ABSTRACT
This paper presents the effects of various slag contents on the residual compressive strength and physical properties of ambient air-cured fly ash-slag blended geopolymers after exposure to various elevated temperatures up to 800°C. The results showed an increasing trend in the compressive strength of ambient air-cured geopolymers with increase in the slag contents after exposure to 400 and 600°C temperatures. This trend deviated, however, at 800°C. Nevertheless, all the geopolymers showed reductions in control compressive strength at ambient temperature after exposure to elevated temperatures. The reductions were much higher at 600 and 800°C compared to 400°C. All the geopolymers exhibited significant damage in terms of cracking after exposure to a temperature of 800°C compared to 400 and 600°C and significant damage occurred at slag contents of 15–30%. Scanning electron microscopic (SEM) images of the above geopolymers also showed higher porosity at 800°C compared to 400 and 600°C. Traces of calcite/calcium silicate hydrate (CSH) peaks are observed in the X-ray diffraction (XRD) analysis of fly ash-slag geopolymers, and the intensity of those peaks increased with increases in slag contents. After exposure to elevated temperatures, the calcite/CSH peaks disappeared and new phases of nepheline and gehlenite were formed at 800°C in all the fly ash-slag geopolymers.
Introduction
Geopolymers are a promising environmentally friendly binder due to their superior mechanical, durability and fire-resistance properties as well as their lower carbon footprint than the ordinary concrete. With the abundant generation of fly ash, as a by-product of coal-fired power stations around the world, its use in geopolymer cement can significantly relieve the land use associated with dumping and the contamination of air and groundwater. Fly ash geopolymers require moderate heat to activate the geopolymer reaction in either a steam curing chamber or oven, which limits their in-situ applications in actual projects. In addition, the heat curing also negatively affects their carbon footprint. According to Turner and Collins [Citation1], heat curing accounts for about 12.5% of total CO2 emissions from heat cured fly ash geopolymers. Developing ambient air-cured fly ash geopolymers can therefore reduce their carbon footprint further. Different researchers have used different source materials for partial replacement of class F fly ash to develop ambient air-cured geopolymers including ordinary Portland cement (OPC), slag, metakaolin and lime powder, for example. The above CaO-rich source materials form calcium-silica-hydrate (C-S-H) gels in addition to sodium-aluminate-silicate-hydrate (N-A-S-H) geopolymer gels and form complex C-(N)-A-S-H geopolymer gels which help to set and harden concrete at ambient temperatures. A number of studies have evaluated the mechanical properties of ambient air-cured fly ash geopolymers [Citation2–Citation6]. All the studies observed that the compressive strength of ambient air-cured fly ash geopolymers increases with increases in the slag contents [Citation3–Citation6], due to increased formation of C-(N)-A-S-H gels resulting from the slag contents.
In several studies, heat-cured fly ash geopolymers exhibited better fire resistance and residual mechanical properties after exposure to elevated temperatures than their concrete counterparts [Citation7–Citation12]. The stable crystalline phases of mullite, nepheline, albite and tridymite in fly ash geopolymers play a significant role in retaining room temperature strength at elevated temperatures and maintain high-temperature stability up to 1840°C [Citation13]. On the other hand, the dehydration and dissociation of cement hydration products (e.g. Ca(OH)2 and C-S-H) at elevated temperatures cause significant loss of strength in concrete. As CaO-rich source materials are used for partial replacement of fly ash in ambient air-cured fly ash geopolymers, their behaviour at elevated temperatures is expected to be different from that of heat-cured fly ash geopolymers. The effect of elevated temperatures on the residual strength of ambient air-cured fly ash geopolymers was reported in a limited study. Ren et al. [Citation14] studied the effect of various elevated temperatures up to 800°C on fly ash-slag blended ambient air-cured geopolymers containing 55% slag as a partial replacement for fly ash. They observed reductions in compressive strength of about 14, 19 and 70% at 400, 600 and 800°C, respectively. Guerrieri and Sanjayan [Citation6] studied the effects of 35, 50 and 65% slag on the elevated temperature behaviour of fly ash-slag blended geopolymers which were cured at 80°C and RH of 95%. They reported a loss of compressive strength of about 90 and 70% in fly ash-slag geopolymers with an initial compressive strength of 83 and 28 MPa after exposure to an elevated temperature of 800°C. On the other hand, fly ash-slag geopolymers with very low strength of about 7.6 MPa exhibited a gain in compressive strength of about 90% after exposure to an elevated temperature of 800°C. In a recent study, Kashani et al. [Citation15] reported a reduction of about 62% in the compressive strength of ambient air-cured fly ash geopolymers containing 20% slag as partial replacement for fly ash after exposure to a temperature of 1000°C. Vasquez-Molina et al. [Citation16] reported a comparative study of fly ash-cement blended geopolymers cured at ambient temperature and heat-cured fly ash geopolymers subjected to elevated temperatures of 700°C. It is shown in their study that the residual strength at 700°C is about 92% of the initial strength in fly ash-cement blended geopolymers and about 113% of the initial strength in fly ash geopolymers. In high calcium fly ash geopolymers which were ambient air-cured, Cheng-Yong et al. [Citation17] also observed reductions of about 67, 71 and 88% in compressive strength at 400, 600 and 800°C, respectively. Ranjbar et al. [Citation18] reported a study in which fly ash-palm oil fuel ash geopolymers cured at 65°C for 24 hrs containing different quantities of palm oil fuel ash as a partial replacement for fly ash were subjected to various elevated temperatures up to 1000°C. It was observed that the compressive strength at all elevated temperatures decreased with increases in palm oil fuel ash contents. It can be seen from the above-reported studies that different CaO-rich source materials in different quantities are used in ambient air-cured fly ash geopolymers. In some studies such as in [Citation6] heat curing was applied. Different types of alkali activators are also used in various concentrations and amounts.
