Abstract
Type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD), obesity (OB) and hypertension (HT) are categorized as metabolic disorders (MDs), which develop independently without distinct borders. Herein, we examined the gut microbiota (GM) and Saururus chinensis (SC) to confirm their therapeutic effects via integrated pharmacology. The overlapping targets from the four diseases were determined to be key protein coding genes. The protein–protein interaction (PPI) networks, and the SC, GM, signalling pathway, target and metabolite (SGSTM) networks were analysed via RPackage. Additionally, molecular docking tests (MDTs) and density functional theory (DFT) analysis were conducted to determine the affinity and stability of the conformer(s). TNF was the main target in the PPI analysis, and equol derived from Lactobacillus paracasei JS1 was the most effective agent for the formation of the TNF complex. The SC agonism (PPAR signalling pathway), and antagonism (neurotrophin signalling pathway) by SC were identified as agonistic bioactives (aromadendrane, stigmasta-5,22-dien-3-ol, 3,6,6-trimethyl-3,4,5,7,8,9-hexahydro-1H-2-benzoxepine, 4α-5α-epoxycholestane and kinic acid), and antagonistic bioactives (STK734327 and piclamilast), respectively, via MDT. Finally, STK734327-MAPK1 was the most favourable conformer according to DFT. Overall, the seven bioactives from SC and equol that can be produced by Lactobacillus paracasei JS1 can exert synergistic effects on these four diseases.
Introduction
Commonly, type 2 diabetes mellitus (T2DM), nonalcoholic fatty liver disease (NAFLD) and obesity (OB) have been strongly linked to its occurrence and development [Citation1]; furthermore, hypertension (HT) causes T2DM [Citation2], NAFLD [Citation3] and OB [Citation4] without consistent patterns. Its aetiological spectrum involves in the intersecting target(s) in the context of therapeutics. In this unexplored project, we focused on medicinal plants to unravel complicated syndromes because the multiple chemicals can exert multiple effects via interactions between two plant species. Among the medicinal plants, Saururus chinensis (SC) is an invaluable resource for treating four diseases: T2DM [Citation5], NAFLD [Citation6], OB [Citation7] and HT [Citation8]. Notably, SC has been utilized as a traditional medication in Asia for a long time without any specific toxicity [Citation9]. It is believed that the gut microbiota (GM) might be a significant agent for ameliorating metabolic disorders (MDs) due to its anti-inflammatory, antioxidant and anticholesterolemic effects [Citation10,Citation11]. Additionally, our previous study has been revealed its combined effects (OB [Citation12], NAFLD [Citation13], alcoholic liver disease (ALD) [Citation14] and liver regeneration [Citation15]) in combination with the use of favourable natural herbal plants. In fact, the GM plays important roles in modulating the physiological system of the host via certain mechanisms [Citation16]. Some probiotics metabolize fewer drug-like molecules (DLMs) into more DLMs via their own biosynthetic pathway. For instance, Bifidobacterium dentium can metabolize rutin (molecular weight: 610.52 g) into quercetin (molecular weight: 302.24 g), implying that quercetin might have better bioavailability than rutin [Citation17,Citation18]. As a flavonoid derivative can produce from isoflavone via Lactobacillus paracasei JS1, revealing that equol has the greatest antioxidant effect on all isoflavone species [Citation19,Citation20]. Some studies confirming the efficacy of metabolites (known as postbiotics) via inflammatory mechanisms have reported inconsistent experimental results due to diverse metabolites and their derivatives [Citation21].
Accordingly, key prebiotics, probiotics and metabolite(s) need to be established for administration in the context of therapeutics.
In this study, significant components were revealed to maintain the consistency of the experimental results in the selection of the bioactive compounds from SC and favourable metabolite(s) via potential GM. Accordingly, our study aimed to determine the key bioactive compounds produced by SC and the GM to confirm the lack of effects on T2DM, NAFLD, OB and HT. To perform the project, we adopted the network pharmacology (NP) concept to decode the critical elements: key molecule(s), target(s), mechanism(s) and GM. NPs might serve as a decoder to elucidate the orchestration of multiple-compounds-targets-mechanisms-GM [Citation22]. Indeed, NPs decrypt the entangled associations between molecules, targets and multiple diseases in conundrums. In fact, combined GM and NP analysis was used to clarify the ability of the GM to ameliorate diarrhoea-predominant irritable bowel syndrome disease (IBSD), suggesting that combination treatment with 18 GM and traditional herbal medicine was effective at relieving IBSD [Citation23].
