Abstract
Liposomes are considered among the most versatile and advanced nanoparticle delivery systems used to target drugs to specific cells and tissues. Structurally, liposomes are sphere-like vesicles of phospholipid molecules that are surrounded by equal number of aqueous compartments. The spherical shell encapsulates an aqueous interior which contains substances such as peptides and proteins, hormones, enzymes, antibiotics, antifungal and anticancer agents. This structural property of liposomes makes it an important nano-carrier for drug delivery. Extrusion is one of the most frequently used technique for preparing monodisperse uni-lamellar liposomes as the technique is used to control vesicle size. The process involves passage of lipid suspension through polycarbonate membrane with a fixed pore size to produce vesicles with a diameter near the pore size of the membrane used in preparing them. An advantage of this technique is that there is no need to remove the organic solvent or detergent from the final preparation. This review focuses on composition of liposome formulation with special emphasis on factors affecting drug release and drug-loading.
HIGHLIGHT POINTS
Liposomes are among the most effective and multifunctional nanocarriers. However, they possess certain prevalent limitations such as lack of targeting strategies, production challenges and slow overall transition of approved therapies into clinic. Improving drug loading and release capabilities of liposomal drug formulations with efficient delivery optimisation can be the most effective path in designing this class of nanoparticle drugs. Considering the numerous applications of liposomes, the drug delivery research community must utilise these nanocarriers to their maximum potential in an attempt to introduce novel medications against life threatening diseases.
Introduction
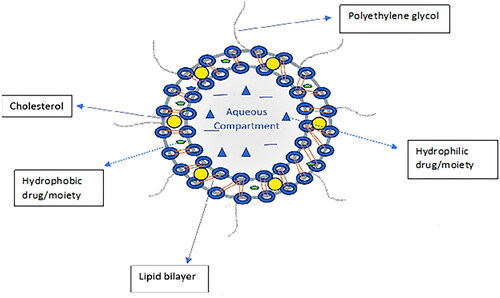
Liposomes are spherical vesicles comprised of lipid bilayer shells surrounding aqueous interior cores and are formed spontaneously when amphiphilic lipids are dispersed in water [Citation1–3]. A general structure of liposome can be seen in .
Among different types of nanoparticles for drug delivery, liposomes are the most developed and established drug delivery system available clinically [Citation2, Citation4, Citation5]. A brief review of the advantages and disadvantages of liposomes reported in literature can be seen below [Citation6, Citation7].
Lipids used in the formulation of liposomes
The importance of liposomes as drug carriers has been well established over the years. It is important to understand the composition of liposomes especially with respect to the lipid components used in their formation. The structure of liposomes consists of lipid bilayer surrounding an aqueous core with the size ranging from 20 to 1000 nm [Citation8].
Phospholipids
Phospholipids are majorly found in the structural composition of biological membranes and are used in the preparation of liposomes extensively [Citation7]. The biocompatibility and amphiphilicity properties of phospholipids contribute to the formation of liposomes and thereby potentiate their desirability as drug carriers [Citation9]. Phospholipids are used in preparation of drug carriers specifically liposomes [Citation10]. Liposomes made with phospholipids possessing multi-functional properties include stimuli sensitive liposomes, targeted liposomes, pH sensitive liposomes and thermosensitive liposomes [Citation10].
Phospholipids are categorised into glycerophospholipids, and sphingomyelins based on the alcohol groups present in their structures [Citation9]. The most used glycerophospholipids in formation of liposomes are Phosphatidylcholine (PC), Phosphatidyl ethanolamine (PE), Phosphatidylserine (PS), Phosphatidylinositol (PI) and Phosphatidylglycerol (PG) [Citation7, Citation8]. Hydrogenated Soy Phosphatidylcholine (HSPC) a glycerophospholipid is a saturated phospholipid which is hydrogenated and possesses greater physicochemical stability compared to unsaturated lipids [Citation11]. The degree of unsaturation, fatty acid side chains and phase transition temperature of phospholipids determine the stability of liposomes [Citation12]. An important property of phospholipids especially in the formation of liposomes in the phase transition temperature (TC). The phase transition temperature of phospholipids is the temperature at which the state of phospholipids changes from gel to liquid crystalline phase [Citation9, Citation13]. The phospholipids which are tightly packed in the bilayer region of liposomes start loosening up and become more permeable when the phase transition temperature of phospholipids is reached [Citation13]. This change causes more empty spaces between the phospholipids thereby aiding in the film formation step of liposome preparation [Citation13]. The phase transition temperature of HSPC is 53 °C and phospholipids with high transition temperature provide increased physical stability at room temperature [Citation9, Citation14].
Cholesterol
Another component in the formation of liposomes is cholesterol. During the preparation of liposomes, cholesterol molecules position themselves with their hydroxyl group oriented towards the aqueous core whereas the hydrophobic group is inserted in the hydrocarbon core region of the bilayer [Citation7, Citation8]. Introduction of cholesterol imparts rigidity into the liposome and thereby reduces the permeability of hydrophilic compounds through the liposomal membrane [Citation7]. Cholesterol causes increased rigidity of phospholipids in the bilayer thereby increasing the mechanical strength of the lipid bilayer [Citation15]. In the absence of cholesterol, liposomes tend to have a less rigid or fluidic bilayer that results in the destabilisation of these drug carriers [Citation7, Citation16]. Thus, cholesterol is a part of the lipid component of liposomes and is subsequently used in their preparation.
Polyethylene glycol and the enhanced permeability and retention (EPR) effect
It is a fact that tumour blood vessels are more permeable to macromolecules as compared to normal healthy blood vessels [Citation17]. Tumours have increased demand of blood supply that results in rapid vascularisation. The subsequent release of angiogenic factors by tumours leads to leaky and defective blood vessels that have incomplete endothelial lining and basement membrane [Citation18]. Due to the highly permeable blood vessels that supply tumours, there is passage of macromolecules and drug carriers leading to the entrapment and accumulation of drug carriers for prolonged periods owing to poor lymphatic drainage in tumour cells [Citation18–20]. This phenomenon is known as the enhanced permeability and retention (EPR) effect [Citation20–22]. The EPR effect is the primary mechanism of passive targeting of nanoparticles to tumours [Citation20, Citation23].
Along with phospholipids and cholesterol, polyethylene glycol (PEG) has been included in preparation of several formulations of drug-loaded liposomes [Citation24]. An effective strategy has been the development of steric stabilised liposomes using polyethylene glycol as part of the formulation [Citation24, Citation25]. PEG is a hydrophilic polymer that prevents the access and binding of blood plasma opsonins to liposomes thereby avoiding their elimination from blood circulation [Citation22]. PEGylated liposomes increase the nanoparticle circulation times and minimises its elimination by the reticuloendothelial system [Citation24, Citation26, Citation27]. It was also observed that PEGylated liposomes improved the overall pharmacokinetics of liposomes and showed minimum interaction with healthy cells in the body [Citation28, Citation29]. PEGylation to achieve prolonged circulation times is essential for nanoparticle drug delivery by passive targeting.
Methods of liposome preparation
A variety of methods have been used in the preparation of liposomes. The selection of method of liposome preparation is based on the type of drug loading, i.e. Passive or Active loading. Passive loading includes two methods of preparation [Citation6, Citation30]: Mechanical dispersion method and Solvent dispersion method.
Mechanical dispersion method includes lipid film hydration, extrusion, sonication, French pressure cell method, freeze thaw. Solvent dispersion method includes ether injection, ethanol injection, reverse phase evaporation and supercritical fluids in the preparation of liposomes [Citation6, Citation30]. Among the mechanical dispersion methods, sonication and lipid film hydration are most widely used in preparation of liposomes [Citation6]. In sonication, the multilamellar vesicles (MLV) are sonicated with use of bath or probe sonicator to form unilamellar vesicles. The method has disadvantages such as non-uniformity in size, low drug encapsulation efficiency [Citation6]. Lipid film hydration along with extrusion can be used to overcome the disadvantages of sonication while achieving passive drug loading [Citation30, Citation31].
Thin-film hydration followed by extrusion method
Thin-film hydration followed by extrusion is one of the simplest and widely used methods of preparing liposomes [Citation32]. The method involves formation of thin film of lipids by evaporation of organic solvent followed by hydration of dried lipid film with aqueous phase and extruding through set of polycarbonate filters to produce unilamellar vesicles [Citation32, Citation33]. Advantages of thin film hydration method for preparation of liposomes are as follows [Citation32, Citation34]:
The method is simple to perform and widely accepted in preparation of liposomes.