Ground granulated blast furnace slag, commonly known as “slag” is another widely available industrial by-product and a good candidate for use in fly ash geopolymers to achieve ambient temperature curing. In most of the studies very high amounts of slag are used in fly ash-slag geopolymers (e.g. [Citation6,Citation14]) and significant loss in strength of the above geopolymers is observed after exposure to elevated temperatures. Insufficient research has been reported to study the residual strength properties of fly ash geopolymers containing lower amounts of slag [Citation15]. This research is designed to study the effect of various slag contents ranging from 5% to 30% as partial replacement for fly ash on the residual compressive strength and physical properties of ambient air-cured fly ash geopolymers. Microstructural investigations involving scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, mercury intrusion porosimetry (MIP) and thermogravimetric analysis (TGA) were also conducted to study the changes in the microstructures and reaction phases occurring when forming various geopolymer reaction products and their phases as well as their effects on the pore structure both before and after exposure to elevated temperatures. A better understanding was therefore established of the changes in microstructure and the compressive strength and physical properties of the above fly ash geopolymers containing various slag contents. The above properties were also benchmarked with the most widely used heat-cured fly ash geopolymers.
Experimental program
This study considered five ambient air-cured fly ash-slag blended geopolymer mixes containing 5, 10, 15, 20 and 30% slag as partial replacement for fly ash. They were termed S1, S2, S3, S4 and S5, respectively. All five geopolymers were subjected to similar elevated temperature exposure at 400, 600 and 800°C. In addition, heat-cured fly ash geopolymers (Mix ID S0) were also considered for the purposes of the comparison, details of which can be found in Haque and Shaikh [Citation19].
Materials, mixing method and curing
Class F fly ash supplied by the Gladstone Power Station of Queensland, Australia, was used as a source material to prepare the heat-cured fly ash geopolymers, and the same fly ash and slag were used in ambient air-cured geopolymers. shows the chemical compositions of the class F fly ash and slag. The activating alkali liquids consisted of 8 M sodium hydroxide (NaOH) and D-Grade sodium silicate (Na2SiO3) solutions. NaOH solution was prepared with a concentration of 8 M using NaOH beads of 97% purity and tap water. The D-Grade Na2SiO3 solution which was supplied by PQ Australia had a specific gravity of 1.51 and a modulus ratio (Ms) equal to 2.0 (where Ms = SiO2/Na2O, Na2O = 14.7%, SiO2 = 29.4% and water = 55.9%). The NaOH and Na2SiO3 solutions were mixed together in a Na2SiO3/NaOH mass ratio of 2.5. shows the mixing proportions of the fly ash-slag blended geopolymers studied in this study.
Table 1. Chemical compositions of class F fly ash and blast furnace slag.
Table 2. Mixing proportions of fly ash-slag blended ambient air-cured geopolymers.
All geopolymer pastes were prepared in a Hobart mixer. In the case of ambient air-cured geopolymers, the fly ash and slag were first dry-mixed for 1 min in a Hobart mixer after which alkaline activators were added and mixed for approximately 4 min. The fresh geopolymer pastes were cast into standard 50‐mm plastic cube moulds and compacted using a vibrating table. The specimens were demoulded the next day and left in the open air at ambient temperature inside the laboratory for 2 weeks. The fly ash geopolymers on the other hand were subjected to heat curing in an oven. In this stage, all the moulds were sealed to minimise moisture loss and placed in the oven at 70°C for 24 h. At the end of the heat curing period, the specimens were removed from the oven and left undisturbed until cool down and then removed from the moulds and stored in the laboratory in the open air at ambient temperature for 2 weeks.
Test methods
The compressive strength of all the specimens was measured according to AS 1012.9 [Citation20] at 14 days. For each mix, at least six specimens were tested in order to check the variability of performance under compression as well as the volumetric shrinkage and mass loss. The specimens in each series were placed in an electric kiln after 14 days of curing to expose them to various elevated temperatures. The volumetric shrinkage of the geopolymer samples was determined by measuring the length of three sides of the cubes before and after heating using Vernier callipers at the respective elevated temperatures. The differences in volume changes indicated the volumetric shrinkage of each cube. As for mass loss of the geopolymer pastes, the mass of the cube specimens in each series was measured using a sensitive weighing machine before and after exposure to the respective elevated temperatures.
For the XRD analysis, the samples were measured with a D8 Advance Diffractometer (Bruker‐AXS) using copper radiation and a Lynx Eye position sensitive detector. The diffractometer scanned the samples from 7° to 70° (2θ) in steps of 0.015° at a scanning rate of 0.5°/min. XRD patterns were obtained using Cu Ka lines (k = 1.5406 Å). A knife edge collimator was fitted to reduce air scatter. SEM analyses were performed using a Zeiss EVO 40XVP microscope equipped with an energy dispersive X-ray analyser.