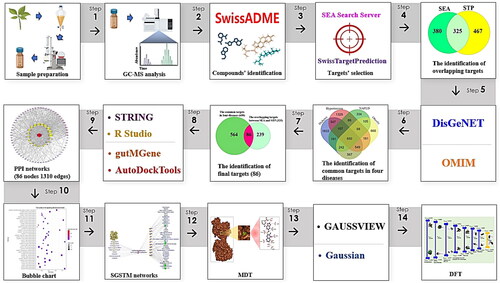
It is thought that the NP is a driver to solve the therapeutic hurdle in this puzzle, paving the way for the combined application of medicinal plants and GM. As previously mentioned, our analysis revealed that combination treatment with SC and GM might be an alternative therapeutic for treating T2DM, NAFLD, OB and HT. Hence, this analysis might provide crucial insight for the clinical tests and the development of combined SC and GM treatments. The workflow of this study is depicted in .
Materials and methods
Sample preparation and analysis
S. chinensis was collected from (latitude: 36.032765, longitude: 128.82696) Gyeongsangbuk-do, South Korea, in September 2022, and the plant was identified by Dr. Ki-Kwang Oh, a phytochemist and research professor, at the Institute for Liver and Digestive Diseases, College of Medicine, Hallym University, Chuncheon, South Korea. As research material, there is no need for permission to access or conduct SC studies. The plant was stored in the herbal repository cabinet of the laboratory. The voucher number (HHP 13-003) was deposited at the Institute for Liver and Digestive Diseases, College of Medicine, Hallym University, and the herbal plant was only allowed to be used as a research resource, not for commercial purposes. The SC was dried in a darkened room at between 20 and 22 °C for 10 days, and the dried SC was powdered with a mortar and pestle. AC powder 20 g was soaked in 1 L of methyl alcohol (Daejung, Siheung, South Korea) for 2 weeks, and the process was repeated twice to obtain a higher yield. The eluted solvent extract was filtered using paper no. 1 (Whatman, model no. WF1-1850, Maidstone, UK), and the methyl alcohol extract was evaporated using a rotary evaporator (IKA-RV8, Staufen city, Germany) at 40 °C. The crude methanolic extract of SC (MESC, 6 g), which was stored in a refrigerator (−20 °C) for further gas chromatography–mass spectrometry (GC–MS) analysis.
The GC–MS setup
An Agilent 7890A GC–MS instrument model (Agilent, Santa Clara, CA) was used to identify the molecules in SC, and the column was equipped with DB-5 (30 m × 0.25 mm × 0.25 µm) (Agilent, Santa Clara, CA). The conditions for GC–MS analysis were profiled in Method S1.
The screening of molecules in SCs
The chemical constituents in SC were detected via GC–MS, and converted into a Simplified Molecular Input Line Entry System (SMILES) file via the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) (accessed on 2 October 2023). Then, SwissADME (http://www.swiss adme.ch/) (accessed on 2 October 2023) was employed to determine the DLMs in the SC chemical constituents. In addition, we noted that the topological polar surface area (TPSA) promoted cell penetrability, and its cutoff value was less than 140 [Citation24].
The identification of targets on DLMs in SM
The targets related to DLMs were identified by similarity ensemble approach (SEA) (accessed on 3 October 2023) [Citation25], and SwissTargetPrediction (STP) (accessed on 3 October 2023) [Citation26] as cross-target searching browsers in the “Homo sapiens” mode. The relationships between the targets and the DLMs were examined in the two repositories, which confirmed their applicability as significant platforms to be validated through experimentation. Approximately, 80% of the identified effector candidates were identified in the SEA database; furthermore, the potential agents bound to cudraflavone C were gathered by STP repository, and the results were confirmed by experiments [Citation27]. On this basis, it is believed that SEA and STP might be significant mediators for obtaining conclusive results before in vivo testing. In addition, the intersecting targets between SEA and STP were selected to pursue more rigorous criteria.
Identification of overlapping targets in T2DM, NAFLD, OB and HT
The targets associated with the four diseases were retrieved from DisGeNET (https://www.disgenet.org/) (accessed on 4 October 2023) [Citation28] and OMIM (https://www.omim.org/) (accessed on 4 October 2023) [Citation29]. The common targets in the four diseases were defined as important protein-coding genes for ameliorating the multiple syndromes.
The final targets of the four diseases that responded to the DLMs of SC
The final targets were identified by the overlapping targets between SEA and STP, and the intersecting targets in T2DM, NAFLD, OB and HT. This means that the final targets related directly to the occurrence and development of the four diseases responded to the DLMs from SC.