It utilises common organic solvents (chloroform, methanol) for dissolution of lipids.
Homogenous unilamellar vesicles are formed after extrusion of hydrated lipid phase.
Formulation of single and dual drug-loaded liposomes by passive loading is possible using thin film hydration method.
There has been considerable work done in the preparation of liposomes by thin film hydration-extrusion method. Everolimus loaded liposomes were prepared using thin film method and characterised for particle size, zeta potential and other morphological evaluations [Citation33]. Results showed that drug-loaded liposomes were efficiently prepared with homogenous vesicle size distribution. A study for preparation of dual drug-loaded liposomes included co-encapsulation of resveratrol and 5-fluorouracil into PEGylated liposomal formulation prepared using thin film hydration method [Citation35]. In addition to evaluation of chemotherapeutic efficacy of the dual drug-loaded formulation, physicochemical evaluation was also done after preparing the formulation using thin film hydration method. Another study for development of liposomal gemcitabine with high drug loading capacity utilised the thin-film hydration method for preparation of liposomes [Citation36]. Owing to the vesicle formation and size consistency, thin film hydration followed by extrusion is effective technique in preparation of liposomes.
Factors affecting drug release from liposomes
Liposomes are the most successful nanoparticle delivery systems developed to target drugs to the site of action. There has been tremendous development in the field of liposomal drug delivery which has led to various clinically approved formulations that are efficacious, biocompatible and possess improved pharmacokinetics [Citation37, Citation38]. The drugs loaded in liposomes become bioavailable only when they are released [Citation39]. Therefore, to have optimum therapeutic activity, modifying/controlling the release rate of drug from liposomes is very essential. There are different methods described in literature for improving and optimising rate of drug release from liposomes. Drug release from liposomes is affected by the following factors [Citation40–42]:
Cholesterol content of the liposomes
Nature of encapsulated drug
Membrane composition of liposomes
Application of external stimuli conditions
Cholesterol content used in the formation of liposomes:
Liposomes that are composed of phospholipids and cholesterol have shown decrease in bilayer permeability with increasing cholesterol content [Citation15, Citation43–46]. Incorporation of cholesterol or sphingomyelin into liposomes provides rigidity to the bilayer and improves retention of drugs into liposomes [Citation7, Citation39, Citation47]. A study of the influence of cholesterol content on liposome stability and in vitro release has been discussed [Citation48]. The study describes the potential advantages of cholesterol as stabiliser which helps in providing strength to the bilayer thereby reducing its permeability to electrolyte and non-electrolyte solutes. The study involved hydrophilic drug (atenolol) and hydrophobic drug (quinine) to be loaded in the liposomes. Liposomes in this study were prepared in five different lipid: cholesterol ratios using the thin-film hydration method. All the formulations were evaluated for particle size, (%) encapsulation efficiency and in vitro drug release profiles. Results showed that formulations with high cholesterol (as high as 50%) had poor encapsulation and drug release profiles. The optimised formulation with 30% cholesterol content showed the best encapsulation and drug release profile. Thus, optimising cholesterol to lipid ratio while formulating drug-loaded liposomes helps in obtaining high encapsulation and drug loading values. Studies have suggested that cholesterol increases the hydrophobicity in the central region of the membrane bilayer which can favour the inclusion of hydrophobic molecules [Citation49–51]. As cholesterol and the hydrophobic drug prefer to accommodate themselves in the bilayer, the competition between the two results in decreased drug encapsulation and loading efficiencies [Citation48, Citation49, Citation52]. Another study described the importance of controlling cholesterol content in evaluating the effects of different lipid for determining the in vitro release kinetics of liposomes [Citation14]. The aim of the study was to evaluate the best lipid used in formulating liposomes and cholesterol content required for improving the overall release kinetics of liposomes. Liposomes were prepared by sonication method and 14C radiolabeled inulin was used as marker to simulate drug encapsulation and release. Results showed that 21% cholesterol was finalised to optimise the stability and release profile of the liposomes. Formulations made with DSPC as lipid showed highest encapsulation efficiency and sustained release for 48h. Therefore, the studies explained so far have shown the importance optimising cholesterol content to obtain improved drug release profiles in liposomes.
Nature of encapsulated drug
Another important factor affecting drug release from liposomes is seen in choosing drugs whose physical characteristics favour retention of drug in liposomes [Citation37, Citation39]. Liposomes possess the ability to entrap both hydrophilic and hydrophobic drug due to their unique structural organisation [Citation14, Citation39, Citation53, Citation54]. There are several drugs that have been successfully delivered through liposomes and have been approved by the FDA for clinical applications. Some of the key examples include doxorubicin, vincristine, paclitaxel, bupivacaine, amphotericin B and irinotecan [Citation7, Citation37, Citation55]. The physicochemical characteristics of drug of choice and the biocompatibility of lipids are the two main considerations in development of a stable and efficacious liposome system [Citation55, Citation56]. A study describing the transcutaneous permeation of three drugs: amphotericin B, imiquimod and indole encapsulated by liposomes effectively explained the comparison of the penetration ability of the three drugs through their encapsulation into ultra-flexible liposomes [Citation57]. Among the drugs encapsulated in liposomes for this study, amphotericin B and imiquimod had poor water solubility whereas indole was water soluble. The study stated that formation and encapsulation of the three drugs into ultra-flexible liposomes increased the overall pharmacokinetics of the drugs with the three drugs showing efficient skin penetration. An effective strategy in designing liposomal drug delivery is selection of drugs that can be loaded to retain inside the liposome and selectively release at the site of action to achieve site specific drug delivery [Citation37, Citation58].
Membrane composition of liposomes
Membrane composition of liposomes plays a major role in the release of drug from liposomes. Since liposomes can encapsulate both hydrophilic and hydrophobic drugs in their structure it is important to study the composition of liposomes during formulation for effective drug release [Citation59]. A study describing the improved liposomal drug retention was conducted by adding dihydrosphingomyelin (DHSM) in the lipid component during the formulation of liposomes [Citation60]. Vincristine-loaded liposomes were prepared by replacing egg sphingomyelin with DHSM in sphingomyelin: cholesterol (55:45 mol/mol) which resulted in a substantial increase in drug encapsulation and showed sustained drug release. Additionally, there was a three-fold increase in drug release half-life compared to liposomes without DHSM. Studies have also suggested that the formation of molecular complexes within the liposomes has resulted in improved retention of drugs in liposomes. Formation of molecular complexes can help in modulating the release of drug from liposome and could also help in preventing leakage of drug from vesicles [Citation39, Citation61]. The design of liposomal co-encapsulation of oleanolic acid and doxorubicin for evaluation of antitumor efficacy utilised three different lipid compositions in formulation of liposomes [Citation62]. Liposomes for this study were prepared by ethanolic injection method and composed of the different lipid ratios as predicted by the statistical design. Results showed that the highest encapsulation efficiency and sustained drug release was achieved by controlling the cholesterol content in the formulation.
Application of external stimuli
Studies have shown that in order to obtain controlled release, there are several stimuli-based strategies that allow rapid release of drug at the tumour site [Citation42, Citation63, Citation64]. The use of local stimuli strategies for improving drug release from liposomes has been extensively studied. This strategy utilises the small changes occurring in the tumour microenvironment such as change in pH, difference in temperature or the overexpression of some proteolytic enzymes for improving the rate of drug release [Citation40]. Formulation of thermosensitive liposomes is an effective strategy in controlling the release of drugs from the nano-lipid vesicles [Citation65–67]. Thermosensitive liposomes have shown tremendous potential when administered with local hyperthermia. There are various studies that have reported the benefits of drug targeting with thermosensitive liposomes. Temperature triggered drug delivery of thermosensitive liposomes using pre and post hyperthermia mechanism has shown promising results [Citation68, Citation69]. A study describing the formulation of lysolipid containing thermosensitive liposomes in combination with local hyperthermia was used to deliver cytotoxic proteins thereby explaining the mechanism of triggered drug release [Citation70]. Liposomes were prepared by thin film hydration and extrusion technique. Liposomes were prepared with different compositions of lipids. The selected formulation (86:10:4 %mol DPPC: MSPC: DSPE-PEG2000) showed efficient drug release with mild hyperthermia and was thereby considered a promising local tumour delivery strategy for cytotoxic proteins. A study describing the release of doxorubicin based on mild hyperthermia-mediated release from thermosensitive liposomes was explored and studied [Citation71]. Briefly, the study consisted of preparation of thermosensitive liposomes for evaluating the release profile of doxorubicin. Results showed that the injected thermosensitive liposomes loaded with doxorubicin released the drug efficiently with mild hyperthermia at 43 °C and was monitored real time with fibred confocal fluorescence microscopy.
