?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Nanotechnology holds substantial promise in the innovative therapies for rheumatoid arthritis (RA). The current study was designed to synthesize and characterize a new graphene titanate nanocomposite (GTNc) and explore its anti-arthritic, anti-inflammatory, and antioxidant potencies against Complete Freund’s adjuvant (CFA)-induced arthritis in rats, as well as investigate the underlying molecular mechanisms. Our characterization methods included XRD, FT-IR, SEM, EDX, zeta potential, practical size, and XRF to characterize the novel GTNc. Our findings revealed that arthritic rats treated with GTNc exhibited lower levels of RF, CRP, IL-1β, TNF-α, IL-17, and ADAMTS-5, and higher levels of IL-4 and TIMP-3. In arthritic rats, GTNc reduced LPO levels while increasing GSH content and GST antioxidant activity. Additionally, GTNc decreased the expression of the TGF-β mRNA gene in arthritic rats. Histopathological investigation showed that GTNc reduced inflammatory cell infiltration, cartilage degradation, and bone destruction in joint injuries caused by CFA in the arthritic rats. Collectively, the anti-arthritic, anti-inflammatory, and antioxidant properties of GTNc appear promising for future arthritis treatments and bone disability research.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by persistent inflammation and can damage joint function [Citation1]. Although the specific cause of RA is still unknown, it is driven by various risk factors such as genetics, hormones, and environmental factors (e.g. infections, smoking) [Citation2]. Autoantibodies, specifically anti-citrullinated protein antibodies, confirm the autoimmune origin of RA [Citation3,Citation4]. RA affects about 0.5–1% of people worldwide, with a higher prevalence among women [Citation5]. The pathophysiology of RA is influenced by pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, that stimulate chronic inflammatory synovitis and cause joint tissue degeneration [Citation6]. Inhibiting these cytokines has positive effects on RA patients [Citation7]. Additionally, immune-mediated bone diseases that cause inflammation are related to inflammatory cytokines [Citation8]. Oxidative stress contributes to the pathophysiology of RA by causing membrane oxidation, protein and DNA damage, cartilage degradation, and bone resorption [Citation9]. Oxidative stress and inflammation positively regulate each other; thus, RA patients may benefit from therapies that enhance the body’s antioxidant defense system and reduce immunological inflammation [Citation10].
The unique physicochemical properties of graphene, carbon atoms arranged in a single layer, have garnered increasing attention in various fields, including chemistry, physics, and biomedicine. Graphene oxide, one of graphene’s most popular compounds, has applications in biomedicine, electronics, and photocatalysis [Citation11]. Both graphene oxide (Go) and reduced graphene oxide (rGO) offer a significant amount of untapped promise in the field of medicine, particularly in the areas of tissue engineering and molecular drug delivery [Citation12–14]. Graphene also has biological characteristics that make it suitable for bioassays, small-molecule drug delivery, and cancer treatment [Citation14,Citation15]. In the field of bone healing and organ regeneration, graphene-based materials have shown promise [Citation16,Citation17]. Graphene oxide has been demonstrated to improve composites’ antibacterial characteristics and promote cell proliferation and drug loading [Citation18]. Furthermore, graphene is an excellent platform for tissue engineering due to its biocompatibility and ease of functionalization [Citation14]. Titanium is a commonly used biocompatible metallic material in implants and bone replacements [Citation19]. Commercial titanium can be treated with a potent alkali to create a porous surface. When dopamine is added to the surface, it can form a covering of graphene oxide [Citation20]. Graphene titanate (GTN) is not only biocompatible but also can stimulate the expression of osteogenic genes and the mineralization of extracellular matrix [Citation21]. Graphene also has biological characteristics that make it suitable for bioassays, small-molecule drug delivery, and cancer treatment [Citation22]. In the fields of bone healing and organ regeneration, graphene-based materials have shown promise [Citation23]. Graphene oxide has been demonstrated to improve composites’ antibacterial characteristics and promote cell proliferation and drug loading [Citation24]. Therefore, the study was designed to synthesize and characterize a new GTNc and explore its anti-arthritic, anti-inflammatory, and antioxidant potencies in CFA-induced arthritic rats, with a focus on the underlying molecular mechanisms and the roles of TNF-α, ADAMTS-5, TIMP-3 and TGF-β.
Materials and methods
Chemicals
The Complete Freund’s adjuvant (CFA, Sigma Chemical Co., St. Louis, Mo., USA) was supplied by Sigma Chemical Company. The remainders of the compounds are of analytical quality.
Preparation of nano-sized graphene titanate nanocomposites (GTNc)
As in our previously reported approach [Citation25], additional purification was unnecessary since all the reagents and solvents were of analytical purity. Pure anatase phase TiO2 powder (10 g) was combined with NaOH aqueous solutions (500 ml, 10 N) for around 30 min to produce a milky white solution. In contrast, an ultrasonic probe (20 KHz, pulsed mode, 9.9 s [on] and 3 s [off]) was used to sonicate 1 g of graphite for 30 min after it had been dissolved in 100 ml of an aqueous solution of 40% ethanol. After mixing the titanate and exfoliated graphite solutions, another 30 min of sonication was conducted. After that, the combination was put into an autoclave. The autoclave was then heated to a temperature of 160 °C for a period of 24 h. The reaction was followed by air cooling of the autoclave chamber to room temperature. The precipitates that had formed were collected after being washed several times with distilled water and dried for two hours at a temperature of 80 °C. The resulting composite was subjected to a strong HCl treatment to investigate the nature of the interaction between graphene and titanate and make appropriate adjustments to the ratio of graphene to titanate as required.
Characterizations of GTNc
Fourier transformation infra-red (FTIR) spectroscopy
FTIR-FT Raman spectrometer (Bruker vertex 70, serial number 1341, Germany) was used to analyse the chemical bond vibrations of GTNc in the 400–4000 cm−1 range.
X-ray diffraction (XRD)
The X-ray powder diffraction method was used to determine the crystal structure of the samples (Cu Kα radiation (λ = 1.54 Å), a scanning range of 10-70, 30 mA as current, 40 kV (power 1200 W) as voltage, and a scanning speed of 2/min). The crystallite size was calculated using Scherrer’s equation,
(1)
(1)
where D stands for the average crystallite size, λ presented as the wavelength of the incident light, β displays the diffraction peak full width at half maximum, and the angle of Bragg’s diffraction belongs to θ.
Scanning electron microscopy (SEM)
SEM analysis was employed to characterize the morphology and microstructure of all samples (SEM, Quanta FEG 250).
