?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The oxygen reduction reaction (ORR) activity and cation valences of Mn-based Ni–Mn–Fe and Mg–Mn layered double hydroxides (LDHs) with various compositions were studied. The valences of the 3D transition metals (Mn, Fe, and Ni) were examined by X-ray absorption. The average valence of Ni was approximately 2, and that of Mn was approximately 3 in the LDHs investigated in this study. The valence of Fe decreased with increases in the Mn/Fe ratio. Increasing the amount of Mn enhanced the ORR activity, while the valences and surface areas of the LDHs had no significant effect on the activity.
1. Introduction
Alkaline fuel cells [Citation1,Citation2] and metal air batteries [Citation3,Citation4] have attracted significant attention as next-generation power sources. The oxygen reduction reaction (ORR) is an important electrochemical reaction occurring in the air electrodes of fuel cells and metal-air batteries. Pt and Pt-based nanoparticles can act as catalysts for ORRs, but these nanoparticles is very expensive. Development of a catalyst using 3D transition metals with small overpotential is consequentially essential. Manganese compounds such as α-MnO2 [Citation5], LaMnO3 [Citation6], and Mn (O, N) [Citation7] have been reported as Pt-free ORR catalysts in alkaline solutions. The correlation between electronic structures and ORR activity has been studied extensively, and a single electron in the antibonding state achieved by trivalent manganese in octahedral coordination, was determined to be favorable for high ORR activity [Citation6,Citation7].
Layered double hydroxides (LDHs) have the general formula [M2+1-xM3+x(OH)2]+[An−x/n yH2O], where M2+ is divalent cations (Mg2+, Mn2+, Co2+, Ni2+, or Zn2+), M3+ is trivalent cations (Al3+, Cr3+, Fe3+, or Co3+), and An− is anions such as CO32− and Cl−. The conductivity of hydroxide ions [Citation8–Citation11] and a large specific surface area [Citation12,Citation13] are favorable factors for ORR in alkaline solutions. In addition, multiple metal ions occupying octahedral sites afford flexibility in their electronic structure. Thus, LDHs are interesting motifs for investigating the correlation between electronic structures and ORR activity.
The valence of transition metals in LDHs is mostly investigated by X-ray photoelectron spectroscopy (XPS) with Al or Mg X-ray sources under high-vacuum conditions [Citation14]. Nonetheless, valence assignment in the bulk state is difficult because XPS is a surface-sensitive analysis and the spectra must be interpreted using overlapping signals with slightly different energies (typically <1–3 eV). XPS analysis of the surface relies on irradiated X-rays; moreover, since LDHs have low electronic conductivity. X-ray absorption spectroscopy is a powerful technique for examining the valence of metals in bulk states regardless of their electronic conductivity. This technique has sufficient energy resolution to estimate the valence and has been utilized to examine the correlation between electronic structures and catalytic activity in oxides and oxynitrides [Citation6,Citation7,Citation15].
Reports regarding ORR and oxygen evolution reaction (OER) catalysis by LDH have recently drawn attention. Since LDHs have low electron conductivity, their combination with an electronic conductor, typically carbon, is essential. The addition of Ni–Mn–Fe LDH to air electrodes enhanced the current generated by the ORR activity on Pt nanoparticles in Vulcan® carbon [Citation16]. LDHs mixed with reduced graphene oxides (rGO) and multiwall carbon nanotubes (MWCNTs) such as Ni–Mn LDH/rGO [Citation17], Ni–Mn LDH/MWCNTs, and Co–Mn LDH/MWCNTs [Citation18] have shown excellent OER activity. Co–Mn–Ni LDH with rGO [Citation19] and Ni–Fe–Mn LDH with carbon black [Citation20] have been used as ORR catalysts. However, the influence of the Mn content and electronic state of LDHs on ORR activity has not yet been investigated.
In this study, we investigated the valences of the transition metals and ORR activity in Ni–Mn–Fe and Mg–Mn LDHs. We found that the average valence of Mn was approximately 3 in all LDHs investigated in the study. Their ORR catalytic activity was independent of the Mn valence but was enhanced by increasing the Mn ratio.