Decrease in pH in cellular lysosomes has been successful in improving the rate of drug release in pH sensitive doxorubicin loaded liposomes [Citation72]. The formulation of pH sensitive liposomes was achieved by encapsulating a precursor that had the ability to generate gas bubbles in situ in acidic pH. The bubble generation in acidic pH led to the rapid release of doxorubicin thereby showing antitumor effect at the targeted site. A study involving a novel acid-labile PEGB-Hz-DPPE conjugate in developing dual pH-responsive strategy was part of the preparation of dual pH-sensitive liposomes with enhanced tumour targeted drug delivery [Citation73]. The effectiveness of the study was tested against pancreatic cancer cells. Results showed that as the pH in lysosomes reduced there was efficient release of doxorubicin whose effectiveness increased with the use of ultrasound waves that substantially reduced the viability of cancer cells. Thereby the study concluded by emphasising the potential of pH triggered drug release in liposomes. Thus, there are various techniques/modifications that can be done to improve and control the rate of drug release from liposomes.
Factors affecting drug loading in liposomes
Liposomes are a multifunctional class of nanoparticles that possess the ability of loading and delivering one or more drugs/agents to the targeted site within the body [Citation74–78]. Factors affecting drug loading in liposomes include the following [Citation79–81]:
Method of liposome preparation and its impact on drug loading
Composition of phospholipid to cholesterol ratio in liposomes
Nature and amount of drug used in liposome formulations
Drug to lipid ratio and its effect on liposomal drug loading
Method of drug loading during liposome preparation
Drug loading can vary with the nature of the drug, lipid composition, size and type of liposomes, drug-lipid ratio, and method of preparation [Citation39, Citation77, Citation82]. The flexibility in the composition of lipid bilayer assists in preparing stable drug-loaded liposomes by modification of the integrity of bilayer [Citation39, Citation83–86]. It is also known that with respect to the type of liposomes, small unilamellar vesicles (SUV) have low trapped volume compared to large unilamellar and multilamellar vesicles (LUV and MLV) [Citation87–90]. It is also known that many drugs due to their natural hydrophobicity are unable to be loaded in liposomes by conventional passive loading techniques and hence need to be loaded by different methods of drug loading [Citation81, Citation91]. Based on the factors affecting drug loading in liposomes, many studies have been carried out to highlight the importance of each mentioned factor in improving the drug loading capacity of liposome formulations.
Method of liposome preparation and its impact on drug loading
Liposomes have served as an important class of nanoparticle drug delivery systems particularly in the development of various formulations that have been clinically approved over the years. Literature has shown several different ways to prepare liposomes with the effect of each preparation technique on the drug loading capacity of liposomes [Citation79, Citation92–94]. A study describing the development of docetaxel loaded PEGylated liposomes for investigating the anticancer efficacy involved the use of various methods of liposome preparation to maximise drug loading of docetaxel [Citation95]. The methods of preparation involved in this study were ethanol injection, thin film hydration and modified hydration. Results showed that the optimised formulation prepared with modified hydration method was stable and had high docetaxel loading compared to the other preparation techniques. Additionally, the formulation also showed sustained drug release of 40% over 48 h. It is therefore important to consider the method of liposome preparation as an important factor which affects drug loading. A study describing the various physiochemical factors affecting drug loading in low density lipoprotein (LDL) particles showed the effect of formulation factors on the drug loading capacity of LDL-drug conjugates [Citation96]. The study also compared different preparation methods of liposomes for increasing drug loading. Results showed that the variables considered significant in improving drug loading were method of preparation, incubation time, temperature, and LDL-drug molar ratio. A study focusing on using reengineered ethanolic injection and thin film hydration method with and without extrusion to prepare liposomes for improving drug loading described the effect of both scenarios on loading and encapsulation of doxorubicin [Citation77]. Results showed that drug loaded liposomes prepared with extrusion had better doxorubicin loading than the ones prepared without extrusion. The study further states that an optimised ethanolic injection method for preparation of liposomes showed the highest encapsulation efficiency among all the methods used in the study. The importance of co-encapsulating two or more drugs into liposomes using active loading technique was seen in the encapsulation of doxorubicin, 5-Fluoro-2-Indolyl Deschlorohalopemide and D-Alpha Tocopheryl Acid Succinate into liposomes [Citation97]. The main aim of this study was to develop a stable multifunctional liposomal formulation for anticancer activity. Liposomes for this study were prepared by thin film hydration method and pH gradient method which effectively involved the use of extrusion for preparation of unilamellar vesicles. The prepared formulation was evaluated for (%) drug loading, (%) encapsulation efficiency and in vitro drug release. Results showed satisfactory drug loading levels for all three encapsulated moieties. Similarly, the in vitro drug release profile showed that there was no burst release and about 20% of the drug released after 48 h which indicated sustained release behaviour.
Composition of phospholipid to cholesterol ratio in liposomes
The importance of phospholipid and cholesterol in the preparation of liposomes has been established. Both materials are essential in the formation of liposomes as they represent the lipid component of liposomes [Citation9, Citation51, Citation98]. Ratio of phospholipid to cholesterol has been an important factor in improving (%) drug loading in liposomes. A study describing the formulation and optimisation of thermosensitive liposomes involved the use of different phospholipid to cholesterol ratios to evaluate the effect of each ratio used on percent drug loading and in vitro release profile [Citation99]. Liposomes were prepared by thin-film hydration and extrusion method. Docetaxel-loaded liposome formulations with different phospholipid to cholesterol ratios were evaluated for in vitro drug release and loading efficiency. The study was particularly useful as it evaluated the effect of each lipid component used on drug loading and release profiles. A study describing the formulation of lamivudine loaded liposomes was done using various phospholipids and their combinations with cholesterol to improve loading of lamivudine [Citation100]. Liposomes loaded with lamivudine was also tested for in vitro drug release for which the drug loaded formulation showed stable release profile. The influence of varied compositions and ratios of phospholipid: cholesterol on the formation of pramipexole loaded liposomes was evaluated [Citation101]. Liposomes were prepared using the thin film hydration method followed by sonication to obtain unilamellar vesicles. Among the eight formulations prepared, the formulation with 1:1(phospholipid to cholesterol) had the highest drug encapsulation and optimum vesicle size. The in vitro drug release profile showed release of pramipexole for 8h. Liposomes designed to incorporate and retain peptide nucleic acid oligomers was tested using different combinations of phospholipid: cholesterol ratios [Citation102]. This study explained the use of different types and amounts of phospholipids with cholesterol in preparation of liposomes to achieve high loading efficiency for peptide nucleic acid. In order to achieve high and efficient drug loading the appropriate amount of phospholipid and cholesterol need to be established as part of the formulation studies. A study describing the effect of cholesterol on drug loading and release of hydrophobic drugs observed that increase in cholesterol content in the formulation caused a reduction in loading of drugs and impacted drug release profile [Citation103]. Albumin encapsulated liposomes were prepared with the goal of improving loading and encapsulation efficiency of bovine serum albumin using different molar ratios of phospholipids, cholesterol and DSPE-PEG2000 [Citation104]. Liposomes for this study were prepared by thin film hydration method followed by extrusion. There was a total of ten formulations prepared with different lipid molar ratios and evaluated for bovine serum albumin loading and encapsulation efficiencies. The formulation with highest loading and encapsulation efficiency was chosen for further studies.