Zetasizer and zeta potential studies
The colloidal stability was evaluated by assessing the zeta potential of the disseminated GTNc. Additionally, the Zetasizer (hydrodynamic size) was measured with the ZS90 Zetasizer instrument with the use of a dynamic light scattering (DLS) approach or photon correlation spectroscopy (Malvern, UK). Zeta potential changes were measured using the 1.330 refractive indexes of the water dispersant.
Experimental animals
Thirty male Wistar rats weighing between 120 and 150 g were donated by the Egyptian Organization for Biological Products and Vaccines (VACSERA) in Helwan, Cairo, Egypt. The animals had two weeks of intensive supervision before the studies to ensure that any infections were treated. Rats were kept in climate-controlled circumstances in highly precise polypropylene cages with a relative humidity of about 55.5%, a daily illumination schedule (10–12 h per day), average room temperatures (20–25 °C), and free access to food and drink. The experiments followed animal care guidelines and the recommendations of the Experimental Animal Ethics Committee of the Faculty of Science, Beni-Suef University, Egypt (Ethical Approval number: 022-387).
Arthritis induction and animal grouping
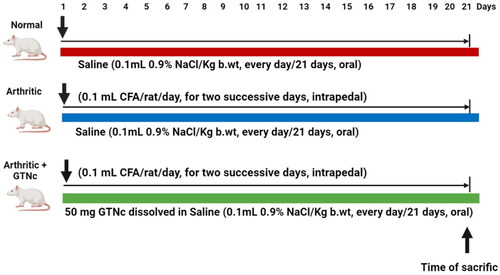
To cause arthritis, the right hind paw footpad of the animal received daily intrapedal injections of 0.1 ml of CFA solution for two days in a row [Citation26]. After achieving RA with CFA, 30 Wistar rats were randomly assigned to one of three groups, with ten rats each ().
Group 1: (Normal control rats), for 21 days, this group received an oral dose of the same amount of vehicle (saline).
Group 2: (Arthritic control group) In this group, saline was administered orally daily for 21 days to treat CFA-induced arthritis in the same manner as the control group.
Group 3: (Arthritic + GTNc) GTNc was administered orally to patients with CFA-induced arthritis at a portion of 50 mg/kg b.wt day [Citation27].
Paw oedema assessment
By evaluating the paw oedema rate and swelling, the paw volume is assessed for each group to monitor the development of arthritis. Following the arthritis induction, measures were taken on Days 3, 7, and 21 after the CFA injection started on Day 0. An electronic calliper was used to determine the size of the hind paw.
Sampling of blood and tissue
Diethyl ether was used to mildly anaesthetize the animals for 21 days before they were slaughtered. Sterile samples of serum were taken, placed in sterile containers, and stored at a temperature of Sera were collected in sterile containers and kept at −20 °C for testing the levels of CRP, RF, 1 L-1β, 1 L-4, 1 L-17, TNF-α, TIMP-3 and ADAMTS-5 in blood samples centrifugation at 3000 rpm for 15 min after clotting. Rats from each group were decapitated by head dislocation, and the right leg’s posterior ankle joints were removed. One tissue sample was mixed with 25% w/v phosphate buffer, followed by 15 min of centrifugation at 4 °C (3000 rpm). Before use, the supernatants were kept at −30 °C to investigate antioxidant activities and oxidative stress. The second tissue piece was stored at 70 °C for RNA extraction and (RT-PCR) tests. One day after being fixed in 10% NBF, a third tissue was sectioned and stained with haematoxylin and eosin (H&E) for histological examination.
Biochemical investigations
The serum ratio of CRP, RF, 1 L-1β, 1 L-4, 1 L-17, TNF-α, TIMP-3, and ADAMTS-5 was obtained using an ELISA kit (R&D Systems, Minneapolis, MN, USA) by the manufacturer’s instructions.
Total RNA isolation of and the inverse transcription–quantitative PCR (RT-qPCR)
Utilizing TRIzol reagent (Gibco, Invitrogen; Eugene, OR; USA) according to the manufacturer’s instructions, total RNA was derived from lung tissue homogenates. At 260 nm, RNA absorbance was determined. Using the method from Sthoeger et al. [Citation28]. The quality of the RNA was evaluated by calculating the ratio of the absorbances measured at 260 and 280 nm to one another (referred to as the 260/280 ratio). With the help of Applied Biosystems®' high-capacity cDNA reverse transcription kit, Foster City, CA, USA, the first strand of cDNA was created. The ABI Prism 7500 System and 96-well optical reaction plates were utilized for quantitative PCR studies of the target genes’ mRNA expression. In brief, the cDNA was amplified by PCR (Applied Biosystems) using a 25 μl reaction mixture including 0.1 μl of 10 μM forward primer and 0.1 μl of 10 μM reverse primer (final concentration of each primer was 40 nM.), 11.05 μ1 of nuclease-free water, 12.5 μ1 of SYBR Green Universal Master mix, and 1.25 μ1 of cDNA sample. displays the primers used for these assays. Comparison between TGF- β expression and the expression of rat GAPDH was made using the 2 − ΔΔCT method to find the appropriate level of normalization.
Table 1. Primers used for TGF-β gene expression.
Oxidative stress and antioxidant state estimation
Employing kits from Bio-diagnostic, LPO was identified as malondialdehyde (MDA) level, decreased glutathione (GSH), and glutathione-S-transferase (GST) (Dokki, Giza, Egypt).
Histopathological examination
Rats were euthanized on day 21 following arthritis induction for the study’s conclusion. Four rats per group had their right ankle joints removed and stored in 10% NBF for 48 h. Bone decalcification was completed at 10% formic acid. This treatment was administered for a period of two weeks. During that time, it was changed twice per week, and the decalcification process was physically evaluated with the assistance of a surgical blade. After being decalcified, the specimens were washed in PBS and then dehydrated using a graded ethanol series before incorporating in paraffin wax. Then, 5-micron-thick sagittal slices were produced and covered with H&E.
A pathologist was conducting histological examinations, including bone damage, cartilage damage, and synovial inflammation. Sancho et al. [Citation29] proposed a system for grading sections for bone erosion, cartilage injury, synovial hypertrophy (pannus development), and inflammation (mononuclear cell infiltration). On a scale ranging from 0 to 3, where 0 represents normal, 1 represents mild inflammation, 2 represents moderate inflammation, 3 represents severe inflammation, and 12 represents the maximum potential score, each parameter was given a grade.