The thermal stability of the samples was studied by TGA. A Mettler Toledo TGA one star system analyser was used for all these measurements. Samples weighing 25 mg were placed in an alumina crucible and the tests were carried out in an Argon atmosphere at a heating rate of 10°C/min from 25 to 1000°C.
MIP was used to measure the pore volume and pore size distribution of the geopolymer samples. The pore diameter and intruded mercury volume were recorded at each pressure point over a pressure range of 0.0083 to 207 MPa. The pressure values were converted into equivalent pore diameters using the Washburn expression [Citation21], as expressed in Equation (1):
where d is the pore diameter (μm), γ is the surface tension (mN/m), θ is the contact angle between mercury and the pore wall (°), and P is the net pressure across the mercury meniscus at the time of the cumulative intrusion measurement (MPa).
Elevated temperature exposure
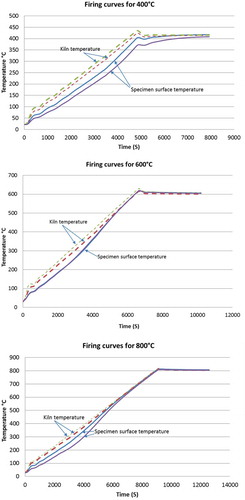
A locally manufactured kiln was used to heat the geopolymer specimens to temperatures from 200°C to 800°C. The specimens were positioned inside the kiln with two thermocouples touching them. Two more thermocouples were placed inside the kiln to monitor the kiln air temperature. The thermocouples were connected to the data logger and used to monitor the temperature on the specimen surfaces and the kiln air, as shown in . A heating rate of 5°C per minute was selected, which is very close to the RILEM [Citation22] recommended heating rate. During the heating process, the temperatures of all four thermocouples were monitored. Once the specimens surfaces reached the target temperature, the temperature inside the kiln was maintained for 1 h. The rate of temperature increases in the kiln and the specimens are shown in . As can be seen in the figure, there is a significant lag between the surface temperature of the cubes and the air temperature inside the kiln, particularly for the 400°C temperature profiles. This was attributable to the heat capacity of the specimens and the rate at which they were able to absorb heat. However, the differences in temperatures between the kiln and the cubes at 600°C and 800°C were smaller. Even though differences in temperature were present between the kiln and the cubes, the target test temperatures in all the cubes were maintained for 1 h, as shown by the thermocouple readings in .
Results and discussion
The microstructure of ambient air-cured geopolymer
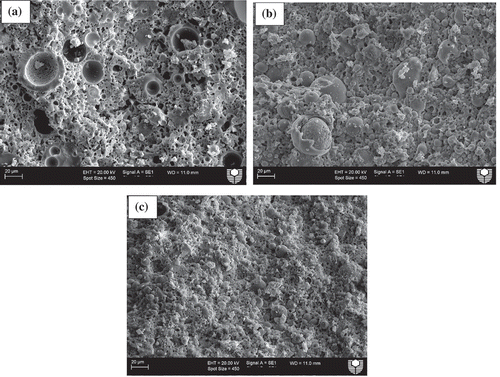
Microstructural analysis was conducted using SEM images to evaluate the effects of various slag contents on the microstructures of ambient air-cured geopolymers matrices as well as those exposed to various elevated temperatures. shows SEM images of ambient air-cured geopolymers containing 5, 15 and 30% slag. It can be seen by comparing ()) with (,)) that the microstructure of the former is denser than those of the the latter geopolymers. This can be attributed to the formation of more C-(N)-A-S-H geopolymer gels due to the increase in slag contents from 5 and 15% to 30%, as shown in . shows the energy dispersive X-ray spectroscopy (EDS) analysis of the reaction products of those three geopolymers and it is apparent from the figure that the “Ca” peak is higher in geopolymers containing 30% slag than in those containing 5 and 15% slag. The predominant calcium, sodium, aluminium and silica peaks in the EDS spectra of those fly ash-slag geopolymers are a clear indication of the formation of C-(N)-A-S-H geopolymer gels. The presence of unreacted fly ash particles is less apparent in the SEM images of the geopolymers containing 30% slag than in those containing 5 and 15% slag, which is also an indication of the dissolution of fly ash particles in geopolymer reaction products. The SEM images of geopolymers containing 5, 15 and 30% slag after exposure to 400, 600 and 800°C temperatures are also shown in –. Analysis of the images reveals an increasing number of pores/voids in the geopolymers containing slag with increases in the elevated temperatures. It can be seen that the pores become slightly larger with the increases in temperature, accompanied by increases in the numbers of pores as well as of microcracks. These pores and cracks might have been left behind by escaping water during the temperature exposure. A relatively smoother geopolymer matrix can still be seen, however, after exposure to 400°C. It can also be seen that the microstructure of geopolymers containing 30% slag after exposure to elevated temperatures is more compact and less damaged than the microstructures of those containing 5 and 15% slag.
Figure 3. SEM images of ambient air-cured geopolymers containing (a) 5%, (b) 15% and (c) 30% slag as partial replacement for fly ash.