Nature and amount of drug used in liposomal formulations
The capacity of liposomes to carry vaccines, enzymes and drugs makes these nanoparticles ideal candidates for drug delivery [Citation9, Citation105]. The structure of liposomes allows for the incorporation of both hydrophilic and hydrophobic drugs which thereby increases the therapeutic capacity of these lipid nanoparticles [Citation74, Citation75, Citation77, Citation106–108]. Nature of the drug to be encapsulated in liposomes plays an important role in determining the method of drug loading to be used [Citation107, Citation109–111]. A study describing the encapsulation of hydrophobic molecule ß-caryophyllene into liposomes was done using different amounts of the hydrophobic drug with respect to soy phosphatidylcholine (SPC) [Citation106]. Liposomes for this study were prepared by thin film hydration method followed by extrusion and evaluated for drug loading with different drug amounts. Results showed that formulations with low drug amounts significantly improved the drug loading capacity and thereby showed increased cytotoxicity. Formulations with high drug content had poor drug loading and release due to interactions between the drug and lipid membrane. Additionally, there have been studies that have used different drug loading techniques based on the nature of the drug. For instance, a study describing the loading of doxorubicin, an amphipathic weak base into liposomes by forming a transmembrane MnSO4 gradient does justify the use of that loading technique considering the nature of doxorubicin [Citation112]. The study stated that the transmembrane loading method used was successful in encapsulating the drug and providing increased drug retention to improve the in vitro drug release profile. Similarly, there have been many commercial liposomal drugs approved by the FDA for clinical use that have emphasised the importance of amount and nature of drug to be used for formulation [Citation37, Citation107, Citation113, Citation114]. Therefore, the amount and nature of drug to be loaded in liposomes affects the drug loading capacity.
Drug to lipid ratio and its effect on liposomal drug loading
In addition to the factors mentioned earlier, regulating the ratio of drug to lipid is of equal importance in formulation of liposomes. Drug to lipid ratio is often described as the moles of drug per moles of phospholipid and calculated as the ratio of mole fraction of the entrapped drug and moles of lipid [Citation37, Citation59, Citation112, Citation115–117]. Several studies have shown the precipitation of drugs within the liposomal formulation which is undesirable and mainly caused due to high drug to lipid ratios [Citation56, Citation109, Citation111, Citation115]. A study describing the influence of drug to lipid ratio on drug release and loading of doxorubicin into liposomes highlights the importance of regulating the ratio during preparation of liposomes [Citation115]. The formulated liposomes were evaluated for in vitro drug release and loading efficiency. Results showed that increasing the drug to lipid ratio in formulations led to drug precipitation and membrane disruption which is undesirable and does not account for a stable drug loaded liposomal formulation. Similarly, at low drug to lipid ratio the formulations showed improved drug loading and stable drug release without any membrane disruption. Another study describing the formulation of vincristine loaded liposomes using various drug to lipid ratios was done to improve drug loading and achieve stable drug release [Citation117]. Drug loaded liposomes were prepared and evaluated for (%) encapsulation efficiency and in vitro drug release with drug to lipid ratio (1:10, 1:5, 1:2). Among the three ratios, formulation with drug to lipid ratio of 1:5 showed the highest encapsulation efficiency and stable drug release profile. Preparation of baicalein loaded liposomes was done optimise phospholipid to cholesterol and drug to lipid ratio required for improving the loading of baicalein in liposomes [Citation9]. Liposomes were prepared by thin film hydration for this study. Along with drug loading the formulation was also tested for in vitro drug release. Results showed that drug loading for the formulated liposomes was 4.41 ± 0.02% and in vitro drug release was 65% after 48h. The loading of platinum-chloroquine into PEGylated liposomes was achieved by formulating the liposomes with different drug to lipid ratios [Citation118]. Liposomes were prepared by thin-film hydration method followed by extrusion. Three drug: lipid ratios (1:2.5, 1:5 and 1:7 w/w) were used to load platinum-chloroquine into liposomes. After evaluating all the formulations, drug: lipid ratio of 1:7 was chosen for future studies as the selected ratio gave the highest drug loading and encapsulation values. Additionally, the in vitro drug release profile was stable and sustained for 72h. Therefore, preparation of liposomes by regulating the drug: lipid ratio can contribute to improved drug loading and release profiles.
Method of drug loading during liposome preparation
Drug loading in liposomes can be mainly accomplished by passive loading and active loading methods [Citation39, Citation79, Citation82, Citation119]. A diagrammatic representation of drug loading into liposomes () explains the development and characterisation of solvent-assisted active loading technology for liposomal loading of poorly water-soluble compounds [Citation92].
Figure 2. Drug loading techniques in liposomes [Citation92].
![Figure 2. Drug loading techniques in liposomes [Citation92].](/cms/asset/67bd8b50-3289-4465-b9d4-b9d5d50fbed2/ianb_a_2247036_f0002_c.jpg)
Passive loading includes all techniques in which during liposome preparation the lipid and drug are dispersed in an aqueous buffer, thus achieving entrapment while the liposomes are being formed [Citation39, Citation87, Citation92, Citation94].
Active loading on the other hand, refers to the entrapment of hydrophilic or hydrophobic drugs in the bilayer or aqueous core of liposomes respectively due to nature of the drug and lipids [Citation56, Citation79]. A variety of studies have efficiently explained the use of active and passive drug loading techniques in preparation of drug loaded liposomes. An article explaining the effects of formulation and process parameters on drug loading and release behaviour of doxorubicin liposomes describes the use of active loading and drug: lipid ratio optimisation that contributed towards high drug loading efficiencies [Citation103]. Development of liposomal gemcitabine was achieved with emphasis on improving loading capacity of gemcitabine by combining remote loading and small volume loading approaches [Citation36]. During remote loading, drug diffuses through the lipid bilayer into intravesicular spaces resulting in a subsequent chemical modification of the drug. This modification prevents the removal of drug from the membrane resulting in accumulation of drug in liposomes. Remote loading can encapsulate drugs with high loading efficiency although the technique is not applicable to all weak acid or base drugs. The encapsulation of gemcitabine has shown difficulty in encapsulation with poor drug loading using remote loading method. According to the study, small volume loading involves the incubation of preformed liposomes in high concentration of gemcitabine solution. The high external gemcitabine concentration generates maximum concentration gradient across liposomal membrane. Liposomes for this study were prepared by thin film hydration-extrusion method. Drug loading was done by passive loading, remote loading, small volume loading and combination of remote and small volume loading. Additionally, drug release of gemcitabine loaded liposomes was also evaluated. Results showed that the combination of remote loading and small volume loading helped in achieving high loading capacity of around 10% w/w with sustained drug release over 120 h. Improving drug loading capacity helps in achieving the required therapeutic potential and is explored in a variety of liposomal drug formulations. A study describing the encapsulation of rapamycin into liposomes highlighted the importance of using a pre-loading technique for rapamycin which significantly improved the (%) encapsulation efficiency and drug loading of the subsequent formulations prepared for the study [Citation120]. The study was further modified in a way that the prepared liposomes were subsequently dispersed and formulated into a hydrogel.
Cytotoxicity studies of drug-loaded liposomes
Liposomes hold great potential as drug delivery vehicles due to their biocompatibility and the ability to effectively encapsulate both hydrophilic and hydrophobic drugs [Citation7]. Several studies involving liposomes loaded with anticancer drugs have been developed and evaluated for cytotoxicity. A study describing the evaluation of in vitro cytotoxicity of gemcitabine and cisplatin co-loaded thermosensitive liposomes in two pancreatic cancer cell lines: MiaPaCa-2 and BxPC-3 resulted in drug-loaded liposomes showing increased anticancer efficacy compared to single drug treatment [Citation121]. The cytotoxicity of the dual drug-loaded liposomes was evaluated using flow cytometry. Another study describing the formulation of everolimus and vinorelbine loaded liposomes was tested for in vitro cytotoxicity in two different renal cell cancer cell lines [Citation122]. The cell viability (%) for this study was measured using Cell titre 96 Aqueous One Solution Cell Proliferation Assay. The dual drug loaded liposomes demonstrated excellent tumour-specific uptake and showed significantly higher inhibition of tumour growth compared to single drug-loaded liposomes. The in vitro cytotoxicity and intracellular uptake efficacy of CPT-11 and Panobinostat co-loaded antibody conjugated immunoliposomes was evaluated using flow cytometry analysis [Citation123]. Results showed that there was a 6-fold increase in drug uptake efficiency for liposomes according to flow cytometry and confocal microscopy. The immunoliposomes possessed cytotoxicity against U87DR cells in vitro with 1.6-fold increase of apoptosis rate.