Results
Characterization of the GTNc
FTIR studies
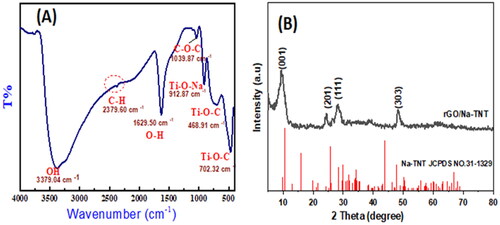
FTIR analysis was conducted to indicate the molecular structure and functional groups of GTNc. displays the FT-IR spectrum of GTNc. The bands obtained at 3379.04 and 1629.50 cm−1 are ascribed to O-H group stretching and bending vibrations in water molecules adsorbed on the surface of titanate nanowires [Citation30,Citation31]. The stretching bands characteristic for Ti-O and Ti-O-C are determined at 468.91 cm−1 and 702.32. These peaks prove that covalent bonds can be formed between titanate and graphene networks and Ti–O bonds in TiO6 octahedra for Na-TNT [Citation32]. The located peak at 1039.87 cm−1 is associated with the C–O–C vibration, which ensures the functionalization of graphene nanosheets with oxygen groups [Citation33]. The peak at 912.87 is attributed to the bond between alkali metal (Na+) and octahedral titanium in the titanate structure.
Qualitative analysis of XRD
XRD analysis is applied to define the crystallinity of the as-prepared GTNc. The XRD diffraction patterns of the as-prepared GTNc sample are presented in . As shown in , most of the diffraction peaks of the GTNc correspond to the Na-TNT (Na2Ti3O7) phase structure (JCPDS no. 31-1329). The same results were reported in the literature [Citation34]. The results reveal that the nanocomposite has an excellent crystal structure and diffraction peaks at 10.5, 24.3, 28.3, 49.9 by the (001), (201), (111), and (300) planes, respectively, which can match well with the typical Na-TNT pattern. As we can see, the XRD pattern of GTNc agrees well with that of the monoclinic Na2Ti3O7(Na-TNT) (JCPDS no. 31-1329) [Citation35]. The characteristic broad peak for pure graphene nanosheets should be noted at 24°, and its weak appearance suggests that Na-TNT is well dispersed.
Scanning electron microscopy (SEM)
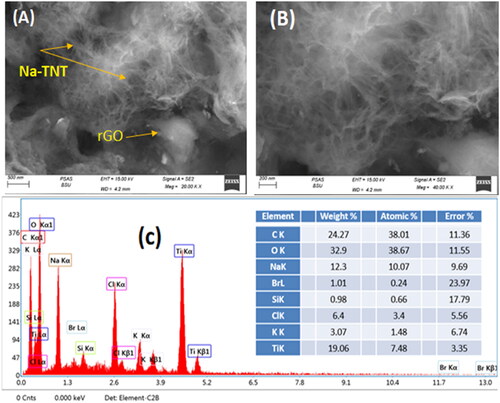
GTNc surface morphology and particle size were displayed by SEM measurements. presents SEM images of the GTNc at different magnifications. It has been revealed that a highly cross-connected Na-TNT nanowire with a high aspect ratio interweaves and is embedded randomly into the graphene nanosheets forming a 3D porous spider web-like network, which is very much like the solvothermal generated Na2Ti3O7 nanowires reported in the literature [Citation36]. The diameter of the Na-TNT nanowires was 70–100 nm, and its length could reach higher than 1 µ. With higher magnifications, more nanowires can be highly observed on the graphene surface (). EDX result () proved the existence of target elements C, O, Ti, and Na species with a ratio 1: 1: 0.5: 0.36 with fewer amounts of C, Br, Si, Cl, and K.
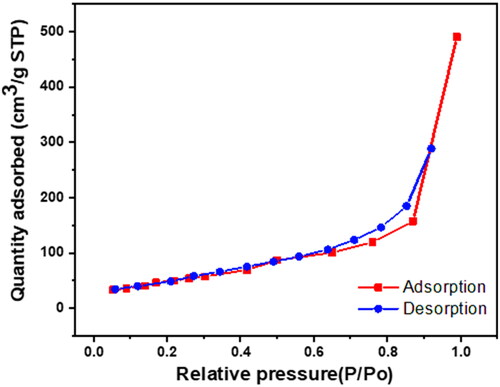
To evaluate the GTNc’s textural parameters (pore size distribution, pore volume, and specific surface area) nitrogen adsorption/desorption measurements were conducted (). It is clear that N2 sorption isotherms of the GTNc present the isotherm pattern IV type with a hysteresis loop observed for mesoporous structures [Citation37]. By IUPAC classifications, the GTNc hysteresis loop classification is H1 and H3 types combination. As H1 type can be observed at relative pressure P/P0 > = 0.6 which indicates that desorption isotherm doesn’t show the exact behaviour of adsorption [Citation30]. H1 type is characteristic of mesoporous structures with a uniform narrow cylindrical-like pore. This interpretation confirms the Na-TNT nanowire structure. On the other hand, the H3 type refers to slit-shaped pores in a wide range with nonuniformed size; such pores arise from solids with non-rigid agglomerates or plate-like particles aggregates. The textural properties are observed in . It was determined that the surface area and pore volume were raised from 154.85 m2/g and 0.21732 cm3/g (Na-TNT) to 187.2465 m2/g and 0.709799 cm3/g (GTNc), respectively. It identifies the porous nature of rGO and is consistent with previous findings.
Table 2. Textural parameters of GTNc.
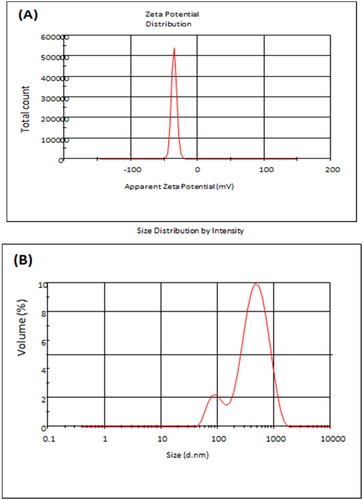
Zetasizer and zeta potential analysis
Both the zeta potential and particle size of GTNc were analysed, and the results are presented in . Zeta potential analysis can determine the surface charges of nanoparticles and the repulsion degree between the charged particles in the colloid system. As the nanoparticles were dispersed in deionized water at 25 C°, one peak appeared and had an average zeta potential of about −35.7 ± 4.8 mV for all the analysed nanoparticles. As is seen from that the sample was negatively charged because of the negatively charged carboxyl groups coming from the combination of graphene oxide functional groups. The particle size of the GTNc presents a narrow particle size distribution with an average diameter of 312.9 nm (PDI = 0.38).