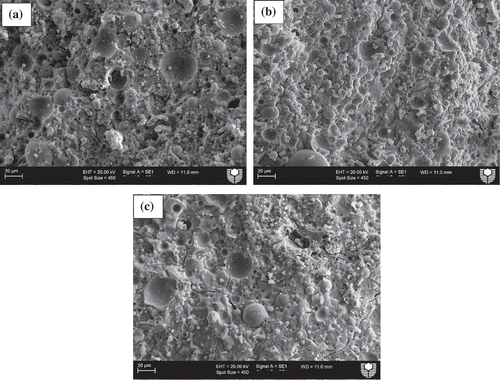
Figure 4. Energy-dispersive X-ray spectroscopy (EDS) analysis of ambient air-cured geopolymers containing (a) 5%, (b) 15% and (c) 30% slag as partial replacement for fly ash.
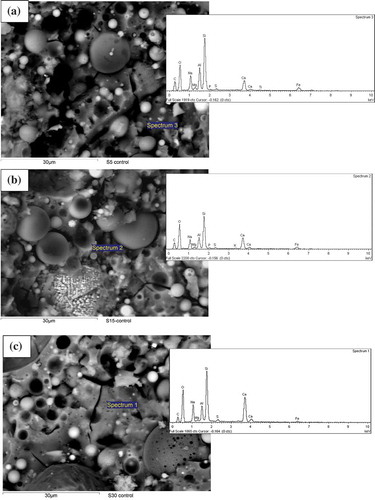
Figure 5. SEM images of ambient air-cured geopolymers containing (a)5%, (b) 15% and (c) 30% slag as partial replacement for fly ash after exposure to a temperature of 400°C.
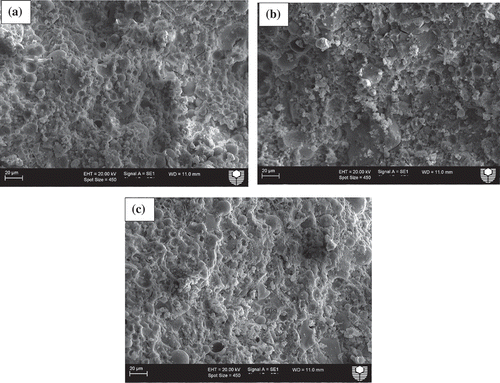
Figure 6. SEM images of ambient air-cured geopolymers containing (a)5%, (b) 15% and (c)30% slag as partial replacement for fly ash after exposure to a temperature of 600°C.
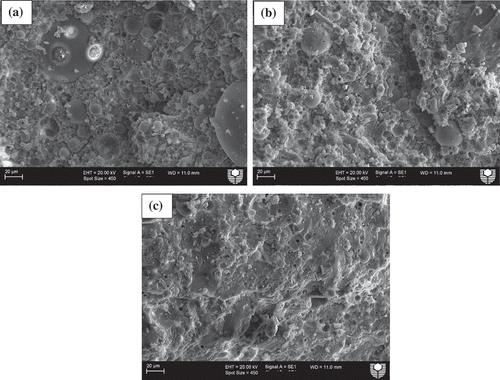
Figure 7. SEM images of ambient air-cured geopolymers containing (a)5%, (b) 15% and (c) 30% slag as partial replacement for fly ash after exposure to a temperature of 800°C.
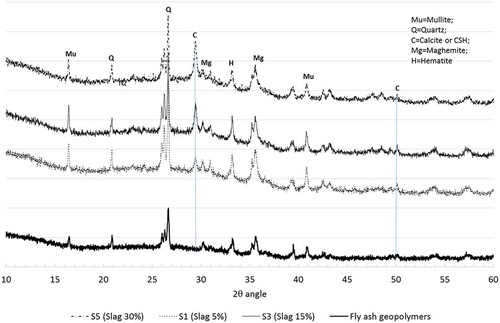
presents XRD diffractograms of geopolymers containing various slag contents. The XRD diffractogram of fly ash geopolymers is shown in the same figure for purposes of benchmarking. A number of crystalline phases (e.g. mullite, quartz, maghemite and hematite phases) can be seen in the geopolymers containing slag, which are also observed in heat-cured fly ash geopolymers. In the case of geopolymers containing slag, however, new crystalline peaks of “calcite” and “calcium silicate hydrate (CSH)” are noticed at the 2θ angles of 29.4° and 50.17°, and the height of the calcite/CSH peaks also increases with increases in slag contents indicating the formation of more calcium-based reaction products in the C-(N)-A-S-H gels, which also correlates well with the denser microstructure observed in the geopolymers containing 30% slag than in the other geopolymers observed in the above SEM analysis. It has been reported by numerous studies that semi-crystalline CSH and calcite peaks overlap at 2-theta angles of 29.45° and 50.17° [Citation23-Citation24].
Figure 8. XRD analysis of ambient air-cured geopolymers containing 5, 15 and 30% slag and fly ash geopolymers.