Commercially available liposomal formulations and recent developments in therapies
The entrapment of anticancer agents and delivery through liposomes is a promising strategy that has gained tremendous potential in treatment of cancers. Apart from cancers, there are multiple targets and disease conditions for which liposomal drug delivery has shown effectiveness. Moreover, liposomes are non-toxic, biocompatible, and biodegradable, approved by the Food and drug administration (FDA) for delivery of various drugs/moieties for treatment of different conditions [Citation2, Citation108, Citation124]. Within various drug delivery systems, liposomes represent a versatile and advanced nano-delivery system for a wide range of biologically active compounds [Citation125, Citation126].
PEGylated liposomes are effective drug delivery vehicles as they facilitate high-drug loading capabilities, improved biocompatibility, and long circulating properties with improved stability [Citation74, Citation127, Citation128]. Variety of anticancer drugs have been incorporated into liposomes with remarkable clinical success [Citation74, Citation129]. Some of the FDA approved liposome formulations include Ambisome (Amphotericin B), Doxil (Doxorubicin) and Marquibo (Vincristine) thereby emphasising the advantages of liposomes as a drug delivery system [Citation114, Citation127]. A list of commercially available drug loaded liposomes can be seen in .
Table 1. Liposomal formulations available commercially.
There have been numerous studies about the capability of liposomes to be loaded with single or multiple drugs [Citation4, Citation136]. A study describing the formulation of gemcitabine loaded thermosensitive liposomes for antitumor activity explained the feasibility of loading and improving the release of gemcitabine to tumour cells and emphasised the potential of liposomes as a drug delivery vehicle [Citation124]. Paclitaxel, a highly potent anticancer drug for breast and ovarian cancer was delivered through liposomes for increasing total drug content with a stable formulation [Citation137]. Doxorubicin, a widely used anticancer drug for prostate cancer is known for its severe side effects including tissue cytotoxicity and cardiotoxicity was formulated into a thermosensitive liposomal formulation [Citation138]. Results showed that there was stable and controlled drug release from thermoresponsive liposomes with enhanced cellular uptake [Citation138]. Thus, liposomes are explored as nano carriers for single and dual drug-loaded formulations.
Recent developments in liposome drug delivery include applications in nucleic acid delivery [Citation125]. A comprehensive review of different nucleic acid-based liposome formulations in various clinical stages effectively sums up the current state and potential applications of liposomes as drug carriers [Citation125]. There are various strategies being developed in the form of nucleic acid-based liposome formulations to facilitate effective therapies in treatment of diseases involving defective or mutated genes (Barba et al. 2019). Nucleic acid-based drugs including siRNA, mRNA, Oligonucleotides, and other chemically synthesised molecules are designed to be delivered using lipid-based carriers as part of gene therapy (Barba et al. 2019). Lipid nanoparticles (LNP) represent one of the most robust and versatile drug carrier systems for delivery of genetic drugs [Citation139]. The first siRNA-LNP formulation approved by the FDA was Patisiran (Onpattro) by Alnylam Pharmaceuticals in 2018 and since then there are close to 10 new lipid-based formulations under development [Citation139, Citation140].
There are several applications which are utilising liposomes as carriers with projects currently ongoing at different phases of clinical trials [Citation141]. These projects include several targets ranging from Hepatocellular carcinoma, Post-operative pain, Solid tumours, Pulmonary infections, Eye infections, Influenza, COVID-19 and more [Citation141]. Some miscellaneous applications of liposomes which are currently used include Super paramagnetic liposomes which are used as MRI contrast agents [Citation141, Citation142]. In summary there are multiple novel liposome drug therapies under clinical development that target complex and rare disease states with an aim to improve overall patient care and replace conventional drug therapies.
Conclusion
Liposomes are multifunctional drug carriers especially in targeted drug delivery. The structural formation of liposomes makes them a versatile nano-carrier with capabilities of loading and delivering multiple drugs/moieties for specific disease states. Understanding the importance of each structural component in formulation of liposomes is crucial in the development of a stable, efficacious liposomal drug formulation. Prospective studies need to be carried out on the biocompatibility, circulation rate and toxicity parameters of potential drug-loaded liposome formulations to optimise the drug development process. Additionally, since the advent of combination drug therapy and development of novel drug products using liposomal formulations have shown significant advantages over conventional drug therapies, there is tremendous potential in this class of drugs to be considered as a preferred drug delivery strategy.
Author contribution
Dr. Shantanu Pande is the sole author of this review article. This section certifies that Dr. Shantanu Pande (Sole Author) is responsible for the conception and design of this article. Similarly, the drafting, revision and final approval of this article was also done by Dr. Shantanu Pande.
Acknowledgments
This manuscript has been derived from my doctoral dissertation. The link to the published dissertation is https://www.proquest.com/docview/2586987897
Data availability statement
The data that support the findings of this review article are available in PubMed Central [PMC] at [https://pubmed.ncbi.nlm.nih.gov/] and Scopus [https://www.scopus.com/home.uri]. These data were cited from the following resources available in the public domain: [https://pubmed.ncbi.nlm.nih.gov/https://www.scopus.com/home.uri]
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Manna S, Wu Y, Wang Y, et al. Probing the mechanism of bupivacaine drug release from multivesicular liposomes. J Control Release. 2019;294:279–287. doi: 10.1016/j.jconrel.2018.12.029.
- Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286.
- Ta T, Porter TM. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J Control Release. 2013;169(1-2):112–125. doi: 10.1016/j.jconrel.2013.03.036.
- Hu CMJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1(2):323–334. doi: 10.4155/tde.10.13.
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. doi: 10.1186/s12951-018-0392-8.
- Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102.
- Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: what is available and what is yet to come. Pharmacol Rev. 2016;68(3):701–787. doi: 10.1124/pr.115.012070.
- Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):381–391. doi: 10.3109/21691401.2014.953633.
- Li J, Wang X, Zhang T, et al. A review on phospholipids and their main applications in drug delivery systems. Asian J Pharm Sci. 2015;10(2):81–98. doi: 10.1016/j.ajps.2014.09.004.
- Drescher S, van Hoogevest P. The phospholipid research center: current research in phospholipids and their use in drug delivery. Pharmaceutics. 2020;12(12):1–36. doi: 10.3390/pharmaceutics12121235.
- Franzé S, et al. Lyophilization of liposomal formulations: still necessary, still challenging. Pharmaceutics. 2018;10(3):139. doi: 10.3390/pharmaceutics10030139.
- Olusanya TOB, et al. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23(4):907. doi: 10.3390/molecules23040907.
- Chen W, Duša F, Witos J, et al. Determination of the main phase transition temperature of phospholipids by nanoplasmonic sensing. Sci Rep. 2018;8(1):14815. doi: 10.1038/s41598-018-33107-5.
- Anderson M, Omri A. The effect of different lipid components on the in vitro stability and release kinetics of liposome formulations. Drug Deliv. 2004;11(1):33–39. doi: 10.1080/10717540490265243.
- Magarkar A, Dhawan V, Kallinteri P, et al. Cholesterol level affects surface charge of lipid membranes in saline solution. Sci Rep. 2014;4(1):5005. doi: 10.1038/srep05005.
- Kirby C, Clarke J, Gregoriadis G. Cholesterol content of small unilamellar liposomes controls phospholipid loss to high density lipoproteins in the presence of serum. FEBS Lett. 1980;111(2):324–328. doi: 10.1016/0014-5793(80)80819-2.
- Nakamura Y, Mochida A, Choyke PL, et al. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem. 2016;27(10):2225–2238. doi: 10.1021/acs.bioconjchem.6b00437.
- Rajani C, Borisa P, Karanwad T, et al. Cancer-targeted chemotherapy: emerging role of the folate anchored dendrimer as drug delivery nanocarrier. Pharmaceut Appl Dendrimer. 2019;2020:151–198.
- Golombek SK, May J-N, Theek B, et al. Tumor targeting via EPR: strategies to enhance patient responses. Adv Drug Deliv Rev. 2018;130:17–38. doi: 10.1016/j.addr.2018.07.007.
- Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi: 10.1016/s0168-3659(99)00248-5.
- Adesina SK, Holly A, Kramer-Marek G, et al. Polylactide-based paclitaxel-loaded nanoparticles fabricated by dispersion polymerization: characterization, evaluation in cancer cell lines, and preliminary biodistribution studies. J Pharm Sci. 2014;103(8):2546–2555. doi: 10.1002/jps.24061.