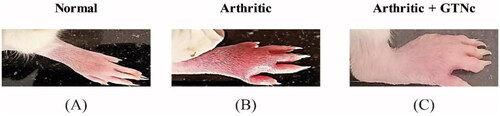
The effect of GTNc on paw and ankle joint gross lesions
It was observed that the arthritic of the ankles and right hind paws induced by CFA displayed redness, swelling, and oedema, all of which are possible outward manifestations of the disease. The arthritic groups that received GTNc obtained a significant reduction in these visible lesions ().
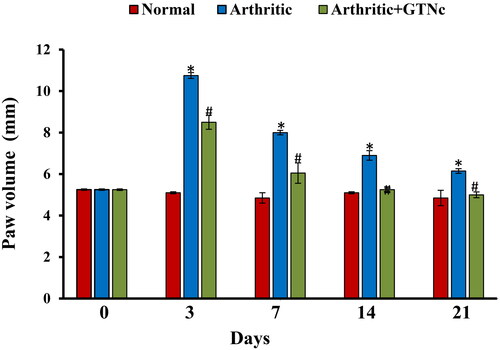
The effect of GTNc on the size of right hind paws
The rats with CFA-induced arthritic demonstrated a considerably enlarged paw size (oedema), which was comparable to the group that served as the control. In contrast, the arthritic rats treated with GTNc had smaller paws than the arthritic group (). The size of the arthritic was measured using a digital calliper to compare the swelling rates of different paw groups.
Figure 7. Effect of GTNc on the size of the right hind paws of rats with CFA-induced arthritis. Results are presented as mean values ± SE. *p < 0.05: significant difference compared to the control group, #p < 0.05: significant compared to GTNc-treated arthritic rats.
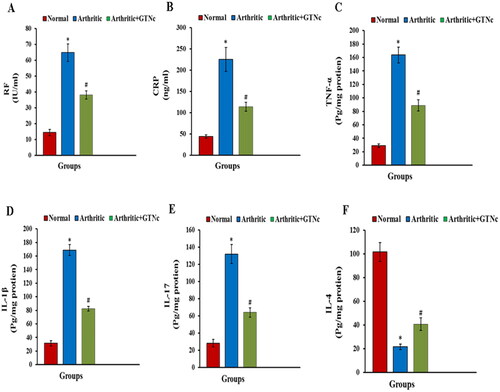
The influence of GTNc on serum levels of RF, TNF-α, CRP, IL-1β, IL-17 and IL-4
CFA induced in rats raised (P ˂ 0.05) RF, CRP, TNF-α, IL-1β, and IL-17 serum levels, while decreasing serum levels of IL-4 (P ˂ 0.05) (). These responses as well were significantly retreated by GTNc when compared to the arthritis rats.
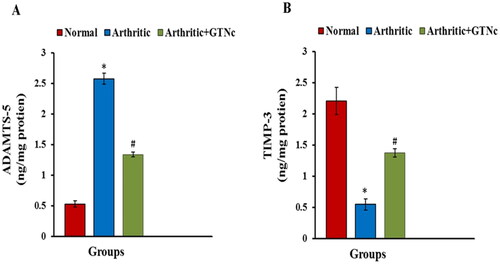
The influence of GTNc on serum ADAMTS-5 and TIMP3 levels
Animals given CFA showed a higher level of ADAMTS-5 and decreased TIMP3 level. GTNc treatment caused decreased ADAMTS-5 as well as increased TIMP3 levels when compared with CFA-induced arthritic rats ().
Figure 9. Influence of GTNc on serum levels (A) ADAMTS-5 and (B) TIMP-3. Results are presented as mean values ± SE. *p < 0.05: significant difference compared to the control group, #p < 0.05: significant compared to GTNc-treated arthritic rats.
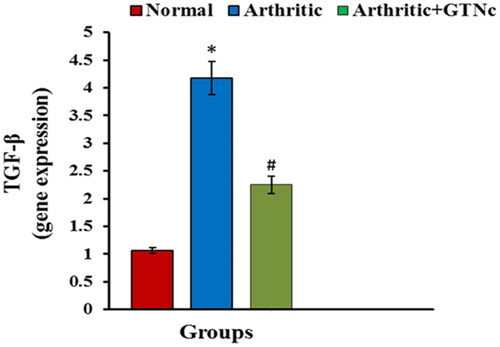
The influence of GTNc on TGF-β mRNA expression
Arthritic rats Animals given CFA showed upregulated expression of TGF-β; these responses as well were significantly reversed by GTNc ().
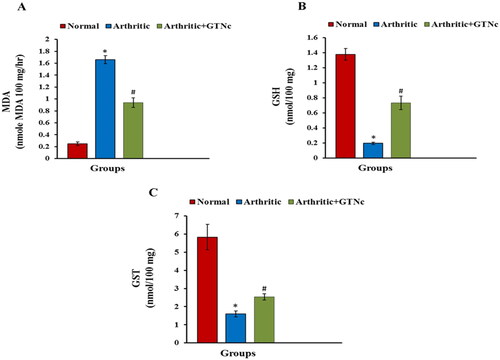
The influence of GTNc on oxidative stress and the antioxidant status
CFA management elevation MDA and reduced GSH levels and GST activity compared to normal rats. GTNc supplementation decreases MDA while increasing GSH amount and GST activity compared to arthritic rats ().
Histopathological alterations and arthritic score
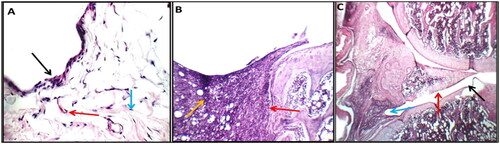
The right ankle joint sections of the hind leg of healthy control rats showed no signs of inflammation when stained with haematoxylin and eosin (H&E). In contrast, the synovium of the arthritic rats showed hyperplasia combined with significant infiltration of inflammatory cells, substantial cartilage degradation, and pannus development. However, rats given the GTNc therapy both developed mild forms of arthritis without any inflammation ().
Figure 12. In control negative normal rats (a), higher power view showing average synovial lining with a single layer of flat synovial cells (black arrow) and intermediate sub-synovial connective tissue (blue arrow) and average blood vessels (red arrow) (H&E X 400). in control positive arthritic rats (B), high power view showing destructing pannus on and on menisci (red arrow) and a yellow arrow pointing to a sub-synovial inflammatory infiltrate (H&E X 200). in GTNc (C), showing small non-destructing pannus on articular cartilage (black arrow) and intact synovial lining (red arrow) with mild sub-synovial inflammatory infiltrate (blue arrow) (H&E X 200).