2. Experimental
2.1. Chemicals
The following chemicals were used without further purification: Mg(NO3)2 6H2O (Wako Pure Chemical, 99.0%), Ni(NO3)2 6HO (Wako Pure Chemical, 98.0%), Fe(NO3)3 9H2O (Wako Pure Chemical, 99.9%), Mn(NO3)2 6H2O (Sigma-Aldrich, 98.0%), NaOH (Kanto Chemical, 97.0%), and Na2CO3 (Wako Pure Chemical, 99.8%).
2.2. Preparation
Ni–Mn–Fe LDH and Mg–Mn LDH were synthesized using the co-precipitation method. An aqueous solution with 0.05 M metal nitrates was dropped into a Na2CO3 solution under a nitrogen or ambient atmosphere to obtain precipitates. During the synthesis, NaOH solution was added dropwise to maintain the pH at 10. Thereafter, the mixed solution was kept at room temperature for 1 h for the Ni–Mn–Fe LDH sample and 18 h for the Ni–Fe LDH and Mg–Mn LDH samples. The precipitates were subsequently filtered, washed with distilled water, and dried under vacuum at room temperature for 18 h.
2.3. Characterization
X-ray diffraction (XRD) patterns were measured using an X-ray diffractometer (Rigaku, MiniFlex 600) with CuKα radiation (λ = 0.15418 nm). The morphology of the samples was observed with a field emission scanning electron microscope (FE-SEM, JEOL, JSM-6500F). The nitrogen adsorption isotherms were measured after the samples were dried at 80°C for one day. The composition of metals in the synthesized LDHs was determined by inductively coupled plasma (ICP, SHIMADZU ICPE-9000). The X-ray absorption spectra were measured in the transmission mode at BL5S1 and BL6N1 at the Aichi Synchrotron Radiation Center (Proposal number 201606008 and 201704041) to evaluate the valences of the transition metals in the LDHs.
2.4. Electrochemical measurement
A catalyst suspension was prepared by mixing 2.0 mg of LDH, 2.0 mg of Vulcan® (Cabot Corporation), 60 μL of 5.0 wt.% 1-propanol solution of anion exchange resin (Tokuyama), and 540 μL of C2H5OH (Kanto Chemical, 95.5%). After the suspension was sonicated for 30 minutes, thin-film electrodes on glassy carbon disks (4 mm in diameter) were prepared by five-times repeated pipetting and drying of 1.5 µL of the suspension. The above procedure yielded a catalyst density loading of 200 μg LDH/cm2 disk.
Electrochemical measurements were performed using a three-electrode setup with a rotating disc electrode (RDE) device (BAS, RRDE-3A). A Hg/HgO electrode was used for the reference electrode, a Pt electrode was used for the counter-electrode, and an RDE was used for the working electrode. The reference potential was converted to reference the reversible hydrogen electrode (RHE) by applying the Nernst equation [Citation7] as shown in Equation (1):
1 M KOH solution was used as the electrolyte for the electrochemical measurements. Simultaneously with the application of the catalyst ink, oxygen gas was flowed into the electrolytic solution to induce an oxygen-saturated state. Subsequently, linear sweep voltammetry was performed at a scan rate of 5 mV s−1.
The number of reaction electrons (n), a key parameter of ORR activity, was calculated based on the Koutecky–Levich (KL) equations [Citation21] as shown in Equation (2):
B = 0.62 ・n・F・C0・D02/3・v−1/6,
where J (mA cm−2) is the measured current density; JK and JL (mA cm−2) are the kinetic- and diffusion-limiting current densities, respectively; ω is the angular velocity (ω = 2πf, where f is rotation rate (rpm)); F is the Faraday constant (96,485 C mol−1); D0 is the diffusion coefficient (1.80 × 10−5 cm2 s−1); v is the kinematic viscosity of the electrolyte (1.0 × 10−2 cm2 s−1); and C0 is the bulk concentration of O2 (7.8 × 10−7 mol cm−3) [Citation22].
