaceutical activities, is a natural product of Vinca minor leaves [Citation30]. Vincamine, a powerful antioxidant, neuroprotective, and anti-tumour agent, works against ischaemia and hypoxia by generating ATP via oxygen and glucose [Citation31]. It is also known to carry oxygen in living cells [Citation32]. It is recommended as a dietary supplement (40–80 mg/d for 20–40 d without noticeable side effects) to stimulate the CNS and enhance memory [Citation32]. It downregulates the NF-κB-mediated iNOS expression after upregulating the Nrf2/HO-1 mediated signalling pathway(s) that eventually regulate the expression of antioxidant genes like SOD, CAT, and HO-1 [Citation33]. Vincamine also depletes the intracellular iron concentration (and hence dysregulates IRP-2) [Citation34] and disrupts mitochondrial membrane potential for directly activating caspase-3 [Citation35]. Nanoparticles and hydrophilic moieties of vincamine and IgG antibodies can avoid recognition by the reticuloendothelial system (RES). Their accumulation in solid tumours is enhanced by the permeation and retention (EPR) effect with prolonged circulation time in the bloodstream. The bioconjugation of drugs with NPs also prevents non-specific interactions.
Due to their maximum internalisation, the best-suited NPs against cancer range from 20-50 nm. The as-synthesised anionic MTX-IgGAuNPs were found to internalise via caveolae-mediated endocytosis, followed the trajectory with microtubules, and penetrated the ER. Eventually, they got entry into the cytoplasm, and via the nuclear pore complex, they entered the nucleus without fusing with lysosomes. This trajectory prevents drugs from degrading and allows them to arrive in other organelles. Furthermore, MTX-IgGAuNPs also follow Clathrin-caveolae-independent, Arf6-dependent, Cdc42-dependent, and Rhoa-dependent pathways of endocytosis [Citation35]. Therefore, poorly soluble and bioavailable methotrexate was delivered into the nucleus unaltered, unmodified, and non-degraded, where it inhibited dihydrofolate reductase and halted the propagation of cancer cells. The methotrexate-treated patients also experienced resistance. The MTX-IgGAuNPs internalised through the Clathrin-caveolae independent pathway were found to remain in the cytoplasm and initiate the Cyt-c mediated intrinsic apoptotic pathway by disturbing mitochondrial membrane potential. Though MTX-IgGAuNPs were found to be non-toxic against NRK cells.
Conclusion
The concept of ADCs brought about the concept of ANDCs. This new and improved concept overcame the limitations, drawbacks, and shortcomings of ADCs. The nanomaterials have pathways and strategies to internalise into different cells. The functionalised nanomaterials also prevent drug molecule aggregation, precipitation, and non-specific interaction. They also simultaneously deliver drugs into the nucleus and cytoplasm and act synergistically with bioconjugated antibodies and drugs. In the given nanomedicine, MTX-IgGAuNPs have the intrinsic anticancer properties of vincamine and gold nanomaterials, along with the therapeutic potential of IgG and methotrexate. The functionalised IgGAuGNPs did not alter the 3D structure of IgG. Also, administering these nanoemulsions in the blood does not require any solvent. The future of ANDCs relies on the strategies and selection of drugs in combination with antibodies.
Author contributions
Conception and design- Asad Syed, Mohd Sajid Khan, Salim S.Al-Rejaie; Analysis and interpretation of the data- Abu Baker, Abdallah M. Elgorban, Mohamed Mohany; The drafting of the paper- Asad Syed, Abdallah M.Elgorban, Mohd Sajid Khan; Revising it critically for intellectual content: Mohd Sajid Khan, Salim S.Al-Rejaie and the final approval of the version to be published; All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, SAR, upon reasonable request.
Additional information
Funding
References
- Baker A, Wahid I, Hassan Baig M, et al. Silk cocoon-derived protein bioinspired gold nanoparticles as a formidable anticancer agent. J Biomed Nanotechnol. 2021;17(4):615–626. doi: 10.1166/jbn.2021.3053.
- Crous A, Abrahamse H. Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int J Mol Sci. 2020;21:3742. doi: 10.3390/ijms21113742.
- Sasaki H, Tamura K, Naito Y, et al. Patient perceptions of symptoms and concerns during cancer chemotherapy:‘affects my family’is the most important. Int J Clin Oncol. 2017;22(4):793–800. doi: 10.1007/s10147-017-1117-y.
- Yang J, Wang X, Wang B, et al. Challenging the fundamental conjectures in nanoparticle drug delivery for chemotherapy treatment of solid cancers. Adv Drug Deliv Rev. 2022;190:114525. doi: 10.1016/j.addr.2022.114525.
- Scicluna MC, Vella-Zarb L. Evolution of nanocarrier drug-delivery systems and recent advancements in covalent organic framework–drug systems. ACS Appl Nano Mater. 2020;3(4):3097–3115. doi: 10.1021/acsanm.9b02603.
- Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer, trends. Trends Pharmacol Sci. 2009;30(11):592–599. doi: 10.1016/j.tips.2009.08.004.
- Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26. doi: 10.1155/2019/3702518.
- Bertrand N, Wu J, Xu X, et al. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009.
- Mack F, Ritchie M, Sapra P. The next generation of antibody drug conjugates. Semin Oncol. 2014;41(5):637–652. doi: 10.1053/j.seminoncol.2014.08.001.
- Heemskerk N, Gruijs M, Temming AR, et al. Augmented antibody-based anticancer therapeutics boost neutrophil cytotoxicity. J Clin Invest. 2021;131:e134680.