- Cunha CRAd, Silva LCNd, Almeida FJF, et al. Encapsulation into stealth liposomes enhances the antitumor action of recombinant cratylia mollis lectin expressed in Escherichia coli. Front Microbiol. 2016;7:1355. doi: 10.3389/fmicb.2016.01355.
- Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7(2):223–225.
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1(3):297–315.
- Lasic DD. Mechanisms of liposome formation. J Liposome Res. 1995;5(3):431–441. doi: 10.3109/08982109509010233.
- Gao W, et al. Liposome-like nanostructures for drug delivery. J Mater Chem B. 2013;1(48):6569–6585. doi: 10.1039/C3TB21238F.
- Rovira-Bru M, Thompson DH, Szleifer I. Size and structure of spontaneously forming liposomes in lipid/PEG-lipid mixtures. Biophys J. 2002;83(5):2419–2439. doi: 10.1016/S0006-3495(02)75255-7.
- Belfiore L, Saunders DN, Ranson M, et al. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J Control Release. 2018;277:1–13. doi: 10.1016/j.jconrel.2018.02.040.
- Huwyler J, Drewe J, Krähenbühl S. Tumor targeting using liposomal antineoplastic drugs. Int J Nanomedicine. 2008;3(1):21–29.
- Monteiro N, Martins A, Reis RL, et al. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11(101):20140459. doi: 10.1098/rsif.2014.0459.
- Lapinski MM, Castro-Forero A, Greiner AJ, et al. Comparison of liposomes formed by sonication and extrusion: rotational and translational diffusion of an embedded chromophore. Langmuir. 2007;23, 23(23):11677–11683. doi: 10.1021/la7020963.
- Zhang H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol Biol. 2017;1522:17–22. doi: 10.1007/978-1-4939-6591-5_2.
- Torres-Flores G, Gonzalez-Horta A, Vega-Cantu YI, et al. Preparation and characterization of liposomal everolimus by thin-film hydration technique. Adv Polym Tech. 2020;2020:1–9. doi: 10.1155/2020/5462949.
- Sharma M, et al. 2020. Liposome-A comprehensive approach for researchers. In: Catala A and Ahmad U, editors. Molecular pharmacology. IntechOpen. doi: 10.5772/intechopen.93256.
- Mohan A, Narayanan S, Sethuraman S, et al. Novel resveratrol and 5-fluorouracil coencapsulated in PEGylated nanoliposomes improve chemotherapeutic efficacy of combination against head and neck squamous cell carcinoma. Biomed Res Int. 2014;2014:424239. doi: 10.1155/2014/424239.
- Tamam H, Park J, Gadalla HH, et al. Development of liposomal gemcitabine with high drug loading capacity. Mol Pharm. 2019;16(7):2858–2871. doi: 10.1021/acs.molpharmaceut.8b01284.
- Bulbake U, et al. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi: 10.3390/pharmaceutics9020012.
- Yadav D, Sandeep K, Pandey D, et al. Liposomes for drug delivery. J Biotechnol Biomater. 2017;07(04):1–8. doi: 10.4172/2155-952X.1000276.
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–999. doi: 10.2147/IJN.S68861.
- Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond). 2013;8(9):1509–1528. doi: 10.2217/nnm.13.118.
- Lindner LH, Hossann M. Factors affecting drug release from liposomes. Curr Opin Drug Discov Devel. 2010;13(1):111–123.
- Ponce A, Wright A, Dewhirst M, et al. Targeted bioavailability of drugs by triggered release from liposomes. Future Lipidology. 2006;1(1):25–34. doi: 10.2217/17460875.1.1.25.
- Drummond DC, Meyer O, Hong K, et al. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743.,
- Jovanović AA, Balanč BD, Ota A, et al. Comparative effects of cholesterol and β-sitosterol on the liposome membrane characteristics. Eur J Lipid Sci Technol. 2018;120(9):1800039. doi: 10.1002/ejlt.201800039.
- Kaddah S, Khreich N, Kaddah F, et al. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem Toxicol. 2018;113:40–48. doi: 10.1016/j.fct.2018.01.017.
- Saito H, Shinoda W. Cholesterol effect on water permeability through DPPC and PSM lipid bilayers: a molecular dynamics study. J Phys Chem B. 2011;115(51):15241–15250. doi: 10.1021/jp201611p.
- Coderch L, Fonollosa J, De Pera M, et al. Influence of cholesterol on liposome fluidity by EPR. Relationship with percutaneous absorption. J Control Release. 2000;68(1):85–95. doi: 10.1016/s0168-3659(00)00240-6.
- Briuglia M-L, Rotella C, McFarlane A, et al. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv Transl Res. 2015;5(3):231–242. doi: 10.1007/s13346-015-0220-8.
- Deniz A, Sade A, Severcan F, et al. Celecoxib-loaded liposomes: effect of cholesterol on encapsulation and in vitro release characteristics. Biosci Rep. 2010;30(5):365–373. doi: 10.1042/BSR20090104.
- Khajeh A, Modarress H. The influence of cholesterol on interactions and dynamics of ibuprofen in a lipid bilayer. Biochim Biophys Acta. 2014;1838(10):2431–2438. doi: 10.1016/j.bbamem.2014.05.029.
- Melzak K, Melzak S, Gizeli E, et al. Cholesterol organization in phosphatidylcholine liposomes: a surface plasmon resonance study. Materials. 2012;5(11):2306–2325. doi: 10.3390/ma5112306.
- Lombardo D, Calandra P, Barreca D, et al. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials. 2016;6(7):125. doi: 10.3390/nano6070125.
- Blume G, Cevc G. Liposomes for the sustained drug release in vivo. Biochim Biophys Acta. 1990;1029(1):91–97. doi: 10.1016/0005-2736(90)90440-y.
- Loew S, Fahr A, May S. Modeling the release kinetics of poorly water-soluble drug molecules from liposomal nanocarriers. J Drug Deliv. 2011;2011:376548. doi: 10.1155/2011/376548.
- Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, et al. Nanomedicine review: clinical developments in liposomal applications. Cancer Nano. 2019;10(1):1–40. doi: 10.1186/s12645-019-0055-y.
- Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: in vivo evidence and recent approaches. Pharmaceutics. 2020;12(3):264. doi: 10.3390/pharmaceutics12030264.
- Peralta MF, Guzmán ML, Pérez AP, et al. Liposomes can both enhance or reduce drugs penetration through the skin. Sci Rep. 2018;8(1):13253. doi: 10.1038/s41598-018-31693-y.
- Zhang W, Falconer JR, Baguley BC, et al. Improving drug retention in liposomes by aging with the aid of glucose. Int J Pharm. 2016;505(1-2):194–203. doi: 10.1016/j.ijpharm.2016.03.044.
- Chountoulesi M, Naziris N, Pippa N, et al. The significance of drug-to-lipid ratio to the development of optimized liposomal formulation. J Liposome Res. 2018;28(3):249–258. doi: 10.1080/08982104.2017.1343836.
- Johnston MJW, Semple SC, Klimuk SK, et al. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim Biophys Acta. 2007;1768(5):1121–1127. doi: 10.1016/j.bbamem.2007.01.019.
- Lee Y, Thompson DH. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(5):. doi: 10.1002/wnan.1450.
- Sarfraz M, Afzal A, Raza SM, et al. Liposomal co-delivered oleanolic acid attenuates doxorubicininduced multi-organ toxicity in hepatocellular carcinoma. Oncotarget. 2017;8(29):47136–47153. doi: 10.18632/oncotarget.17559.
- Knepp VM, Hinz RS, Szoka FC, et al. Controlled drug release from a novel liposomal delivery system. I. Investigation of transdermal potential. J Controlled Release. 1987;5(3):211–221. doi: 10.1016/0168-3659(88)90020-X.
- Suchyta DJ, Schoenfisch MH. Controlled release of nitric oxide from liposomes. ACS Biomater Sci Eng. 2017;3(9):2136–2143. doi: 10.1021/acsbiomaterials.7b00255.
- Blueschke G, Boico A, Negussie AH, et al. Enhanced drug delivery to the skin using liposomes. Plast Reconstr Surg Glob Open. 2018;6(7):e1739. doi: 10.1097/GOX.0000000000001739.
- Mazzotta E, Tavano L, Muzzalupo R. Thermo-Sensitive vesicles in controlled drug delivery for chemotherapy. Pharmaceutics. 2018;10(3):150. doi: 10.3390/pharmaceutics10030150.