3. Results
3.1. Structural characterization of LDHs
shows the XRD patterns of the LDHs. Since LDH peaks were observed in all the compositions, it was confirmed that LDHs were formed as the main phase in the samples. In Ni–Mn–Fe LDH, the diffraction peak 012 shifted to a lower angle as the Mn ratio increased, presumably due to a reduction in Fe ions, as described later. Weak peaks corresponding to Mn3O4 were also confirmed at x = 0.80. The stabilities of LDHs in 1.0 M KOH solution for 24 h were examined by XRD. While the XRD patterns (Figure S1 in the supporting information) of LDHs with x = 0–0.60 showed the peaks assigned as LDHs, Mn3O4 was formed in the LDH with x = 0.80. shows the compositions, lattice parameters, and specific surface areas of the tested LDHs. The chemical compositions of LDHs determined by ICP were nearly identical to the ratio of metal nitrates in the starting materials. In Ni–Mn–Fe LDH, the lattice parameters of the a-axis increased with increases in the Mn ratio, and those of the c-axis showed no clear trend. The surface areas of the samples ranged between 30 and 140 m2 g−1, and the lattice parameters of Mg0.67–Mn0.33 LDH were similar to the reported values [Citation23].
Table 1. Structural parameters of LDHs.
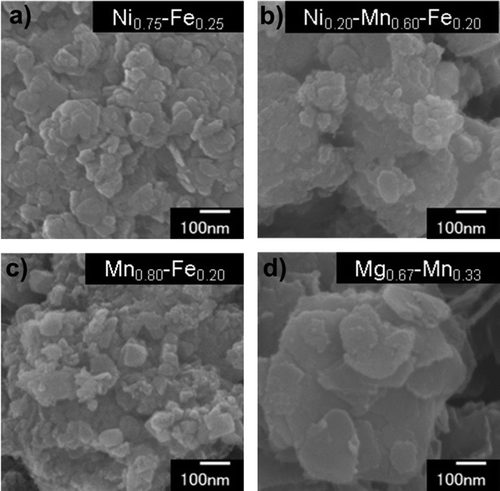
Representative SEM images of the LDHs are shown in . Characteristic plate-like structures were observed in every sample, with particle sizes ranging from 50 to 500 nm.
Figure 2. SEM images of (a) Ni0.75–Fe0.25 LDH, (b) Ni0.20–Mn0.60–Fe0.20 LDH, (c) Mn0.80–Fe0.20 LDH, and (d) Mg0.67–Mn0.33 LDH.
shows the X-ray absorption spectra of the LDHs for purposes of qualitative discussion concerning the valences of transition metals. We discuss the valence of transition metals by using references; the absorption edges in these references are similar to their positions in past references [Citation24–Citation29]. Based on a comparison of the absorption spectra of Ni–Mn–Fe LDH and the standard samples, the average valence of Ni was estimated to be approximately 2 and that of Mn to be approximately 3. The absorption edge of Fe shifted toward a lower energy with increases in the Mn ratio, indicating that the valence of Fe decreased. For samples with x = 0–0.4, the energy of the absorption edges was higher than Fe2O3, indicating that the valence was slightly higher than 3. Conversely, for samples with x = 0.80, the valence was lower than 3. In Mg0.67–Mn0.33 LDH, the average valence of Mn was almost 3, as estimated by the energy of the normalized absorption intensity of 0.5. This was consistent with the estimation in a previous report regarding Mg–Mn LDH [Citation23,Citation30].
Figure 3. X-ray absorption spectra of the (a) Ni–K edge, (b) Mn–K edge, and (c) Fe–K edge in Ni–Mn–Fe LDH and the (d) Mn–K edge in Mg0.67–Mn0.33 LDH. The spectrum of FeO is adopted from a report [Citation25] after adjusting the energy shift using the spectrum of Fe2O3.