Discussion
Arthritis in vivo model was created to research GTNc’s ability to treat arthritis caused by CFA. RF is a significant biomarker linked to immunological disorders, including rheumatoid arthritis, and has been demonstrated to have a part in the etiopathogenesis of arthritis [Citation38]. Another critical indicator of inflammation in rheumatoid arthritis is C-reactive protein (CRP). In the current study, we found an increase in the serum level of both RF and CRP in arthritic rats. This result parallels Sun et al. [Citation10] and Liu and Su [Citation39]. RF and CRP were regarded to be two significant biomarkers of systemic inflammation in RA, indicating a positive inflammatory reaction. They were utilized to evaluate joint activity in rats with RA [Citation40]. RF is one of the numerous antibody systems found to be effective against RA based on the antigens these antibodies bind to [Citation41]. Serum CRP was shown to be closely associated with RA patients [Citation42]. Several researchers have documented that the discharge of inflammatory mediators triggers an immune response in arthritis [Citation43]. GTNc treatment lowered both RF and CRP serum levels, suggesting that GTNc has an anti-inflammatory effect. In the context of immunotherapy, graphene may be regarded as an immunostimulant [Citation44].
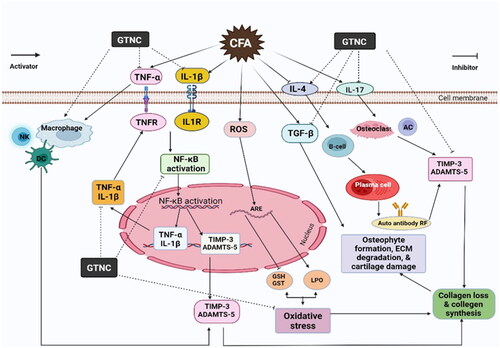
According to all of our previous research, the effects of CFA on rats demonstrate an increase in the serum levels of proinflammatory as TNF-α, IL-1β, and IL-17, and decreased levels of anti-inflammatory cytokines (IL-4). The findings of our study are in agreement with those of Weng et al. [Citation45], and Akhter et al. [Citation46]. ROS accumulates and is released as a result of proinflammatory cytokine overexpression. Oxidative stress disrupts DNA, proteins, and lipids when ROS generation surpasses the body’s natural antioxidant defense system [Citation47]. In the initiation and progression of RA, macrophages and activated T cells release proinflammatory cytokines [Citation48]. Response of T cell-mediated immunity accelerates the formation of antibodies and stimulates the release of pro-inflammatory cytokines responsible for causing damage to the joints. These cytokines are overexpressed, which causes tissue eradication, bone loss, irreversible tissue proliferation, and automated cell death [Citation49]. In inflamed RA joints, polymorphonucleate produces proinflammatory cytokines, TNF-α and IL-1β, and free radicals, which cause damage to bones and cartilage [Citation50]. In the animal model induced by CFA, different cytokines, including TNF-α and IL-1β, are produced by activated macrophages in the chronic inflammation associated with RA [Citation51]. Other inflammatory cells enter the afflicted inflammatory areas, generating pro-inflammatory cytokines; TNF-α, IL-1β, and IL-17 [Citation51]. TNF-α and IL-1β could amplify the inflammatory responses in RA [Citation52]. IL-4 is crucial in controlling the levels of endogenous pro-inflammatory cytokines in RA [Citation53,Citation54]. However, rats given GTNc demonstrated a noticeable decrease in levels of TNF-α, IL-1β, and IL-17 and higher levels of IL-4, confirming that GTNs have an anti-inflammatory effect. We have found that graphene oxide can serve as an antioxidant and reduce inflammation and inflammatory polarisation in macrophages by lowering the amount of intracellular reactive oxygen species (ROS) [Citation55]. Graphene oxide further supports their potential use as attractive biomaterials for bone tissue engineering by creating a favourable immunomodulatory bone environment [Citation56]. Researchers have demonstrated that graphene oxide affects the progression of cytokines such as IL-1 and TNF-α [Citation57]. GTNc diminished inflammatory responses by reduced of proinflammatory cytokines [Citation21].
Matrix metalloproteinases (MMPs), including collagenase matrix metalloproteinase 13 (MMP-13), are involved in type-II collagen degradation [Citation58,Citation59], and ADAMTS-5 is controlled in aggrecan degradation [Citation60,Citation61]. TIMP-3 inhibits aggrecanases, indicating that it works as an endogenous inhibitor of these enzymes [Citation62,Citation63]. As a result, the mechanism by which TIMP-3 protein levels are modulated in individuals with arthritic illnesses merits additional exploration. TIMP-3 may be able to prevent cartilage loss [Citation62,Citation64]. A relationship exists between decreasing TIMP-3 expression and ECM loss, and maintaining TIMP-3 expression improves cartilage deterioration caused by osteoarthritis [Citation65]. CFA-induced animals showed elevated levels of ADAMTS-5 and decreased TIMP3 levels. These results are the same as Szeremeta et al. [Citation66], who hypothesize that the quantitative mismatch between ADAMTS-5 and TIMP-3 leads to fast cartilage matrix degradation in RA. In our study, the GTNc treatment significantly decreased ADAMTS-5, and increased TIMP-3 levels were anti-inflammatory indicators in RA rats’ joints.
TGF-β, as a cytokine, plays multiple roles in haematopoiesis, angiogenesis, differentiation, cell proliferation, migration, and death. Studies in models of arthritis have produced significantly different findings, in contrast to other illnesses where the role of TGF-β is characterized [Citation67]. The rising expression of TGF-β in RA is consistent with the results of Raafat et al. [Citation68]. Many investigations have verified the presence of TGF-β in the synovial fluids and tissues of RA patients; assumptions have been made that TGF-β is involved in the pathophysiology of RA [Citation69]. The expression of TGF-β was decreased after treatment with GTNc. GTNc may reduce inflammation by inhibiting the production of the pro-inflammatory cytokine TGF-β.