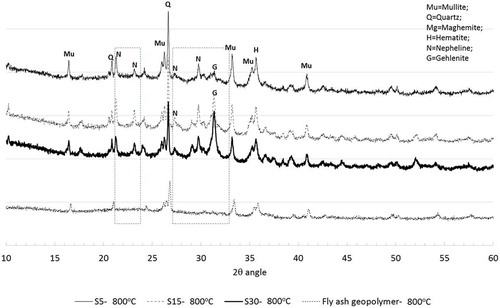
Elevated temperatures increased the tendency towards the formation of more stable crystalline phases in fly ash geopolymers. XRD diffractograms of geopolymers containing 5, 15 and 30% slag after exposure to 400, 600 and 800°C temperatures shown in ()) also show similar crystalline phases which were formed in fly ash geopolymers. In geopolymer containing slag, however, two additional crystalline phases, nepheline (Na4Al4Si4O16) and gehlenite (Ca2Al2SiO7), are also observed especially after exposure to a temperature of 800°C. It can also be seen that the calcite phase observed in geopolymers containing slag disappeared, after exposure to elevated temperatures indicating that this calcium-based reaction product decomposes at those elevated temperatures leaving pores in the geopolymer gels, as observed in the SEM images. The geopolymers exposed to a temperature of 800°C contain more gehlenite and a new alumina silicate phase of nepheline, as shown in . It seems that as the geopolymer matrix decomposes, the alumina silicate species react with the released alkalis and calcium to form crystalline phases of gehlenite and nepheline.
Figure 9. XRD analysis of ambient air-cured geopolymers containing (a) 5%, (b) 15% and (c) 30% slag as partial replacement for fly ash after exposure to 400, 600 and 800°C temperatures.
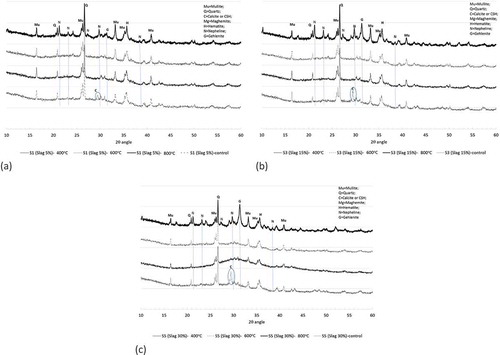
Figure 10. Comparison of XRD analysis of ambient air-cured geopolymers containing various slag contents and fly ash geopolymers after exposure to a temperature of 800°C.
Mass loss of various ambient air-cured geopolymers containing 5, 15 and 30% slag as partial replacement for fly ash from room temperature to 1000°C in TGA is shown in . The TGA curve of fly ash geopolymers is shown in the same figure for purposes of comparison. Generally, the TGA curves of the above geopolymers can be divided into four major parts: (a) evaporation of water, (b) decomposition of geopolymer components (e.g. calcite/CSH) (c) a change phase to nepheline and (d) a stage in which the weight loss is unchanged. It can be seen from the TGA curves that sharp reductions of mass occurred at below 200°C and that the rate of mass loss is almost the same for all three fly ash-slag blended geopolymers. The same figure shows that the rate of mass loss of fly ash geopolymers is slower than that of fly ash-slag blended geopolymers. This mass loss can be attributed to the evaporation of evaporable free water weakly absorbed in the geopolymers and the pores of geopolymers as well as decomposition of CSH and calcite in C-(N)-A-S-H gels. The higher mass loss of ambient air-cured geopolymers containing higher slag contents indicates the presence of larger amounts of calcium-based reaction products formed in C-(N)-A-S-H gels in these geopolymers, a result which agrees well with the reduced porosity of the geopolymers as shown in . The mass loss rate slowed at above 200°C where a small reduction in mass loss occurred at between 200 and 400°C compared to that occurring at below 200°C. This can be attributed to the loss of physically and chemically bound water. A comparison with fly ash geopolymers shows that the rate of mass loss at between 200 and 400°C increases in the fly ash-slag geopolymers with increases in slag contents. The mass loss rate was significantly reduced at above 400°C and no mass loss peak is observed in the region of 450°C, confirming the absence of Portlandite (Ca(OH)2) in the geopolymer gels in fly ash-slag geopolymers. This indicates that Ca(OH)2 is not formed in the alkali activation of CaO-rich slag with fly ash in geopolymers despite very high alkalinity. The rate of mass loss at temperatures from 600°C to 1000°C in fly ash-slag blended geopolymers is very similar to that in fly ash geopolymers.
Figure 11. TGA analysis of ambient air-cured geopolymers containing various slag contents and fly ash geopolymers.
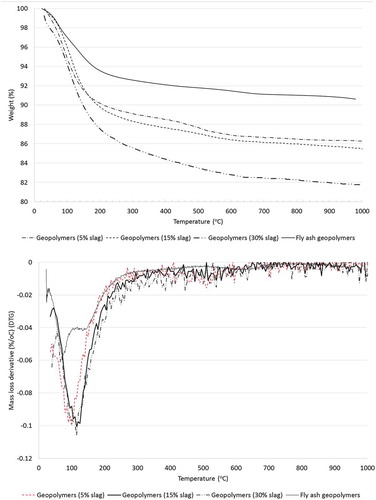
Figure 12. Pore size distribution of various fly ash-slag blended geopolymers and fly ash geopolymers.