![Figure 3. X-ray absorption spectra of the (a) Ni–K edge, (b) Mn–K edge, and (c) Fe–K edge in Ni–Mn–Fe LDH and the (d) Mn–K edge in Mg0.67–Mn0.33 LDH. The spectrum of FeO is adopted from a report [Citation25] after adjusting the energy shift using the spectrum of Fe2O3.](/cms/asset/8991ac08-6e39-4d8f-b522-dc33d04b88cf/tace_a_1581321_f0003_oc.jpg)
3.2. ORR measurement
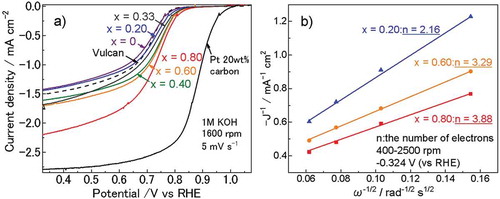
) shows the linear sweep voltammetry results where Ni0.75–Fe0.25 LDH showed ORR activity as low as that for Vulcan® alone without LDH. The onset potential shifted in the direction of the noble metal potential, and the current density increased with increases in the Mn ratio. The Koutecky–Levich plots corresponding to a rotation rate of 400–2500 rpm and −0.324 V vs RHE were linear, and the number of reaction electrons was estimated to be 2.16 for Ni0.60–Mn0.20–Fe0.20 LDH, 3.29 for Ni0.20–Mn0.60–Fe0.20 LDH, and 3.88 for Mn0.80–Fe0.20 LDH ()). Current density therefore increased in LDHs with a high Mn content, the since the main reduction process switched from a two-electron reaction to a four-electron reaction. The chronopotentiometry curves of Ni0.20–Mn0.60–Fe0.20 LDH at a constant current density of −1.0 mA/cm−2 showed a slight decrease in potential for 4 h, indicating moderate catalytic stability (Figure S2). However, further investigation of the stability of the crystal structures and valences of LDHs will be necessary.
Figure 4. (a) Linear-sweep polarization curves of typical LDHs measured with a rotating disc electrode in an O2-saturated 1 M KOH solution at a scan rate of 5 mV s−1. The polarization curves of Pt/C and Vulcan® are shown for comparison. (b) Koutecky–Levich plots at −0.324 V (vs. RHE) and 400–2500 rpm.
4. Discussion
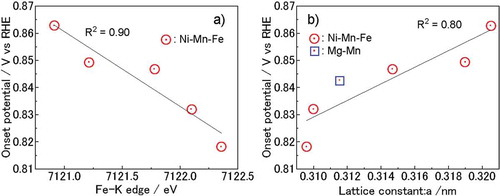
We examined the cation valences and ORR activities of various LDHs with different Mn ratios. The overpotential of the ORRs decreased with increases in the Mn ratio ()). Assuming that the active site of an ORR is a hydroxide ion where Mn is present in the nearest metal site, the number of active sites should increase with increases in the specific surface area, resulting in higher activity. The influence of the Mn ratio and specific surface area on the onset potential was therefore examined in detail. In , the onset potentials (vs RHE) of the LDHs are plotted as a function of the ratio of Mn to total cations, and specific surface area (, respectively). A strong positive correlation between the Mn ratio and onset potential ()) can be observed. On the other hand, there is no clear correlation between the specific surface area and the onset potential ()). Because the actual Mn active site concentration can be expressed as the Mn ratio multiplied by the surface area, the onset potential was also plotted as a function of the product of the Mn ratio and specific surface area ()). The correlation between the onset potential and the product of the Mn ratio and specific surface area is not as strong as the Mn ratio. This indicates that not all Mn on the surfaces of LDHs are highly active sites. The specific configurations of Mn, such as the edges, defects, and interfaces with carbon, likely play an important role in their catalytic activity. Further investigation is necessary.
Figure 5. (a) Onset potential of LDHs plotted as a function of the ratio of Mn to total cations, (b) specific surface area, and (c) product of the Mn ratio and specific surface area.
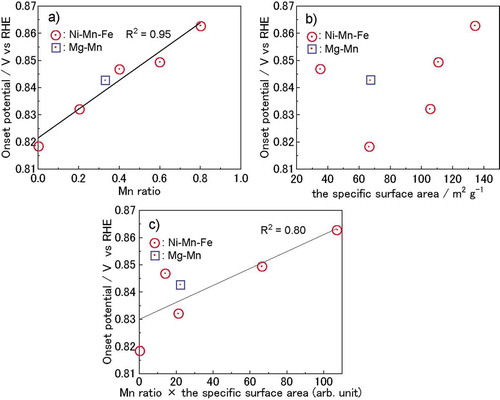
Based on the X-ray absorption spectra in ,), the valences of Mn3+ and Ni2+ ions were estimated to be constant. This indicated that the catalytic capabilities and electronic structures of Mn and Ni were unrelated to each other. On the other hand, because the valence of Fe varied with the Mn ratio, the onset potential was plotted as a function of the Fe–K edge in ). The Fe–K edge is related to the average valence of Fe and shows a strong correlation with the onset potential. With increases in the Mn ratio, the lattice parameter of the a-axis also increased, as shown in . The a-axis parameter is related to the distance between transition metal ions, and the correlation between the onset potential and a-axis parameter is shown in ). The lattice parameter of the a-axis and the onset potentials exhibit a weak correlation, which is lower than the correlation with the Mn ratio. The Mn ratio also affects the lattice parameters and Fe valence. We can therefore conclude that the Fe valence and lattice parameter of the a-axis are not essential to ORR activity, and that the Mn ratio is the most important factor governing ORR activity. In LDHs with an Mn-rich composition (x = 0.80), however, Mn3O4 was formed. Further examination of their electrochemical stability will be necessary, since our results do not guarantee stability during and after the electrochemical reaction.