RA has been attributed to oxidative stress, in which ROS levels increase over time because of increased production or decreased antioxidant defences [Citation70]. Overproduction of ROS at the diseased site stimulates cytokine production, which can interact with ROS, accelerating development and causing severe bone and cartilage degradation [Citation71,Citation72]. One of the factors that contributed to oxidative stress was RA. In the inflammatory and immunological cellular response to RA, free radicals act as secondary messengers, suggesting that they may contribute to joint damage indirectly. T-cells exposed to higher levels of oxidative stress become resistant to various stimuli, including those that promote growth and death, which could prolong the abnormal immune response [Citation73]. Our results agree with those of Ahmed et al. [Citation26] and Singhai and Patil [Citation74]. LPO has been related to the aetiology of inflammatory arthritis [Citation75]. In RA conditions, the ROS are formed by phagocytic cells due to inflammatory events, which results in a reduction in cellular antioxidants and elevated LPO, and increased susceptibility of synovial fluid and collagen to oxygen radical-mediated injury [Citation76,Citation77]. Our findings revealed that the treatment of arthritic rats with GTNc resulted in a significant improvement in antioxidant status, with increased GSH content and GST activity, and a decrease in MDA levels. These data were consistent with the results of Zhang et al. [Citation78], who state that graphene oxide enhances antioxidant enzyme activities. Graphene oxide is assumed to have an antioxidant effect because of its capacity to scavenge free radicals [Citation79].
Conclusion
The novel synthesized GTNc has demonstrated anti-inflammatory, anti-arthritic, and antioxidant effects in CFA-induced arthritis. The study found that GTNc significantly decreased inflammatory markers (RF, CRP, IL-1β, TNF-α, IL-17, TGF-β and ADAMTS-5), elevated anti-inflammatory markers (TIMP-3 and IL-4), reduced oxidative stress (LPO), and improved the antioxidant defence system (GSH content and GST activity) . Additionally, GTNc improved the histopathological implications of arthritic joints in rats. These findings suggest that GTNc may be a promising therapeutic option for RA treatment. However, further studies are required to assess its safety and efficacy before it can be used in humans.
Acknowledgment
The researchers would like to acknowledge Deanship of scientific research, Taif University for funding this work.
Author contributions
The final version of the published work has been seen by all authors, and they have all given their approval.
Institutional review board Statement
The experiments followed animal care guidelines and the recommendations of the Experimental Animal Ethics Committee of the Faculty of Science, Beni-Suef University, Egypt (Ethical Approval number: 022-387).
Informed consent statement
Not applicable.
Supplemental Material
Download MS Word (5 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability Statement
All data can be found in the article.
Additional information
Funding
References
- Conforti A, Di Cola I, Pavlych V, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. 2021;20(2):102735. doi: 10.1016/j.autrev.2020.102735.
- De Molon RS, Rossa C, Thurlings RM, et al. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. IJMS. 2019;20(18):4541–4635. doi: 10.3390/ijms20184541.
- Brink M, Hansson M, Mathsson L, et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013;65(4):899–910. doi: 10.1002/art.37835.
- Van de Sande MG, De Hair MJ, Van Der Leij C, et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann Rheum Dis. 2011;70(5):772–777. doi: 10.1136/ard.2010.139527.
- Asoudeh F, Djafarian K, Akhalghi M, et al. The effect of probiotic cheese consumption on inflammatory and anti-inflammatory markers, disease severity, and symptoms in patients with rheumatoid arthritis: study protocol for a randomized, double-blind, placebo-controlled trial. Trials. 2022;23(1):180. doi: 10.1186/s13063-022-06113-2.
- Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immun. 2017;46(2):183–196. doi: 10.1016/j.immuni.2017.02.006.
- Sweeney SE, Firestein GS. Rheumatoid arthritis: regulation of synovial inflammation. Int J Biochem Cell Biol. 2004;36(3):372–378. doi: 10.1016/s1357-2725(03)00259-0.
- Zuo Y, Deng GM. Fc gamma receptors as regulators of bone destruction in inflammatory arthritis. Front Immunol. 2021;12:688201. doi: 10.3389/fimmu.2021.688201.
- Ponist S, Zloh M, Bauerova K. Impact of oxidative stress on inflammation in rheumatoid and adjuvant arthritis: damage to lipids, proteins, and enzymatic antioxidant defense in plasma and different tissues. Animal Models Med Bio. 2019.
- Sun Y, Liu J, Xin L, et al. Xinfeng capsule inhibits inflammation and oxidative stress in rheumatoid arthritis by up-regulating LINC00638 and activating Nrf2/HO-1 pathway. J Ethnopharmacol. 2023;301:115839. doi: 10.1016/j.jep.2022.115839.
- Behbudi G. Mini review of graphene oxide for medical detection and applications. Adv App Nano Bio-Tech. 2020;1(3):63–66.
- Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88. doi: 10.1016/j.jconrel.2013.10.017.
- Wang XD, Wan XC, Liu AF, et al. Effects of umbilical cord mesenchymal stem cells loaded with graphene oxide granular lubrication on cytokine levels in animal models of knee osteoarthritis. Int Orthop. 2021;45(2):381–390. doi: 10.1007/s00264-020-04584-z.
- Kulshrestha S, Khan S, Meena R, et al. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofou. 2014;30(10):1281–1294. doi: 10.1080/08927014.2014.983093.
- Wang Y, Li Z, Wang J, et al. Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011;29(5):205–212. doi: 10.1016/j.tibtech.2011.01.008.
- Priyadarsini S, Mohanty S, Mukherjee S, et al. Graphene and graphene oxide as nanomaterials for medicine and biology application. J Nanostruct Chem. 2018;8(2):123–137. doi: 10.1007/s40097-018-0265-6.
- Crowder SW, Prasai D, Rath R, et al. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2013;5(10):4171–4176. doi: 10.1039/c3nr00803g.
- Grant JJ, Pillai SC, Hehir S, et al. Biomedical applications of electrospun graphene oxide. ACS Biomater Sci Eng. 2021;7(4):1278–1301. doi: 10.1021/acsbiomaterials.0c01663.
- Jung HS, Choi YJ, Jeong J, et al. Nanoscale graphene coating on commercially pure titanium for accelerated bone regeneration. RSC Adv. 2016;6(32):26719–26724. doi: 10.1039/C6RA03905G.
- Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci USA. 2006;103(35):12999–13003. doi: 10.1073/pnas.0605552103.
- Su J, Du Z, Xiao L, et al. Graphene oxide coated titanium surfaces with osteoimmunomodulatory role to enhance osteogenesis. Mater Sci Eng C Mater Biol Appl. 2020;113:110983. doi: 10.1016/j.msec.2020.110983.