Protein–protein interaction networks and bubble plot (BP)
The obtained final targets were assembled into protein–protein interaction (PPI) networks to determine the most crucial targets. The elements used to the significance of target(s) are degree centrality (DC; the number of connections on each target) [Citation30], betweenness centrality (BC; the number of tracks through a certain node) [Citation31] and closeness centrality (CC; the average of the shortest route between a target and all other targets in the system) [Citation31]. The PPI network was analysed by the R program via inputting the STRING database (https://string-db.org/) fitted less than p value (.05) [Citation32]. The target with the highest DC, BC and CC was considered a critical target for regulation in the PPI network.
A BP of the identified Kyoto Encyclopaedia of Genes and Genomes (KEGG) was constructed by the R program based on the STRING database. The BP shows the enrichment of an agonistic signalling pathway, and an antagonistic signalling pathway (the proportion of DEGs related to the signalling pathway).
SC, GM-signalling pathway–target–metabolite (SGSTM) network
We constructed SGSTM networks to decipher the interconnections of each element: SC or GM, key signalling pathways, targets and metabolites. Collectively, each element is represented as a node with a circle, and their relationships are represented as edges with a grey line. The above elements were merged with Microsoft Excel, and then, the SGSTM networks were constructed by using the R program to identify their interconnectedness against the four diseases via the incorporation of SC or GM.
Molecular docking test (MDT)
The MDT was conducted with AutodockTools-1.5.6 to identify the most stable conformer between key target(s) and molecule(s) (or metabolites) in both SC and GM. As a rule, the threshold of AutodockTools-1.5.6 was −6.0 kcal/mol [Citation33] and the ligand with the lowest binding energy value on each target was considered the most significant effector. The molecules were gathered as .sdf files from PubChem (https://pubchem.ncbi.nlm.nih.gov/) (accessed on 6 October 2023), and converted into .pdb file through the PyMOL visualizer. The .pdb file was translated into a .pdbqt file via AutodockTools-1.5.6 to dock on each target (or protein). Taken together with each .pdbqt file, the docking site was formatted in a square box (x = 40 Å, y = 40 Å and z = 40 Å).
Chemical reactivity description via frontier molecular orbitals
The chemical reactivity of a key molecules was determined by LEE-Yang-Parr (LYP) correlation functional analysis at the 6-31G++(d,p) optimized geometry level [Citation34]. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) were also obtained in the optimized geometry via frontier molecular orbital (FMO) theory. To determine the energy gap (Egap) of each molecule, the LUMO energy was deducted from HOMO energy value. The following formula was used to determine the key parameters for evaluating the chemical reactivity level.
Energy gap (Egap) = HOMO − LUMO;
Hardness (ɳ) = (LUMO − HOMO)/2’
Softness (S) = 1/ɳ;
Electronegativity (χ) = − (LUMO − HOMO)/2.
As mentioned above, the parameters were calculated and visualized by GaussView 6.0 and Gaussian 09 W to determine the electron donor or acceptor. Pinpointedly, a molecule with the lowest energy gap (Egap) and the highest softness (S) was confirmed to be a promising agent for the treatment of these four diseases.
Results and discussion
Profiling of chemical constituents in SC
A total of 71 chemical constituents were detected by GC–MS and key molecules were indicated by their peaks (), including the molecular name, PubChem ID, retention time (RT), area (%) and classification (). A molecule with highest amount in SC was methyl homovanillate (9.97%) classified into pyridines and derivatives, which is followed by 3,4-dimethoxyphenylalanine (7.50%), 3-butylpyridine 1-oxide (5.60%) and piclamilast (4.41%). All 71 chemical constituents were accepted by Lipinski’s five rule; in detail, the TPSA (≤140 Å2) is a criterion for penetrating the cell membrane [Citation35]. Commonly, a violation of only one in Lipinski’s rule is acceptable for drug-likeness properties [Citation36]. Hence, we identified 71 compounds from SC as having drug-like physicochemical properties ().