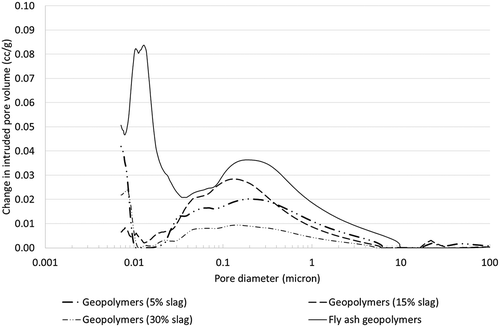
The pore size distribution in shows that the overall pore volumes of fly ash-slag blended geopolymers are much lower than those of fly ash geopolymers. It can be seen that the volume of small diameter pores (e.g. 0.01–0.03 microns) is significantly reduced in fly ash-slag blended geopolymers, presumably due to the formation of calcium-based reaction products in the C-(N)-A-S-H gels evident in the XRD and SEM analyses. It can also be seen that, among fly ash-slag blended geopolymers, geopolymer containing 30% slag exhibited the lowest volume of pores of between 0.03 and 10 microns. In the case of pore sizes 0.01–0.03 microns and 0.35–10 microns mixed results are observed for example for geopolymers containing 5 and 15% slag, the pore volume of geopolymer containing 15% slag is smaller than in those containing 5% slag. On the other hand, an opposite trend is observed in a small band of pore sizes measuring 0.03–0.35 microns.
Physical behaviour at elevated temperatures
The effects of increasing slag contents on changes in the mass, volume, cracking and colour of ambient air-cured geopolymers after exposure to elevated temperatures are shown in –. It is apparent from that the mass loss of ambient air-cured geopolymers increases with increases in slag contents after exposure to a temperature of 400°C. The increase in mass loss of geopolymers containing 10 and 15% slag is very low compared to that containing 5% slag. However, increases in mass loss of about 10 and 15% are observed at 20 and 30% slag contents, respectively. When geopolymers were exposed to higher temperatures such as 600 and 800°C, no significant change in mass loss is noticed for slag contents of 10 and 15%, but a similar level of increase in mass loss as that observed at 400°C is also observed for 20 and 30% slag contents. Nevertheless, it can be seen that the mass loss of geopolymers increases with increases in elevated temperatures for all slag contents. The increasing mass loss with increases in slag contents in ambient air-cured geopolymers can be attributed to the increase in CaO, which results in a larger amount of calcium-based reaction products such as C-(N)-A-S-H gels. In heat cured fly ash geopolymers, on the other hand, the mass loss at 400, 600 and 800°C is 9, 11 and 15%, respectively, much lower than that observed in the case of ambient air-cured fly ash-slag blended geopolymers, presumably due to the absence of calcium-based reaction products in N-A-S-H geopolymer gels [Citation19].
Figure 13. Mass loss of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.
[Note: S0 represents 100% fly ash geopolymers].
![Figure 13. Mass loss of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.[Note: S0 represents 100% fly ash geopolymers].](/cms/asset/42b28cec-1eaf-443e-8bce-78fb3a3c5eea/tace_a_1529013_f0013_oc.jpg)
Figure 14. Volume loss of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.
[Note: S0 represents 100% fly ash geopolymers].
![Figure 14. Volume loss of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.[Note: S0 represents 100% fly ash geopolymers].](/cms/asset/e90da9ad-af51-499c-973b-4c616ae570af/tace_a_1529013_f0014_oc.jpg)
Figure 15. Cracking behaviour of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.

The effects of increasing slag contents on volume changes in geopolymer specimens after exposure to elevated temperatures are shown in . It can be seen from the figure that, unlike for mass loss there is no trend in volume changes of ambient air-cured geopolymers with increases in slag contents at any elevated temperatures except for 600°C where an increasing trend is noticed. At 800°C, however, especially for higher slag contents such as 15, 20 and 30% the volume changes could not be measured due to severe cracking as seen in which shows the effects of increasing slag contents on cracking and colour changes. It can be seen in that no cracking occurred in any geopolymers at up to 600°C regardless of the slag contents. At 800°C, however, cracks appeared in all ambient air-cured geopolymers and the cracks width and depth increased with increases in slag contents. In heat-cured fly ash geopolymers, on the other hand, the intensity of cracking at 800°C is much less than in ambient air-cured geopolymers containing high slag contents [Citation24]. It has been shown in next section that the compressive strengths of ambient air-cured geopolymers containing high slag contents (e.g. 20 and 30%) are higher than those containing 5, 10 and 15% slag. This can be attributed to the formation of larger amounts of calcium-based reaction products in C-(N)-A-S-H geopolymer gels, which are more compact than those of heat-cured fly ash geopolymer matrices. The more compact geopolymer matrices of fly ash-slag geopolymers therefore prevented water vapour from escaping from the inner portion of the matrix in ambient air-cured geopolymers containing high slag contents, and more cracking occurred in these geopolymers due to internal pore pressure. In addition, residual silica provided by the activator which might not have been incorporated into the reaction products (most likely C-(N)-A-S-H gels) may have caused swelling and thermal instability at elevated temperatures [Citation13,Citation25]. A colour change in ambient air-cured fly ash-slag geopolymers is also observed after exposure to elevated temperatures as shown in the same figure . It can be seen that the colour of ambient air-cured geopolymers is changed from dark grey at ambient temperature to grey, light brown and brown at 400, 600 and 800°C, respectively regardless of the slag contents. The same figure shows colour changes in heat-cured fly ash geopolymers and it can be seen that the fly ash geopolymers also changed to a brown colour at 800°C. This change in colour is attributed to the oxidation of iron species within the fly ash particles during heating at elevated temperatures.