- Makuch S, Więcek K, Woźniak M. The immunomodulatory and anti-inflammatory effect of curcumin on immune cell populations, cytokines, and in vivo models of rheumatoid arthritis. Pharmaceuticals. 2021;14(4):309. doi: 10.3390/ph14040309.
- Veronese N, Cooper C, Bruyère O, et al. Multimodal multidisciplinary management of patients with moderate to severe pain in knee osteoarthritis: a need to meet patient expectations. Drugs. 2022;82(13):1347–1355. doi: 10.1007/s40265-022-01773-5.
- Rahman MM, Islam F, Afsana Mim S, et al. Multifunctional therapeutic approach of nanomedicines against inflammation in cancer and aging. J Nanomater. 2022;2022:1–19. doi: 10.1155/2022/4217529.
- Farghali AA, Zaki AH, Khedr MH. Control of selectivity in heterogeneous photocatalysis by tuning TiO2 morphology for water treatment applications. Nanomater Nanotechnol. 2016;6:12. doi: 10.5772/62296.
- Ahmed RH, Galaly SR, Moustafa N, et al. Curcumin and mesenchymal stem cells ameliorate ankle, testis, and ovary deleterious histological changes in arthritic rats via suppression of oxidative stress and inflammation. Stem Cells Int. 2021;2021:3516834–3516820. doi: 10.1155/2021/3516834.
- Wang Y, Chen Z, Ba T, et al. Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small. 2013;9(9-10):1742–1752. doi: 10.1002/smll.201201185.
- Sthoeger Z, Zinger H, Sharabi A, et al. The tolerogenic peptide, hCDR1, down-Regulates the expression of interferon-α in murine and human systemic lupus erythematosus. PLoS One. 2013;8(3):e60394. doi: 10.1371/journal.pone.0060394.
- Sancho D, Gómez M, Viedma F, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-β production in collagen-induced arthritis. J Clin Invest. 2003;112(6):872–882. doi: 10.1172/JCI200319112.
- Lin KS, Cheng HW, Chen WR, et al. Synthesis, characterization, and adsorption kinetics of titania nanotubes for basic dye wastewater treatment. In Proceedings of the Adsorption. 2010;16(1-2):47–56. doi: 10.1007/s10450-010-9216-3.
- Dong W, Hou L, Li T, et al. A dual role of graphene oxide sheet deposition on titanate nanowire scaffolds for osteo-implantation: mechanical hardener and surface activity regulator. Sci Rep. 2015;5(1):18266. doi: 10.1038/srep18266.
- Pan H, Lu X, Yu X, et al. Sodium storage and transport properties in layered Na2Ti 3O7 for room-temperature sodium-ion batteries. Adv Energy Mater. 2013;3(9):1186–1194. doi: 10.1002/aenm.201300139.
- Sang B, Li Z, Li X, et al. Titanate nanotubes decorated graphene oxide nanocomposites: preparation, flame retardancy, and photodegradation. Nanoscale Res Lett. 2017;12(1):441. doi: 10.1186/s11671-017-2211-9.
- Tran NQ, Le TA, Lee H. An ultralight and flexible sodium titanate nanowire aerogel with superior sodium storage. J. Mater. Chem. A. 2018;6(36):17495–17502. doi: 10.1039/C8TA06988C.
- Yan Z, Liu L, Shu H, et al. A tightly integrated sodium titanate-carbon composite as an anode material for rechargeable sodium ion batteries. J Power Source. 2015;274:8–14. doi: 10.1016/j.jpowsour.2014.10.045.
- Zhou Z, Xiao H, Zhang F, et al. Solvothermal synthesis of Na2Ti3O7 nanowires embedded in 3D graphene networks as an anode for high-performance sodium-ion batteries. Electrochim Acta. 2016;211:430–436. doi: 10.1016/j.electacta.2016.06.036.
- Natarajan TS, Bajaj HC, Tayade RJ. Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration. J Colloid Interface Sci. 2014;433:104–114. doi: 10.1016/j.jcis.2014.07.019.
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers. 2013;35(6):727–734. doi: 10.1155/2013/726598.
- Liu T, Su B. Styphnolobium japonicum (L.) schott flower extract alleviates oxidative stress and inflammatory factors in the adjuvant-induced arthritis rat model. J Pain Res. 2021;14:2907–2919. doi: 10.2147/JPR.S325988.
- Arjumand S, Shahzad M, Shabbir A, et al. Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α, IL-1, and NFκB expression levels. Biomed Pharmacother. 2019;111:958–963. doi: 10.1016/j.biopha.2019.01.006.
- van Delft MA, Huizinga TW. An overview of autoantibodies in rheumatoid arthritis. J Autoimmun. 2020;110:102392. doi: 10.1016/j.jaut.2019.102392.
- Pope JE, Choy EH. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51(1):219–229. doi: 10.1016/j.semarthrit.2020.11.005.
- Zhang L, Zhu M, Li M, et al. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget. 2017;8(33):55384–55393. doi: 10.18632/oncotarget.19526.
- Liu Y, Luo M, Xiang X, et al. A graphene oxide and exonuclease-aided amplification immuno-sensor for antigen detection. Chem Commun (Camb). 2014;50(20):2679–2681. doi: 10.1039/c4cc00128a.
- Weng W, Wang F, He X, et al. Protective effect of corynoline on the CFA induced rheumatoid arthritis via attenuation of oxidative and inflammatory mediators. Mol Cell Biochem. 2021;476(2):831–839. doi: 10.1007/s11010-020-03948-8.
- Akhter S, Irfan HM, Jahan S, et al. Nerolidol: a potential approach in rheumatoid arthritis through reduction of TNF-α, IL-1β, IL-6, NF-kB, COX-2 and antioxidant effect in CFA-induced arthritic model. Inflammopharm. 2022; 30 (2): 537–548. doi: 10.1007/s10787-022-00930-2.
- Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, et al. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: a systematic review. Nut. 2019;11(11):2786–3115. doi: 10.3390/nu11112786.
- Vandooren B, Noordenbos T, Ambarus C, et al. Absence of a classically activated macrophage cytokine signature in peripheral spondylarthritis, including psoriatic arthritis. Arthritis Rheum. 2009;60(4):966–975. doi: 10.1002/art.24406.
- Zou CJ, Zhu LJ, Li YH, et al. The association between hepatitis B virus infection and disease activity, synovitis, or joint destruction in rheumatoid arthritis. Clin Rheumatol. 2013;32(6):787–795. doi: 10.1007/s10067-013-2170-1.
- Alivernini S, Firestein GS, McInnes IB. The pathogenesis of rheumatoid arthritis. Immunity. 2022;55(12):2255–2270. doi: 10.1016/j.immuni.2022.11.009.