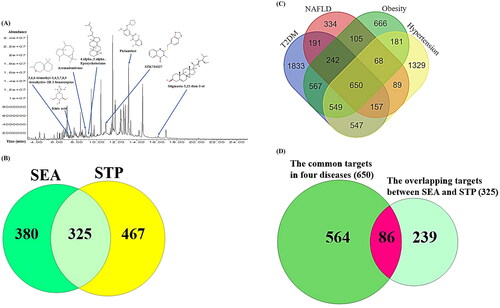
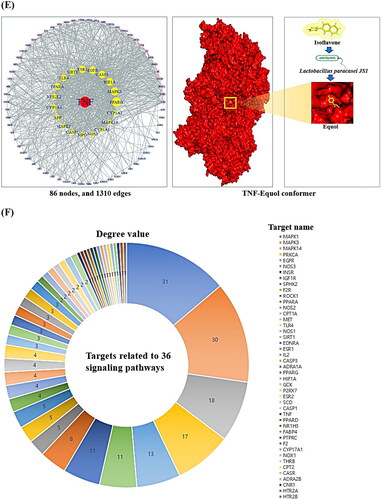
Figure 2. (A) The chromatogram of GC–MS and seven key chemical compounds. (B) The intersecting targets (325 targets) between SEA (705 targets) and STP (792 targets). (C) The common targets (650 targets) in T2DM (4736 targets), NAFLD (1836 targets), obesity (3028 targets) and hypertension (3570 targets). (D) The overlapping targets (86 targets) between 325 targets (B) and 650 targets (C). (E) PPI networks (86 nodes and 1310 edges) and TNF-Equol conformer (–7.6 kcal/mol). (F) The degree value of 45 targets related to 36 signalling pathways.
Table 1. The profiling of chemical compounds in Saururus chinensis (SC).
Table 2. The physicochemical properties of chemical constituents.
Identification of targets related to 71 chemicals from SC
The chemical constituents of each target of the DLMs in SC were selected by SEA (705 targets) and STP (792 targets) (Supplementary Table S1). The Venn diagram represents the intersecting targets (325 targets) between the two databases (Supplementary Table S1) ().
The common targets related to the four diseases and their final targets
The data for T2DM (4736 targets), NAFLD (1836 targets), OB (3028 targets) and HT (3570 targets) were retrieved from the DisGeNET and OMIM human disease databases (Supplementary Table S2). The intersection targets (650 targets) among the four diseases were identified by Venn diagram plotter (Supplementary Table S2) (). This means that the 650 targets are directly interconnected with the four diseases. There were 86 overlapping targets (). The 325 targets (common targets between SEA and STP) and the 650 targets were defined as the final targets for this study.
The protein–protein interaction networks and the uppermost conformer
The 86 final targets were used for PPI network construction to identify most abundant target. In the context of PPI networks, tumour necrosis factor (TNF) functions as a key player in regulating other neighbouring targets. Commonly, TNF has been described as an adipokine in light of metabolic inflammation [Citation37]. Globally, TNF had the highest value in DC, BC and CC with the highest impact among nodes (or targets) (). The dampening of TNF is a significant protein-coding gene that affects these four diseases. Thus, we explored whether the most significant compounds were SC chemical constituent(s) or GM metabolite(s) via MDTs. The two compounds from SC were 3,4-dimethoxy-dl-phenylalanine and 1-benzofuran, with binding energies of −5.9 kcal/mol and −5.5 kcal/mol, respectively. The six metabolites that can be produced by the GM were equol, 3-indolepropionic acid, succinate, butyrate, acetate and trimethylamine oxide, whose binding energies are −7.6 kcal/mol, −5.7 kcal/mol, −4.2 kcal/mol, −3.9 kcal/mol, −2.9 kcal/mol and −2.6 kcal/mol, respectively. Similarly, equol is a metabolite formed by isoflavone species via Lactobacillus paracasei JS1 [Citation19, Citation38]. Specifically, an animal test demonstrated that equol is a potent inhibitor of TNF by dampening of nuclear factor-κB (NF-κB) regulation in mouse macrophages [Citation39]. This result is consistent with our PPI network analysis. This depiction is shown in .
Table 3. The topological analysis of final targets.
Bubble plot to identify key signalling pathway(s)
The BP suggested that 45 out of 86 targets were associated with 36 signalling pathways related to T2DM, NAFLD, OB and HT. The description is based on a rich factor (the total number of targets expressed differentially in the signalling pathway). The PPAR signalling pathway with the highest enrichment factor was defined as agonism, while the neurotrophin signalling pathway with the lowest enrichment factor was described as antagonism. The detailed information is shown in . Notably, the target with the most connections among in the 36 signalling pathways was MAPK1 (31), followed by MAPK3 (30), MAPK14 (18) and PRKCA (17) (). Thus, MAPK1 is a significant target for elucidating the mechanisms related to T2DM, NAFLD, OB and HT.
Table 4. The description of key signalling pathways related to T2DM, NAFLD, OB and HT.
SC, GM, signalling pathways, targets and metabolite networks
The SGSTM networks represented the interconnections between SC or GM (nine nodes, yellow circle), the PPAR signalling pathway (one node, red circle), the neurotrophin signalling pathway (one node, red circle), targets (11 nodes, orange circle) and metabolites (39 nodes, green circle) with 61 nodes and 106 edges (). From this perspective, the four incorporated elements can have therapeutic effects, interplaying with synchronizing features against T2DM, NAFLD, OB and HT.