Residual compressive strength
The measured compressive strengths of ambient air-cured geopolymers containing various amounts of slag before and after exposure to elevated temperatures are shown in . Error bars are shown in the same figure. The compressive strength of heat-cured fly ash geopolymers is also shown in the same figure for purpose of comparison. It can be seen that the measured compressive strength of unexposed geopolymers to elevated temperatures is decreased by 15 and 7% due to increases in the slag content from 5% to 10 and 15%, respectively. At higher slag contents such as 20 and 30%, however, the compressive strength is increased slightly by about 6 and 5%, respectively. The exact reason for the slight reduction in compressive strength for 10 and 15% slag contents is not known, but the microstructural analysis results reveal a slightly more porous matrix in the SEM images and slightly higher pore volumes in the MIP results for geopolymers containing 15% slag than in those containing 5% slag. After these geopolymers are exposed at 400°C, it can be seen that the residual compressive strength increases with increases in slag contents. A similar trend is also observed after exposure to 600°C. After exposure to 800°C, however, no such increasing trend is observed. Rather, the residual compressive strength decreases at higher slag contents. In the case of heat cured fly ash geopolymers, on the other hand, a different trend can be seen in , where the compressive strength is increased at 400°C and decreased negligibly at 600°C, with an approximate 40% reduction at 800°C. shows that fly ash-slag blended geopolymers lost between 60 and 70% of their ambient temperature compressive strength at 800°C. This compares to compressive strength loss of about 22–68% at 600°C. At a temperature of 400°C, however, the compressive strength loss is small at about 12–40%. The significantly higher loss of compressive strength at all temperatures is believed to be due to loss of absorbed and chemically bound water in fly ash-slag geopolymer gels as well as to decomposition of calcium-based reaction products in the geopolymer gels at such high temperatures, which is evident in the TGA results discussed earlier which showed mass loss of fly ash-slag geopolymers at all temperatures to be higher than that of fly ash geopolymers. On the other hand, due to the absence of calcium-based reaction products in fly ash geopolymer gels, the moisture loss and hence, mass loss is lower than for fly ash-slag geopolymers as can be seen in . In addition, due to lower moisture loss, the contraction of fly ash geopolymers is also stable from 200°C to about 600°C as observed in a dilatometry test conducted by Shaikh and Hosan [Citation11]. In the case of fly ash-slag geopolymers, the higher loss of compressive strength at temperatures of 600 and 800°C can be attributed to the higher mass loss at these temperatures than at 400°C and to the higher number of pores observed in the SEM images in fly ash-slag geopolymers at these temperatures than at 400°C, which were discussed above.
Conclusions
In this study, the effects of five slag contents ranging from 5–30% by weight as partial replacement for fly ash in fly ash-slag blended ambient air-cured geopolymers are investigated and their effects on residual compressive strength, physical behaviour and microstructural changes are evaluated and compared with those of heat-cured fly ash geopolymers. The following conclusions can be summarised from the results:
All geopolymers show reductions in ambient temperature compressive strength after exposure to elevated temperatures. The reductions are much higher at 600 and 800°C than at 400°C. The residual compressive strength of ambient air-cured geopolymers increases with increases in slag contents at 400 and 600°C. No such trend is observed at 800°C, where compressive strength loss of about 60–70% is observed.
Mass loss of ambient air-cured geopolymers increases with increases in slag contents at all elevated temperatures. No such trend is observed in the case of volumetric shrinkage.
No significant cracking is observed in ambient air-cured geopolymers at 400 or 600°C. Significant cracking is observed at 800°C especially for 15–30% slag contents presumably due to dissociation of a larger amount of calcium bound product calcite.
SEM images show increases in the density of geopolymer microstructures with increases in slag contents both before and after exposure to elevated temperatures.
A new “calcite/CSH” crystalline peak appears at 2θ angles of 29.4° and 50.17° with increases in slag contents and the height of the calcite peak also increases indicating the formation of more calcium-bearing reaction compounds in the C-(N)-A-S-H gels. After exposure to elevated temperatures, the calcite phase observed in geopolymers containing slags disappears indicating that this calcium compound decomposes at those elevated temperatures, as is also evidenced in the higher number of micropores in the SEM images of fly ash-slag blended geopolymers at 800°C. Two additional crystalline phases, nepheline (Na4Al4Si4O16) and gehlenite (Ca2Al2SiO7), are also observed in all geopolymers containing slag after exposure to a temperature of 800°C.
The rate of mass loss in fly ash-slag blended geopolymers is much higher than that in heat-cured fly ash geopolymers up to 200°C. No mass loss peak is observed in the region of 450°C a result confirming the absence of Portlandite (Ca(OH)2) in the geopolymer gels in fly ash-slag geopolymers. The higher rate of mass loss in fly ash-slag blended geopolymers than in fly ash geopolymers can be attributed to decomposition of calcite formed in C-(N)-A-S-H gels.
The overall pore volume of slag-fly ash blended geopolymers is lower than that of fly ash geopolymers. Geopolymers containing 30% slag exhibited the lowest pore volume among those examined.