- Silpavathi L, Das MK, Das D. Anti-arthritic potentials of aqueous and methanolic leaf extracts of ardisia solanacea on complete Freund’s adjuvant induced rheumatoid arthritis in rats. Adva Trad Med. 2021;1–9.
- Fouser LA, Wright JF, Dunussi-Joannopoulos K, et al. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226(1):87–102. doi: 10.1111/j.1600-065X.2008.00712.x.
- Jing R, Ban Y, Xu W, et al. Therapeutic effects of the total lignans from vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine. 2019;58:152825. doi: 10.1016/j.phymed.2019.152825.
- Saleem A, Saleem M, Akhtar MF, et al. Moringa rivae leaf extracts attenuate complete freund’s adjuvant-induced arthritis in wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharma. 2020;28(1):139–151. doi: 10.1007/s10787-019-00596-3.
- Han J, Kim YS, Lim MY, et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano. 2018;12(2):1959–1977. doi: 10.1021/acsnano.7b09107.
- Xue D, Chen E, Zhong H, et al. ImmunAQomodulatory properties of graphene oxide for osteogenesis and angiogenesis. Int J Nanomedicine. 2018;13:5799–5810. doi: 10.2147/IJN.S170305.
- Jung JH, Cheon DS, Liu F, et al. A graphene oxide based immuno‐biosensor for pathogen detection. Angew Chem Int Ed Engl. 2010;49(33):5708–5711. doi: 10.1002/anie.201001428.
- Onitsuka K, Murata K, Kokubun T, et al. Effects of controlling abnormal joint movement on expression of MMP13 and TIMP-1 in osteoarthritis. Cartilage. 2020;11(1):98–107. doi: 10.1177/1947603518783449.
- Wang M, Sampson ER, Jin H, et al. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res Ther. 2013;15(1):R5. doi: 10.1186/ar4133.
- Larkin J, Lohr TA, Elefante L, et al. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage. 2015;23(8):1254–1266. doi: 10.1016/j.joca.2015.02.778.
- Verma P, Dalal K. ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. J Cell Biochem. 2011;112(12):3507–3514. doi: 10.1002/jcb.23298.
- Li W, Wu M, Jiang S, et al. Expression of ADAMTs-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol. 2014;59(5):524–529. doi: 10.1016/j.archoralbio.2014.02.016.
- Kashiwagi M, Tortorella M, Nagase H, et al. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001;276(16):12501–12504. doi: 10.1074/jbc.C000848200.
- Dunn SL, Wilkinson JM, Crawford A, et al. Cannabinoid WIN-55,212-2 mesylate inhibits interleukin-1β induced matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human chondrocytes. Osteoarthritis Cartilage. 2014;22(1):133–144. doi: 10.1016/j.joca.2013.10.016.
- Lu W, He Z, Shi J, et al. AMD3100 attenuates post-traumatic osteoarthritis by maintaining transforming growth factor-β1-induced expression of tissue inhibitor of metalloproteinase-3 via the phosphatidylinositol 3-kinase/akt pathway. Frontiers Pharmaco. 2020;10:1554.
- Szeremeta A, Jura-Półtorak A, Zoń-Giebel A, et al. Aggrecan turnover in women with rheumatoid arthritis treated with TNF-α inhibitors. JCM. 2020;9(5):1377. doi: 10.3390/jcm9051377.
- Gonzalo-Gil E, Galindo-Izquierdo M. Role of transforming growth factor-beta (TGF) beta in the physiopathology of rheumatoid arthritis. Reumatol Clin. 2014;10(3):174–179. doi: 10.1016/j.reuma.2014.01.009.
- Al-Naqqash SR, Al-Asady ZT, Jawad M, et al. The correlation of TGF-β level and GARP gene expression in Iraqi patients with rheumatoid arthritis treated by biological and chemo-therapy. Inter J Pharm Res. 2020;1.
- Ciregia F, Deroyer C, Cobraiville G, et al. Modulation of αVβ6 integrin in osteoarthritis-related synovitis and the interaction with VTN (381–397 aa) competing for TGF-β1 activation. Exp Mol Med. 2021;53(2):210–222. doi: 10.1038/s12276-021-00558-2.
- Phull AR, Nasir B, Haq I, et al. Oxidative stress, consequences and ROS mediated cellular signaling in rheumatoid arthritis. Chem Biol Interact. 2018;281:121–136. doi: 10.1016/j.cbi.2017.12.024.
- Mateen S, Moin S, Shahzad S, et al. Level of inflammatory cytokines in rheumatoid arthritis patients: correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS One. 2017;12(6):e0178879. doi: 10.1371/journal.pone.0178879.
- Smallwood MJ, Nissim A, Knight AR, et al. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med. 2018;125:3–14. doi: 10.1016/j.freeradbiomed.2018.05.086.
- Hassan SZ, Gheita TA, Kenawy SA, et al. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: relationship to disease manifestations and activity. Int J Rheum Dis. 2011;14(4):325–331. doi: 10.1111/j.1756-185X.2011.01630.x.
- Singhai A, Patil UK. Amelioration of oxidative and inflammatory changes by peganum harmala seeds in experimental arthritis. Clin Phytosci. 2021;7(1):1–11. doi: 10.1186/s40816-020-00243-3.
- Umar S, Kumar A, Sajad M, et al. Hesperidin inhibits collagen-induced arthritis possibly through suppression of free radical load and reduction in neutrophil activation and infiltration. Rheumatol Int. 2013;33(3):657–663. doi: 10.1007/s00296-012-2430-4.
- Singhai A, Ahmad Y. Patil UK phyto-therapeutic potential of aconitum ferox roots in CFA-induced arthritis in rat model. Indian J Pharmaceut Edu Res. 2022;56:725–735.
- Song SN, Iwahashi M, Tomosugi N, et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Res Ther. 2013;15(5):R141. doi: 10.1186/ar4323.
- Zhang P, Guo Z, Luo W, et al. Graphene oxide-induced pH alteration; iron overload, and subsequent oxidative damage in rice (Oryza sativa L.): a new mechanism of nanomaterial phytotoxicity. Environ Sci Technol. 2020;54(6):3181–3190. doi: 10.1021/acs.est.9b05794.
- Tang M, Xing W, Wu J, et al. Graphene as a prominent antioxidant for diolefin elastomers. J. Mater. Chem. A. 2015;3(11):5942–5948. doi: 10.1039/C4TA06991A.