Molecular docking test
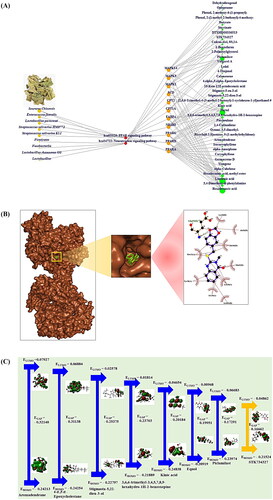
The MDT describes what the potential ligand(s) are involved in the PPAR signalling pathway or neurotrophin signalling pathway, and the critical key compound(s) are originate from either the SC or GM. The sole ligand (10-keto-12Z-octadecenoic acid) on PPARG was derived from linoleic acid (substrate) via Lactobacillus paracasei [Citation40]; however, its affinity had a low stability of −5.7 kcal/mol. Although Enterococcus faecalis, Streptococcus salivarius JIM8772 and Streptococcus salivarius K12 influence on PPARG [Citation41,Citation42], their distinct metabolites have yet to be identified. The other seven PPAR signalling pathway targets (PPARA, PPARD, NR1H3, FABP4, CPT1A, CPT2 and SCD) are still unclear concerning their relevance via the GM. Moreover, six organic compounds (aromadendrane on PPARA; −8.9 kcal/mol, stigmasta-5,22-dien-3-ol on PPARD; −8.6 kcal/mol, 3,6,6-trimethyl-3,4,5,7,8,9-hexahydro-1H-2-benzoxepine on PPARG; −6.9 kcal/mol, 4.alpha.,5.alpha.-epoxycholestane on NR1H3; −11.5 kcal/mol, kinic acid on FABP4; −6.4 kcal/mol, and piclamilast on CPT2; −7.1 kcal/mol, SCD; −6.9 kcal/mol) in SC were formed the stable conformer (Supplementary Table S3).
On the neurotrophin signalling pathway, some reports have shown that Firmicutes, Fusobacteria, Lactobacillus rhamnosus GG and Lactobacillus can produce succinate and butyrate [Citation43–45]. Notably, the two metabolites succinate and butyrate derived from GM were not valid ligands on MAPK1 and MAPK3. The fitted ligand linked to MAPK14 was yet to be identified. All three targets formed the most stable conformers as follows: MAPK1-STK734327 (–7.7 kcal/mol), MAPK3-piclamilast (–7.5 kcal/mol) and MAPK14-STK734327 (–8.1 kcal/mol) (Supplementary Table S3). In this study, a pinpointed conformer was MAPK1-STK734327 () because MAPK had the highest degree value (31) to enable the regulation of 36 signalling pathways against T2DM, NAFLD, OB and HT.
Overall, MDT is an efficient strategy for identifying potential drug candidates, especially, to for searching for a pair of specific targets [Citation46]. Furthermore, computational biology-based drug designs have been developed for anti-inflammatory agents and antitumor effectors [Citation47]. Overall, MDT might be a crucial tool for identifying the novel targets and treating diseases.
DFT verification of key chemical compounds or metabolites
The density functional theory (DFT) of key chemical compounds was adopted to determine their physicochemical reactivity in pharmacological space. Commonly, how a compound functions as a donor or an acceptor to its valence electron can be explored by the LUMO and HOMO energies. Herein, we determined the HOMO–LUMO (energy gap; EGAP) of eight promising therapeutic compounds () and profiled significant parameters (hardness, ɳ; softness, S; electronegativity, χ) to confirm the most promising agent for the orchestration of GM metabolite(s) (). The great physicochemical stability and reactivity can be attributed to the considerable HOMO–LUMO ratio, which is a descriptor used to clarify chemical susceptibility, including that of S and ɳ [Citation48–50]. Among the eight chemical compounds, STK734327 had the greatest kinetic reactivity due to having the lowest EGAP and the highest S. Thus, based on DFT analysis, we concluded that STK734327 is the most promising effector against T2DM, NAFLD, OB and HT.
Table 5. The chemical reactivity parameters of eight key compounds in this study.