Acknowledgments
The author thanks final-year project student Mr. Suneeth Fernandez for his help in casting and testing samples in this study.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Turner LK, Collins FG. Carbon dioxide equivalent (CO2e) emissions: A comparison between geopolymer and OPC cement concrete. Constr Build Mater. 2013;43:125–130.
- Manjunathan GS, Vanugopal RK, Maruthi SV. Strength characteristics of open air-cured geopolymer concrete. Trans Indian ceram soc. 2014;73(2):149–156.
- Nath P, Sarker PK. Flexural strength and elastic modulus of ambient cured blended low-calcium fly ash geopolymer concrete. Constr Build Mater. 2017;130:22–31.
- Hadi MNS, Farhan NA, Sheikh MN. Design of geopolymer concrete with GGBFS at ambient curing condition using Taguchi method. Constr Build Mater. 2017;140:412–431.
- Khan MZN, Shaikh FUA, Hao Y, et al. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr Build Mater. 2016;125:809–820.
- Guerrieri M, Sanjayan JG. Behaviour of combined fly ash-slag based geopolymers when exposed to high temperatures. Fire Mater. 2010;34:163–175.
- Zhao R, Sanjayan J. Geopolymer and portland cement concretes in simulated fire. Mag Concr Res. 2011;63(3):163–173.
- Kong DLY, Sanjayan JG. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem Concr Res. 2010;40(2):334–339.
- Pan Z, Sanjayan J. An investigation of the mechanisms for strength gain or loss of geopolymer mortar after exposure to elevated temperatures. J Mater Sci. 2009;44:1873–1880.
- Shaikh FUA, Vimonsatit V. Compressive strength of fly ash based geopolymer concrete at elevated temperatures. Fire Mater. 2015;39(2):174–188.
- Shaikh FUA, Hosan A. Mechanical properties of steel fibre reinforced geopolymer concretes at elevated temperatures. Construct Build Mater. 2016;114:15‐28.
- Hosan A, Haque S, Shaikh F. Compressive behaviour of sodium and potassium activators synthetised fly ash geopolymer at elevated temperatures: a comparative study. J Build Eng. 2016;8:123–130.
- Rickard WDA, Temuujin J, van-Riessen A. Thermal analysis of geopolymer pastes synthesized from five fly ashes of variable compositions. J Non-Cryst Solids. 2014;358(2012):1830–1839.
- Ren W, Xu J, Bai E. Strength and ultrasonic characteristics of alkali activated fly ash – slag geopolymer concrete after exposure to elevated temperatures. J mater civ eng. 2016;28(2):04015124.
- Kashani A, Ngo TD, Walkley B, et al. Thermal performance of calcium-rich alkali activated materials: A microstructural and mechanical study. Constr Build Mater. 2017;153:225–237.
- Vasquez-Molina D, Mejia-Arcila JM, Gutierrez RM. Mechanical and thermal performance of a geopolymer and hybrid material based on fly ash. DYNA. 2016;83(195):216–223.
- Cheng-Young H, Yun-Ming L, Abdullah MMA, et al. Thermal resistance variations of fly ash geopolymers: foaming responses. Sci Rep. 2017. DOI:10.1038/srep45355
- Ranjbar N, Mehrali M, Alengaram UJ, et al. Compressive strength and microstructure analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr Build Mater. 2014;65:114–121.
- Haque S, Shaikh FUA. Effect of nano silica and fine sand on the compressive strength of sodium and potassium synthetized fly ash geopolymers at elevated temperatures. Fire Mater. 2017. DOI:10.1002/fam.2496
- AS 1012.9. Methods of testing concrete – compressive strength tests – concrete, mortar and grout specimens. Standards Australia; 2014.
- Washburn EW. Note on a method of determining the distribution of pore sizes in a porous material. Proc Natl Acad Sci USA. 1921;7(4):115–116.
- Tc RILEM. 129-MHT, Test methods for mechanical properties of concrete at high temperatures - Compressive strength for service and accident conditions. Mater Struct. 1995;28(1995):410–414.
- Rashad AM, Sadek DM. An investigation on Portland cement replaced by high-volume GGBS pastes modified with micro-sized metakaolin subjected to elevated temperatures. Int J Sustainable Built Environ. 2017;6:91–101.
- de Souza LMS, Fairbairn EDMR, Filho RDT, et al. Influence of initial CaO/SiO2 ratio on the hydration of rice husk ash-Ca(OH)2 and sugar cane bagasse ASH-Ca(OH)2 pastes. Quim. Nova. 2014;37(10):1600–1605.
- Abdulkareem OA, Al-Bakri AMM, Kamarudin H, et al. Effects of elevated temperatures on the thermal behaviour and mechanical performance of fly ash geopolymer paste, mortar and light weight concrete. Constr Build Mater. 2014;50:337–387.


![Figure 16. Compressive strength of ambient air-cured geopolymers containing various slag contents before and after exposure to elevated temperatures.[Note: S0 represents 100% fly ash geopolymers].](/cms/asset/128d05e6-2233-453b-84ae-abc292049230/tace_a_1529013_f0016_oc.jpg)
![Figure 17. Percentage loss of compressive strength of ambient air-cured geopolymers containing various slag contents after exposure to elevated temperatures.[Note: S0 represents 100% fly ash geopolymers].](/cms/asset/cd351e19-d594-4451-ac2c-3410cf39afcf/tace_a_1529013_f0017_b.gif)