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15(6):361–370. doi: 10.1038/nrc3930.
- Reis ES, Mastellos DC, Ricklin D, et al. Complement in cancer: untangling an intricate relationship. Nat Rev Immunol. 2018;18(1):5–18. doi: 10.1038/nri.2017.97.
- Chiossone L, Dumas P-Y, Vienne M, et al. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. doi: 10.1038/s41577-018-0061-z.
- Kaifu T, Nakamura A. Polymorphisms of immunoglobulin receptors and the effects on clinical outcome in cancer immunotherapy and other immune diseases: a general review. Int Immunol. 2017;29(7):319–325. doi: 10.1093/intimm/dxx041.
- Del Re M, Rofi E, Restante G, et al. Implications of KRAS mutations in acquired resistance to treatment in NSCLC. Oncotarget. 2018;9(5):6630–6643. doi: 10.18632/oncotarget.23553.
- Moshera Samy, Sameh Hosam Abd El-Alim, Abd El Gawad Rabia, Amal Amin, Magdy M.H. Ayoub. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. International Journal of Biological Macromolecules. 2020; 156: 783-791. doi: 10.1016/j.ijbiomac.2020.04.112.
- Bedenic B, Topic M, Budimir A, et al. Urinary bactericidal activity of oral antibiotics against common urinary tract pathogens in an ex vivo model. Chemotherapy. 2006;52(6):293–297. doi: 10.1159/000095969.
- Ayyappan S, Sundaraganesan N, Aroulmoji V, et al. Molecular structure, vibrational spectra and DFT molecular orbital calculations (TD-DFT and NMR) of the antiproliferative drug methotrexate. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77(1):264–275. doi: 10.1016/j.saa.2010.05.021.
- Baker A, Khalid M, Uddin I, et al. Targeted non AR mediated smart delivery of abiraterone to the prostate cancer. PLOS One. 2022;17(8):e0272396. doi: 10.1371/journal.pone.0272396.
- Iram S, Zahera M, Khan S, et al. Gold nanoconjugates reinforce the potency of conjugated cisplatin and doxorubicin. Colloids Surf B Biointerfaces. 2017;160:254–264. doi: 10.1016/j.colsurfb.2017.09.017.
- Nachin L, Nannmark U, Nyström T. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J Bacteriol. 2005;187(18):6265–6272. doi: 10.1128/JB.187.18.6265-6272.2005.
- Baker A, Syed A, Iram S, et al. AR independent anticancer potential of enza against prostate cancer. Colloids Surfaces A Physicochem Eng Asp. 2022;642:128598. doi: 10.1016/j.colsurfa.2022.128598.
- Zhou Y, Andersson O, Lindberg P, et al. Reversible hydrophobic barriers introduced by microcontact printing: application to protein microarrays. Microchim Acta. 2004;146(3–4):193–205. doi: 10.1007/s00604-003-0174-2.
- Baker A, Syed A, Alyousef AA, et al. Sericin-functionalized GNPs potentiate the synergistic effect of levofloxacin and balofloxacin against MDR bacteria. Microb Pathog. 2020;148:104467. doi: 10.1016/j.micpath.2020.104467.
- Berne BJ, Pecora R. Dynamic light scattering: with applications to chemistry, biology, and physics. Mineola: Courier Corporation; 2000.
- Rabinovich-Guilatt L, Couvreur P, Lambert G, et al. Extensive surface studies help to analyse zeta potential data: the case of cationic emulsions. Chem Phys Lipids. 2004;131(1):1–13. doi: 10.1016/j.chemphyslip.2004.04.003.
- McKie A, Samuel D, Cohen B, et al. A quantitative immuno-PCR assay for the detection of mumps-specific IgG. J Immunol Methods. 2002;270(1):135–141. doi: 10.1016/s0022-1759(02)00325-3.
- Koźmiński P, Halik PK, Chesori R, et al. Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int J Mol Sci. 2020;21:3483. doi: 10.3390/ijms21103483.
- Senapati S, Mahanta AK, Kumar S, et al. Controlled drug delivery vehicles for cancer treatment and their performance, signal transduct. Target Ther. 2018;3:1–19.
- Nejadmoghaddam M-R, Minai-Tehrani A, Ghahremanzadeh R, et al. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;11:3.
- Munisvaradass R, Kumar S, Govindasamy C, et al. Human CD3+ T-cells with the anti-ERBB2 chimeric antigen receptor exhibit efficient targeting and induce apoptosis in ERBB2 overexpressing breast cancer cells. Int J Mol Sci. 2017;18:1797. doi: 10.3390/ijms18091797.
- Romig AD Jr, Baker AB, Johannes J, et al. An introduction to nanotechnology policy: opportunities and constraints for emerging and established economies. Technol Forecast Soc Change. 2007;74(9):1634–1642. doi: 10.1016/j.techfore.2007.04.003.
- Barua S, Yoo J-W, Kolhar P, et al. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc Natl Acad Sci U S A. 2013;110(9):3270–3275. doi: 10.1073/pnas.1216893110.
- Abedin MR, Powers K, Aiardo R, et al. Antibody–drug nanoparticle induces synergistic treatment efficacies in HER2 positive breast cancer cells. Sci Rep. 2021;11(1):7347. ppdoi: 10.1038/s41598-021-86762-6.
- Launay P, Patry C, Lehuen A, et al. Alternative endocytic pathway for immunoglobulin a Fc receptors (CD89) depends on the lack of FcRγ association and protects against degradation of bound ligand. J Biol Chem. 1999;274(11):7216–7225. doi: 10.1074/jbc.274.11.7216.