5. Conclusion
This study demonstrated that increasing the Mn ratio in LDH is an important factor for improving the ORR activity of the catalysts. The average valence of Ni was approximately 2, and that of Mn approximately 3 in Ni–Mn–Fe LDH. The average valence of Fe decreased with increases in the Mn ratio, and the average valence of Mn in Mg0.67–Mn0.33 LDH was 3. The correlation between the onset potential of the ORR and various parameters indicated that a high Mn ratio was important for reducing the overpotential of the ORR.
Supplemental Material
Download PDF (195.8 KB)Acknowledgments
This research was supported part in through EIG CONCERT-Japan 4th Call under the Strategic International Collaborative Research Program (SICORP) of the Japan Science and Technology Agency (JST).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Gülzow E. Alkaline fuel cells. Fuel Cells. 2004;4(4):251–255.
- Lin BYS, Kirk DW, Thorpe SJ. Performance of alkaline fuel cells: a possible future energy system? J Power Sources. 2006;161(1):474–483.
- Cheng F, Chen J. Metal–air batteries: from oxygen reduction electrochemistry to cathode catalysts. Chem Soc Rev. 2012;41(6):2172–2192.
- Miyazaki K, Nishio K, Abe T, et al. Effects of addition of layered double hydroxide to air electrodes for metal-air batteries. Electrochem. 2012;80(10):728–730.
- Meng Y, Song W, Huang H, et al. Structure−property relationship of bifunctional MnO2 nanostructures: highly efficient, ultra-stable electrochemical water oxidation and oxygen reduction reaction catalysts identified in alkaline media. J Am Chem Soc. 2014;136(32):11452–11464.
- Suntivich J, Gasteiger HA, Yabuuchi N, et al. Design principles for oxygen- reduction activity on perovskite oxide catalysts for fuel cells and metal–air batteries. Nat Chem. 2011;3:546–550.
- Miura A, Navarro CR, Masubuchi Y, et al. Nitrogen-rich manganese oxynitrides with enhanced catalytic activity in the oxygen reduction reaction. Angew Chem Int Edit. 2016;55(28):7963–7967.
- Tadanaga K, Furukawa Y, Hayash A, et al. Direct ethanol fuel cell using hydrotalcite clay as a hydroxide ion conductive electrolyte. Adv Mater. 2010;22(39):4401–4404.
- Furukawa Y, Tadanaga K, Hayashi A, et al. Evaluation of ionic conductivity for Mg–Al layered double hydroxide intercalated with inorganic anions. Solid State Ion. 2011;192(1):185–187.
- Tadanaga K, Furukawa Y, Hayashi A, et al. Effect of Mg/Al ratio on hydroxide ion conductivity for Mg–Al layered double hydroxide and application to direct ethanol fuel cells. J Electrochem Soc. 2012;159(4):B368–B370.
- Kubo D, Tadanaga K, Hayashi A, et al. Hydroxide ion conduction in Ni–Al layered double hydroxide. J Electroanal Chem. 2012;671:102–105.
- Chen C, Wangriya A, Buffeta JC, et al. Tuneable ultra high specific surface area Mg/Al-CO3 layered double hydroxides. Dalton T. 2015;44(37):16392–16398.
- Ezeh CI, Tomatis M, Yang X, et al. Ultrasonic and hydrothermal mediated synthesis routes for functionalized Mg-Al LDH: comparison study on surface morphology, basic site strength, cyclic sorption efficiency and effectiveness. Ultrason Sonochem. 2018;40:341–352.