- Yuba E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J Mater Chem B. 2020;8(6):1093–1107. doi: 10.1039/c9tb02470k.
- Kneidl B, Peller M, Winter G, et al. Thermosensitive liposomal drug delivery systems: state of the art review. Int J Nanomedicine. 2014;9:4387–4398. doi: 10.2147/IJN.S49297.
- Li L, ten Hagen TLM, Bolkestein M, et al. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J Control Release. 2013;167(2):130–137. doi: 10.1016/j.jconrel.2013.01.026.
- de Matos MBC, Beztsinna N, Heyder C, et al. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur J Pharm Biopharm. 2018;132:211–221. doi: 10.1016/j.ejpb.2018.09.010.
- Derieppe M, Escoffre J-M, Denis de Senneville B, et al. Assessment of intratumoral doxorubicin penetration after mild hyperthermia-mediated release from thermosensitive liposomes. Contrast Media Mol Imaging. 2019;2019:2645928. doi: 10.1155/2019/2645928.
- Nahire R, Hossain R, Patel R, et al. PH-triggered echogenicity and contents release from liposomes. Mol Pharm. 2014;11(11):4059–4068. doi: 10.1021/mp500186a.
- Kanamala M, Palmer BD, Jamieson SM, et al. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine (Lond). 2019;14(15):1971–1989. doi: 10.2217/nnm-2018-0510.
- Ashley JD, Quinlan CJ, Schroeder VA, et al. Dual carfilzomib and doxorubicin-loaded liposomal nanoparticles for synergistic efficacy in multiple myeloma. Mol Cancer Ther. 2016;15(7):1452–1459. doi: 10.1158/1535-7163.MCT-15-0867.
- Miatmoko A, Salim HR, Zahro SM, et al. Dual loading of primaquine and chloroquine into liposome. Eur Pharmaceut J. 2019;66(2):18–25. doi: 10.2478/afpuc-2019-0009.
- Park H-B, Kim Y-J, Lee S-M, et al. Dual drug-loaded liposomes for synergistic efficacy in MCF-7 breast cancer cells and cancer stem cells. BSL. 2019;25(2):159–169. doi: 10.15616/BSL.2019.25.2.159.
- Sarfraz M, et al. Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies. Pharmaceutics. 2018;10(3):151. doi: 10.3390/pharmaceutics10030151.
- Sen K, Banerjee S, Mandal M. Dual drug loaded liposome bearing apigenin and 5-fluorouracil for synergistic therapeutic efficacy in colorectal cancer. Colloids Surf B Biointerfaces. 2019;180:9–22. doi: 10.1016/j.colsurfb.2019.04.035.
- Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8(5):565–580. doi: 10.1517/17425247.2011.566552.
- Mohammed AR, Weston N, Coombes AGA, et al. Liposome formulation of poorly water-soluble drugs: optimisation of drug loading and ESEM analysis of stability. Int J Pharm. 2004;285(1-2):23–34. doi: 10.1016/j.ijpharm.2004.07.010.
- Zhang W, Wang G, Falconer JR, et al. Strategies to maximize liposomal drug loading for a poorly water-soluble anticancer drug. Pharm Res. 2015;32(4):1451–1461. doi: 10.1007/s11095-014-1551-8.
- Mayer LD, Bally MB, Hope MJ, et al. Techniques for encapsulating bioactive agents into liposomes. Chem Phys Lipids. 1986;40(2–4):333–345. doi: 10.1016/0009-3084(86)90077-0.
- He H, Lu Y, Qi J, et al. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9(1):36–48. doi: 10.1016/j.apsb.2018.06.005.
- Liu W, Ye A, Liu W, et al. Stability during in vitro digestion of lactoferrin-loaded liposomes prepared from milk fat globule membrane-derived phospholipids. J Dairy Sci. 2013;96(4):2061–2070. doi: 10.3168/jds.2012-6072.
- Routledge SJ, Linney JA, Goddard AD. Liposomes as models for membrane integrity. Biochem Soc Trans. 2019;47(3):919–932. doi: 10.1042/BST20190123.
- Taira MC, Chiaramoni NS, Pecuch KM, et al. Stability of liposomal formulations in physiological conditions for oral drug delivery. Drug Deliv. 2004;11(2):123–128. doi: 10.1080/10717540490280769.
- Cullis PR, Mayer LD, Bally MB, et al. Generating and loading of liposomal systems for drug-delivery applications. Adv Drug Deliv Rev. 1989;3(3):267–282. doi: 10.1016/0169-409X(89)90024-0.
- Hirai M, Kimura R, Takeuchi K, et al. Structure of liposome encapsulating proteins characterized by X-ray scattering and shell-modeling. J Synchrotron Radiat. 2013;20(Pt 6):869–874. doi: 10.1107/S0909049513020827.
- Laouini A, Jaafar-Maalej C, Limayem-Blouza I, et al. Preparation, characterization and applications of liposomes: state of the art. j Coll Sci Biotechnol. 2012;1(2):147–168. doi: 10.1166/jcsb.2012.1020.
- Pandey H, Rani R, Agarwal V. Liposome and their applications in cancer therapy. Braz. arch. biol. technol. 2016;59(0):16150477. doi: 10.1590/1678-4324-2016150477.
- Sur S, Fries AC, Kinzler KW, et al. Remote loading of preencapsulated drugs into stealth liposomes. Proc Natl Acad Sci U S A. 2014;111(6):2283–2288. doi: 10.1073/pnas.1324135111.
- Pauli G, Tang WL, Li SD. Development and characterization of the solvent-assisted active loading technology (SALT) for liposomal loading of poorly water-soluble compounds. Pharmaceutics. 2019;11(9):465. doi: 10.3390/pharmaceutics11090465.
- Popovska O, et al. An overview: methods for preparation and characterization of liposomes as drug delivery systems. Int J Pharmaceut Phytopharmacol Res. 2013;3(3):182–189.
- Wehbe M, Malhotra A, Anantha M, et al. A simple passive equilibration method for loading carboplatin into pre-formed liposomes incubated with ethanol as a temperature dependent permeability enhancer. J Control Release. 2017;252:50–61. doi: 10.1016/j.jconrel.2017.03.010.
- Patel K, Doddapaneni R, Chowdhury N, et al. Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine (Lond). 2016;11(11):1377–1392. doi: 10.2217/nnm.16.37.
- Kader A, Davis PJ, Kara M, et al. Drug targeting using low density lipoprotein (LDL): physicochemical factors affecting drug loading into LDL particles. J Control Release. 1998;55(2-3):231–243. doi: 10.1016/s0168-3659(98)00052-2.
- Song M, Wang J, Lei J, et al. Preparation and evaluation of liposomes co-loaded with doxorubicin, phospholipase D inhibitor 5-fluoro-2-indolyl deschlorohalopemide (FIPI) and d-alpha tocopheryl acid succinate (α-TOS) for anti-metastasis. Nanoscale Res Lett. 2019;14(1):138. doi: 10.1186/s11671-019-2964-4.
- Huang Z, Szoka FC. Sterol-modified phospholipids: cholesterol and phospholipid chimeras with improved biomembrane properties. J Am Chem Soc. 2008;130(46):15702–15712. doi: 10.1021/ja8065557.
- Park SM, Cha JM, Nam J, et al. Formulation optimization and in vivo proof-of-concept study of thermosensitive liposomes balanced by phospholipid, elastin-like polypeptide, and cholesterol. PLoS One. 2014;9(7):e103116. doi: 10.1371/journal.pone.0103116.
- Godbole MD, Mathur VB. Selection of phospholipid and method of formulation for optimum entrapment and release of lamivudine from liposome. J Drug Delivery Ther. 2018;8(5-s):175–183. doi: 10.22270/jddt.v8i5-s.1935.
- Trivedi RV, et al. Influence of egg lecithin composition on physicochemical characteristics of pramipexole liposomes. Int J Res Pharmaceut Sci. 2017;8(1):6–15.
- Ringhieri P, Avitabile C, Saviano M, et al. The influence of liposomal formulation on the incorporation and retention of PNA oligomers. Colloids Surf B Biointerfaces. 2016;145:462–469. doi: 10.1016/j.colsurfb.2016.05.034.