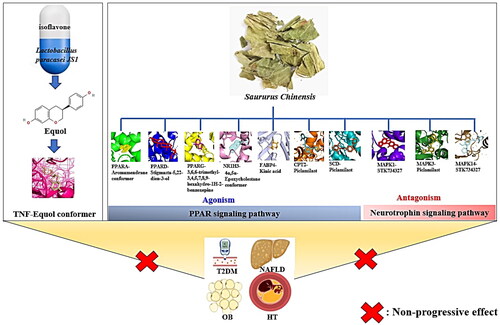
In summary, these findings shed light on the applicability of GM as therapeutic alternative(s), and SC with an adjuvant role with seamless linkage. Accordingly, our research needs to be investigated in clinical trials and prospective investigations. The key findings of this study are shown in .
The pros and cons of this study
Overall, the incorporation of SC and GM to combat T2DM, NAFLD, OB and HT can occur without a consistent interval. To unravel this conundrum, we pioneered SC, GM, signalling pathways, targets and metabolites with the help of bioinformatics, cheminformatics, microbial informatics and computational biology tools. Our results have provided critical clues to support clinical validation via the use of the current data. Certainly, even in the limited theoretical space, identifying key therapeutic components (potential GM, mechanism(s), target(s) and metabolite(s)) is a clue to enhancing the success rate of drug discovery. At this point, a large amount of data has been available to clarify the mechanism of action (MOA) of T2DM, NAFLD, OB and HT. In this unfinished project, our workflow will guide the evaluation of promising indications for T2DM, NAFLD, OB and HT.
Conclusions
The synchronization of the SC and GM might be an optimal therapeutic strategy to drive the nonprogressive phase against T2DM, NAFLD, OB and HT. This study sheds light on the use of prebiotics (isoflavone), probiotics (Lactobacillus paracasei JS1) and postbiotics (equol). In parallel, all seven chemical compounds from SC had drug-like potency, notably, MAPK1-STK734327 (antagonism in the neurotrophin signalling pathway) was the most promising conformer in terms of MDT, and DFT theory.
Author contributions
Ki-Tae Suk, Dong Joon Kim and Ki-Kwang Oh had substantial contributions to the conception or design for the work. Ki-Tae Suk, Ki-Kwang Oh, Sang-Jun Yoon and Seol Hee Song were involved in the acquisition or analysis for the work. Ki-Kwang Oh, Sang-Jun Yoon, Jeong Ha Park and Jeong Su Kim performed the interpretation of data for the work. Ki-Kwang Oh, Seol Hee Song, Jeong Ha Park and Jeong Su Kim performed drafting and reviewing it for important intellectual content. All authors confirmed the final approval of the version to be published. Also, all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Material
Download MS Excel (24.4 KB)Supplemental Material
Download MS Excel (151.9 KB)Supplemental Material
Download MS Excel (27.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Additional information
Funding
References
- Dharmalingam M, Yamasandhi PG. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J Endocrinol Metab. 2018;22(3):421–428. doi: 10.4103/IJEM.IJEM_585_17.
- Alsaadon H, Afroz A, Karim A, et al. Hypertension and its related factors among patients with type 2 diabetes mellitus – a multi-hospital study in Bangladesh. BMC Public Health. 2022;22(1):198. doi: 10.1186/S12889-022-12509-1/TABLES/2.
- Ng CH, Wong ZY, Chew NWS, et al. Hypertension is prevalent in non-alcoholic fatty liver disease and increases all-cause and cardiovascular mortality. Front Cardiovasc Med. 2022;9:942753. doi: 10.3389/FCVM.2022.942753.
- Jiang SZ, Lu W, Zong XF, et al. Obesity and hypertension. Exp Ther Med. 2016;12(4):2395–2399. doi: 10.3892/ETM.2016.3667.
- Hayashi K, Sato K, Ochi S, et al. Inhibitory effects of Saururus chinensis extract on receptor for advanced glycation end-products-dependent inflammation and diabetes-induced dysregulation of vasodilation. Int J Mol Sci. 2022;23(10):5757. doi: 10.3390/IJMS23105757.
- Fang K, Wu F, Chen G, et al. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement Altern Med. 2019;19(1):255. doi: 10.1186/s12906-019-2671-9.
- Cai J, Qiong G, Li C, et al. Manassantin B attenuates obesity by inhibiting adipogenesis and lipogenesis in an AMPK dependent manner. FASEB J. 2021;35(5):e21496. doi: 10.1096/FJ.202002126RR.
- Ryu SY, Oh KS, Kim YS, et al. Antihypertensive, vasorelaxant and inotropic effects of an ethanolic extract of the roots of Saururus chinensis. J Ethnopharmacol. 2008;118(2):284–289. doi: 10.1016/J.JEP.2008.04.011.
- Song MK, Lee SY, Kim M, et al. Saururus chinensis-controlled allergic pulmonary disease through NF-κB/COX-2 and PGE2 pathways. PeerJ. 2020;8:e10043. doi: 10.7717/PEERJ.10043/SUPP-2.