- Qian L, Lu Z, Xu T, et al. Trinary layered double hydroxides as high performance bifunctional materials for oxygen electrocatalysis. Adv Energy Mater. 2015;5(13):1550245.
- Miura A. Low-temperature synthesis and rational design of nitrides and oxynitrides for novel functional material development. J Ceram Soc Jpn. 2017;125(7):552–558.
- Tadanaga K, Igarashi K, Kubota T, et al. Development of alkaline fuel cells using hydroxide-ion conductive layered double hydroxides. ECS Trans. 2015;69(17):385–389.
- Ma W, Ma R, Wu J, et al. Development of efficient electrocatalysts via molecular hybridization of NiMn layered double hydroxide nanosheets and graphene. Nanoscale. 2016;8(19):10425–10432.
- Jia G, Hu Y, Qian Q, et al. Formation of hierarchical structure composed of (Co/Ni)Mn-LDH nanosheets on mwcnt backbones for efficient electrocatalytic water oxidation. ACS Appl Mater Inter. 2016;8(23):14527–14534.
- Jia X, Gao S, Liu T, et al. Fabrication and bifunctional electrocatalytic performance of ternary CoNiMn layered double hydroxides/polypyrrole/reduced graphene oxide composite for oxygen reduction and evolution reactions. Electrochim Acta. 2017;245(10):59–68.
- Lu Z, Qian L, Tian Y, et al. Ternary NiFeMn layered double hydroxides as highly-efficient oxygen evolution catalysts. Chem Commun. 2016;52(5):908–911.
- Brocato S, Lau C, Atanassov P. Mechanistic study of direct electron transfer in bilirubin oxidase. Electrochim Acta. 2012;61:44–49.
- Qiao J, Xu L, Ding L, et al. Effect of KOH concentration on the oxygen reduction kinetics catalyzed by heat-treated co-pyridine/C electrocatalysts. Int J of Electrochem Sci. 2013;8(1):1189–1208.
- Fernandez JM, Barriga C, Ulibarri MA, et al. Preparation and thermal stability of manganese-containing hydrotalcite, [Mg0.75MnⅡ0.04MnⅢ0.21 (OH)2] (CO3)0.11・nH2O. J Mater Chem. 1994;4(7):1117–1121.
- Wang Y, Zhang Y, Yu F, et al. Correlation investigation on the visible-light- driven photocatalytic activity and coordination structure of rutile Sn-Fe-TiO2 nanocrystallites for methylene blue degradation. Catal Today. 2015;258:112–119.
- Yang D, An Y, Wang S, et al. Evidence of the oxygen vacancies-induced roomtemperature ferromagnetism in the (In0.97-xFexSn0.03)2O3 films. RSC Adv. 2014;4(64):33680–33686.
- Fu L, Shozugawa K, Matsuo M. Oxidation of antimony (III) in soil by manganese (IV) oxideusing X-ray absorption fine structure. J Environ Sci. 2018;73:31–37.
- Liang X, Zhong Y, Zhu S, et al. The valence and site occupancy of substituting metals in magnetite spinel structure Fe3−xMxO4 (M = Cr, Mn, Co and Ni) and their influence on thermal stability: an XANES and TG-DSC investigation. Solid State Sci. 2013;15:115–122.
- Rutirawut T, Limphirat W, Sinsarp A, et al. Composition and oxidation state of cobalt- and nickel-iron oxide colloidal nanoparticles in liquid phase. Adv Mat Res. 2015;1103:21–27.
- Ravel B. Muffin-tin potentials in EXAFS analysis. J Synchrotron Radiat. 2015;22(5):1258–1262.
- Tezuka S, Chitrakar R, Sakane K, et al. The synthesis and phosphate adsorptive properties of Mg(II)–Mn(III) layered double hydroxides and their heat-treated materials. B Chem Soc Jpn. 2004;77(11):2101–2107.

![Figure 1. Representative X-ray diffraction pattern of LDHs. [×] indicates the Mn3O4 phase.](/cms/asset/4950ce74-d9c7-4b5f-9ed7-75c5eb64cf64/tace_a_1581321_f0001_oc.jpg)