- Ali MH, Kirby DJ, Mohammed AR, et al. Solubilisation of drugs within liposomal bilayers: alternatives to cholesterol as a membrane stabilizing agent. J Pharm Pharmacol. 2010;62(11):1646–1655. doi: 10.1111/j.2042-7158.2010.01090.x.
- Okamoto Y, Taguchi K, Yamasaki K, et al. Albumin-encapsulated liposomes: a novel drug delivery carrier with hydrophobic drugs encapsulated in the inner aqueous core. J Pharm Sci. 2018;107(1):436–445. doi: 10.1016/j.xphs.2017.08.003.
- Wodlej C, Riedl S, Rinner B, et al. Interaction of two antitumor peptides with membrane lipids – influence of phosphatidylserine and cholesterol on specificity for melanoma cells. PLoS One. 2019;14(1):e0211187. doi: 10.1371/journal.pone.0211187.
- Di Sotto A, et al. SPC liposomes as possible delivery systems for improving bioavailability of the natural sesquiterpene β-caryophyllene: lamellarity and drug-loading as key features for a rational drug delivery design. Pharmaceutics. 2018;10(4):274. doi: 10.3390/pharmaceutics10040274.
- El-Hammadi MM, Arias JL. An update on liposomes in drug delivery: a patent review (2014-2018). Expert Opin Ther Pat. 2019;29(11):891–907. doi: 10.1080/13543776.2019.1679767.
- Nogueira E, Gomes AC, Preto A, et al. Design of liposomal formulations for cell targeting. Colloids Surf B Biointerfaces. 2015;136:514–526. doi: 10.1016/j.colsurfb.2015.09.034.
- Modi S, Xiang TX, Anderson BD. Enhanced active liposomal loading of a poorly soluble ionizable drug using supersaturated drug solutions. J Control Release. 2012;162(2):330–339. doi: 10.1016/j.jconrel.2012.07.001.
- Nam JH, Kim SY, Seong H. Investigation on physicochemical characteristics of a nanoliposome-based system for dual drug delivery. Nanoscale Res Lett. 2018;13(1):101. doi: 10.1186/s11671-018-2519-0.
- Schnyder A, Huwyler J. Drug transport to brain with targeted liposomes. NeuroRx. 2005;2(1):99–107. doi: 10.1602/neurorx.2.1.99.
- Cheung BC, Sun TH, Leenhouts JM, et al. Loading of doxorubicin into liposomes by forming Mn2+-drug complexes. Biochim Biophys Acta. 1998;1414(1-2):205–216. doi: 10.1016/s0005-2736(98)00168-0.
- Jain A, Gulbake A, Jain A, et al. Dual drug delivery using ‘smart’ liposomes for triggered release of anticancer agents. J Nanopart Res. 2013;15(7):1–12. doi: 10.1007/s11051-013-1772-5.
- Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23(9):3319–3329. doi: 10.1080/10717544.2016.1177136.
- Johnston MJW, Edwards K, Karlsson G, et al. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J Liposome Res. 2008;18(2):145–157. doi: 10.1080/08982100802129372.
- Johnston MJW, Semple SC, Klimuk SK, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta. 2006;1758(1):55–64. doi: 10.1016/j.bbamem.2006.01.009.
- Mao W, Wu F, Lee RJ, et al. Development of a stable single-vial liposomal formulation for vincristine. Int J Nanomedicine. 2019;14:4461–4474. doi: 10.2147/IJN.S205276.
- Ibrahim S, Tagami T, Ozeki T. Effective-loading of platinum-chloroquine into PEGylated neutral and cationic liposomes as a drug delivery system for resistant malaria parasites. Biol Pharm Bull. 2017;40(6):815–823. doi: 10.1248/bpb.b16-00914.
- Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metabol Personal Therap. 2019;34(1):20180032. doi: 10.1515/dmpt-2018-0032.
- Yoon HY, Chang IH, Goo YT, et al. Intravesical delivery of rapamycin via folate-modified liposomes dispersed in thermo-reversible hydrogel. IJN. 2019;ume 14:6249–6268. doi: 10.2147/IJN.S216432.
- Emamzadeh M, Emamzadeh M, Pasparakis G. Dual controlled delivery of gemcitabine and cisplatin using polymer-modified thermosensitive liposomes for pancreatic cancer. ACS Appl Bio Mater. 2019;2(3):1298–1309. doi: 10.1021/acsabm.9b00007.
- Pal K, Madamsetty VS, Dutta SK, et al. Co-delivery of everolimus and vinorelbine via a tumor-targeted liposomal formulation inhibits tumor growth and metastasis in RCC. Int J Nanomedicine. 2019;14:5109–5123. doi: 10.2147/IJN.S204221.
- Jose G, Lu Y-J, Hung J-T, et al. Co-delivery of CPT-11 and 31anobinostat with anti-GD2 antibody conjugated immunoliposomes for targeted combination chemotherapy. Cancers. 2020;12(11):3211. doi: 10.3390/cancers12113211.
- Affram K, et al. In vitro and in vivo antitumor activity of gemcitabine loaded thermosensitive liposomal nanoparticles and mild hyperthermia in pancreatic cancer. Int J Adv Res. 2015;3(10):859–874.
- Nsairat H, Alshaer W, Odeh F, et al. Recent advances in using liposomes for delivery of nucleic acid-based therapeutics. OpenNano. 2023;11:100132. doi: 10.1016/j.onano.2023.100132.
- Xu Y, Meng H. Paclitaxel-loaded stealth liposomes: development, characterization, pharmacokinetics, and biodistribution. Artif Cells Nanomed Biotechnol. 2016;44(1):350–355. doi: 10.3109/21691401.2014.951722.
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037.
- Calvagno MG, et al. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters, and in vitro biological activity of gemcitabine-loaded liposomes. Current Drug Delivery. 2006;4(1):89–101.
- Chang HI, Yeh MK. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomedicine. 2012;7:49–60. doi: 10.2147/IJN.S26766.
- Zhao Y, Alakhova DY, Kim JO, et al. A simple way to enhance doxil® therapy: drug release from liposomes at the tumor site by amphiphilic block copolymer. J Control Release. 2013;168(1):61–69. doi: 10.1016/j.jconrel.2013.02.026.
- O’Byrne KJ, et al. A phase I dose-escalating study of daunoxome, liposomal daunorubicin, in metastatic breast cancer. Br J Cancer. 2002;87(1):15–20.
- Salehi B, et al. Liposomal cytarabine as cancer therapy: from chemistry to medicine. Biomolecules. 2019;9(12):773. doi: 10.3390/biom9120773.
- Batist G, Barton J, Chaikin P, et al. Myocet (liposome-encapsulated doxorubicin citrate): a new approach in breast cancer therapy. Expert Opin Pharmacother. 2002;3(12):1739–1751. doi: 10.1517/14656566.3.12.1739.
- Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4.
- Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002;49 Suppl 1(Suppl. S1):21–30. doi: 10.1093/jac/49.suppl_1.21.
- Attia MF, Anton N, Wallyn J, et al. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71(8):1185–1198. doi: 10.1111/jphp.13098.
- Kan P, Tsao C-W, Wang A-J, et al. A liposomal formulation able to incorporate a high content of paclitaxel and exert promising anticancer effect. J Drug Deliv. 2011;2011:629234. doi: 10.1155/2011/629234.
- Eleftheriou K, Kaminari A, Panagiotaki KN, et al. A combination drug delivery system employing thermosensitive liposomes for enhanced cell penetration and improved in vitro efficacy. Int J Pharm. 2020;574:118912. doi: 10.1016/j.ijpharm.2019.118912.
- Kulkarni JA, Cullis PR, van der Meel R, et al. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28(3):146–157. doi: 10.1089/nat.2018.0721.
- Roberts TC, Langer R, Wood MJA, et al. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19(10):673–694. doi: 10.1038/s41573-020-0075-7.
- Andra VVSNL, Pammi SVN, Bhatraju LVKP, et al. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials, and patents. Bionanoscience. 2022;12(1):274–291. doi: 10.1007/s12668-022-00941-x.
- Mochalova EN, Egorova EA, Komarova KS, et al. Comparative study of nanoparticle blood circulation after forced clearance of own erythrocytes (mononuclear phagocyte system-cytoblockade) or administration of cytotoxic doxorubicin- or clodronate-loaded liposomes. IJMS. 2023;24(13):10623. doi: 10.3390/ijms241310623.