- Fabiano GA, Shinn LM, Antunes AEC. Relationship between oat consumption, gut microbiota modulation, and short-chain fatty acid synthesis: an integrative review. Nutrients. 2023;15(16):3534. doi: 10.3390/NU15163534.
- Vourakis M, Mayer G, Rousseau G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int J Mol Sci. 2021;22(15):8074. doi: 10.3390/IJMS22158074.
- Oh KK, Gupta H, Min BH, et al. Elucidation of prebiotics, probiotics, postbiotics, and target from gut microbiota to alleviate obesity via network pharmacology study. Cells. 2022;11(18):2903. doi: 10.3390/CELLS11182903/S1.
- Oh KK, Gupta H, Ganesan R, et al. The seamless integration of dietary plant-derived natural flavonoids and gut microbiota may ameliorate non-alcoholic fatty liver disease: a network pharmacology analysis. Artif Cells Nanomed Biotechnol. 2023;51(1):217–232. doi: 10.1080/21691401.2023.2203734.
- Oh K, Yoon S, Lee S, et al. The juxtaposition of Ilex cornuta fruit and gut microbiota against alcoholic liver disease based on the integrated pharmacology via metabolomics. Clin Transl Med. 2023;13(9):e1392. doi: 10.1002/CTM2.1392.
- Oh KK, Choi I, Gupta H, et al. New insight into gut microbiota-derived metabolites to enhance liver regeneration via network pharmacology study. Artif Cells Nanomed Biotechnol. 2023;51(1):1–12. doi: 10.1080/21691401.2022.2155661.
- Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut–brain communication. Front Endocrinol. 2020;11:25. doi: 10.3389/FENDO.2020.00025.
- Bang SH, Hyun YJ, Shim J, et al. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-l-rhamnosidase from Bifidobacterium dentium. J Microbiol Biotechnol. 2015;25(1):18–25. doi: 10.4014/JMB.1404.04060.
- Manach C, Morand C, Demigné C, et al. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997;409(1):12–16. doi: 10.1016/S0014-5793(97)00467-5.
- Kwon JE, Lim J, Bang I, et al. Fermentation product with new equol-producing Lactobacillus paracasei as a probiotic-like product candidate for prevention of skin and intestinal disorder. J Sci Food Agric. 2019;99(9):4200–4210. doi: 10.1002/JSFA.9648.
- Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140(7):1363S–1368S. doi: 10.3945/JN.109.119784.
- Jastrząb R, Graczyk D, Siedlecki P. Molecular and cellular mechanisms influenced by postbiotics. Int J Mol Sci. 2021;22(24):13475. doi: 10.3390/IJMS222413475.
- Oh KK, Yoon SJ, Lee SB, et al. The convergent application of metabolites from Avena sativa and gut microbiota to ameliorate non-alcoholic fatty liver disease: a network pharmacology study. J Transl Med. 2023;21(1):263. doi: 10.1186/s12967-023-04122-6.
- Zhen Z, Xia L, You H, et al. An integrated gut microbiota and network pharmacology study on Fuzi-Lizhong pill for treating diarrhea-predominant irritable bowel syndrome. Front Pharmacol. 2021;12:746923. doi: 10.3389/FPHAR.2021.746923.
- Matsson P, Kihlberg J. How big is too big for cell permeability? J Med Chem. 2017;60(5):1662–1664. doi: 10.1021/acs.jmedchem.7b00237.
- Keiser MJ, Roth BL, Armbruster BN, et al. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007;25(2):197–206. doi: 10.1038/NBT1284.
- Daina A, Michielin O, Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47(W1):W357–W364. doi: 10.1093/NAR/GKZ382.
- Soo HC, Chung FFL, Lim KH, et al. Cudraflavone C induces tumor-specific apoptosis in colorectal cancer cells through inhibition of the phosphoinositide 3-kinase (PI3K)-AKT pathway. PLOS One. 2017;12(1):e0170551. doi: 10.1371/JOURNAL.PONE.0170551.
- Piñero J, Saüch J, Sanz F, et al. The DisGeNET cytoscape app: exploring and visualizing disease genomics data. Comput Struct Biotechnol J. 2021;19:2960–2967. doi: 10.1016/J.CSBJ.2021.05.015.
- Hamosh A, Scott AF, Amberger JS, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33(Database issue):D514–D517. doi: 10.1093/NAR/GKI033.
- Zeng Q, Li L, Siu W, et al. A combined molecular biology and network pharmacology approach to investigate the multi-target mechanisms of Chaihu Shugan San on Alzheimer’s disease. Biomed Pharmacother. 2019;120:109370. doi: 10.1016/J.BIOPHA.2019.109370.
- Zhang L, Shi X, Huang Z, et al. Network pharmacology approach to uncover the mechanism governing the effect of Radix Achyranthis Bidentatae on osteoarthritis. BMC Complement Med Ther. 2020;20(1):121. doi: 10.1186/S12906-020-02909-4/FIGURES/7.
- Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(D1):D605–D612. doi: 10.1093/NAR/GKAA1074.
- Shityakov S, Förster C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv Appl Bioinform Chem. 2014;7:23–36. doi: 10.2147/AABC.S63749.
- Noureddine O, Gatfaoui S, Brandan SA, et al. Experimental and DFT studies on the molecular structure, spectroscopic properties, and molecular docking of 4-phenylpiperazine-1-ium dihydrogen phosphate. J Mol Struct. 2020;1207:127762. doi: 10.1016/j.molstruc.2020.127762.
- Gillet VJ, Leach AR. Chemoinformatics. Comprehens Med Chem II. 2007;3:235–264. doi: 10.1016/B0-08-045044-X/00085-7.
- Iheagwam FN, Rotimi SO. Computer-aided analysis of multiple SARS-CoV-2 therapeutic targets: identification of potent molecules from African medicinal plants. Scientifica. 2020;2020:1878410. doi: 10.1155/2020/1878410.
- Sethi JK, Hotamisligil GS. Metabolic messengers: tumour necrosis factor. Nat Metab. 2021;3(10):1302–1312. doi: 10.1038/s42255-021-00470-z.
- Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11(9):2231. doi: 10.3390/NU11092231.
- Kang JS, Yoon YD, Han MH, et al. Estrogen receptor-independent inhibition of tumor necrosis factor-α gene expression by phytoestrogen equol is mediated by blocking nuclear factor-κB activation in mouse macrophages. Biochem Pharmacol. 2005;71(1–2):136–143. doi: 10.1016/J.BCP.2005.10.009.
- Goto T, Kim YI, Furuzono T, et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, potently activates PPARγ and stimulates adipogenesis. Biochem Biophys Res Commun. 2015;459(4):597–603. doi: 10.1016/J.BBRC.2015.02.154.
- Are A, Aronsson L, Wang S, et al. Enterococcus faecalis from newborn babies regulate endogenous PPARgamma activity and IL-10 levels in colonic epithelial cells. Proc Natl Acad Sci U S A. 2008;105(6):1943–1948. doi: 10.1073/PNAS.0711734105.
- Couvigny B, De Wouters T, Kaci G, et al. Commensal Streptococcus salivarius modulates PPARγ transcriptional activity in human intestinal epithelial cells. PLOS One. 2015;10(5):e0125371. doi: 10.1371/JOURNAL.PONE.0125371.
- Nepelska M, Cultrone A, Béguet-Crespel F, et al. Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLOS One. 2012;7(12):e52869. doi: 10.1371/JOURNAL.PONE.0052869.
- Giahi L, Aumueller E, Elmadfa I, et al. Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Benef Microbes. 2012;3(2):91–98. doi: 10.3920/BM2011.0052.
- Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi: 10.1038/NM.4102.
- Shah K, Mujwar S, Gupta JK, et al. Molecular docking and in silico cogitation validate mefenamic acid prodrugs as human cyclooxygenase-2 inhibitor. Assay Drug Dev Technol. 2019;17(6):285–291. doi: 10.1089/ADT.2019.943.
- Reddy R, Mutyala R, Aparoy P, et al. Computer aided drug design approaches to develop cyclooxygenase based novel anti-inflammatory and anti-cancer drugs. Curr Pharm Des. 2007;13(34):3505–3517. doi: 10.2174/138161207782794275.
- Shahinozzaman M, Taira N, Ishii T, et al. Anti-inflammatory, anti-diabetic, and anti-Alzheimer’s effects of prenylated flavonoids from Okinawa propolis: an investigation by experimental and computational studies. Molecules. 2018;23(10):2479. doi: 10.3390/MOLECULES23102479.
- Pearson RG. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci U S A. 1986;83(22):8440–8441. doi: 10.1073/PNAS.83.22.8440.
- Lucido MJ, Orlando BJ, Vecchio AJ, et al. Crystal structure of aspirin-acetylated human cyclooxygenase-2: insight into the formation of products with reversed stereochemistry. Biochemistry. 2016;55(8):1226–1238. doi: 10.1021/acs.biochem.5b01378.