?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Nanobioceramic hydroxyapatite (HAp) was synthesized from golden apple snail (Pomacea canaliculata) shell and used to fabricate HAp-based scaffolds with polyvinyl alcohol (PVA), polyethylene oxide (PEO), or polyvinylpyrrolidone (PVP) as the porogen agent. The porogen was removed during the HAp sintering process. The SEM results showed increased agglomeration of HAp particles with increases in concentration of polymeric porogen used in the scaffold fabrication process. The XRD pattern showed the scaffolds to be HAp/β-TCP composites. Thermal analysis of the porogen weight loss at a sintering rate of 32°C/min showed that all the porogen had leached out before the sample attained the maximum sintering temperature. Fourier transform infrared (FTIR) spectra confirmed the absence of porogen functional groups within the scaffold after sintering. The weight concentration of the porogen plays an important role in establishing the homogeneity and interconnectivity of the pores, as well as the mechanical strength of the scaffold.
1. Introduction
Bone is a natural composite consisting of tissues that are capable of regeneration. Damage to bone tissue can be resolved by implantation of prosthetic implant materials such as metals, but metal alone cannot promote the formation of new bone tissues. In addition, the installation of metal bone implants in medical applications is still constrained by the failure of installations due to such material physics problems as the metal wear rate, metal impurities, insufficient integration of bones with implants, and corrosion of the materials over time [Citation1]. Bioceramic materials, such as hydroxyapatite (HAp) from the calcium phosphate family of minerals, are new alternative materials for orthopedic applications. They have many advantages over conventional metal materials because they support the ability of bone tissue to regenerate. The calcium phosphate composition of HAp gives it excellent material properties in terms of biocompatibility and the quality of its integration with bone [Citation2].
HAp has become the preferred material for bone tissue engineering applications due to its compositional similarities to human bone tissue, which give it good biocompatibility and the ability to form bonds with bone tissue [Citation3]. HAp is also the most stable phase of Ca/P crystals. Since most of the mineral fraction in human bone tissue has the HAp structure, HAp can be effective in reconstructing human bone tissue.
HAp can be synthesized from biogenic materials containing large amounts of calcium carbonate by combining the sources of calcium and phosphate. One biogenic material that is high in calcium carbonate is the shell of the golden apple snail (Pomacea canaliculata). Because this material is plentiful and underutilized, it has significant potential for use in the synthesis of bioceramic-based HAp for bone tissue reconstruction [Citation4,Citation5].
The fabrication of scaffolds to support bone growth and vascularization must take into account the porosity of the scaffold material, since the bioactivity, and therefore the effectiveness, of an engineered scaffold is influenced by its porosity and the interconnections between pores [Citation6].
Pores can be created in HAp by infiltration of a porogen, so the focus of the present study was on the use of polymers as porogenic agents in HAp derived from golden apple snail shells. The polymers of interest, which included polyvinyl alcohol (PVA), polyethylene oxide (PEO), and polyvinylpyrrolidone (PVP), are nontoxic, so they will not harm the implant environment. The polymers have low melting point compared to the desired HAp densification temperature, would result in evaporation of the polymeric porogen during the HAp sintering process, thereby leaving pores in the final HAp scaffold. The differences in the characteristic of polymeric porogens may affect the characteristics of scaffold.
2. Methods and procedures
The fabrication is divided into three main stages: raw material preparation, synthesis of powder HAp, and fabrication of porous HAp scaffold. This study characterized the molecular functional groups, crystallographic properties, morphology and pore size, elements contained in material, microhardness, and thermal degradation during the sintering process.
2.1. Raw material preparation
The golden apple snail shells were cleaned, dried, and milled, and the milled product was characterized using scanning electron microscope—electron dispersive X-ray (SEM-EDX) and Fourier transform infrared (FTIR).
2.2. Synthesis of powder HAp
The milled shell powder was then calcined in a furnace at 1000°C for 6 h, at a rate of temperature increase of 32°C/min, to obtain CaO powder. The chemical reaction of the calcination process is shown by Eq. (1).
Calcium oxide (CaOH2; 5 M) was then prepared by the reaction shown in Eq. (2):
A 3 M H3PO4 solution was added dropwise (1 mL/min flow rate) into the 5 M Ca(OH)2 suspension, with stirring at 60°C, for 2 h. The pH was maintained at pH 10 using 25% ammonium hydroxide (NH4OH). The mixture was then left to settle at room temperature for 24 h. The synthesis reaction is shown by Eq. (3). The mixture was then sintered at 1000°C for 6 h.
The resulting bioceramic HAp was characterized by EDX to determine the elements contained in the material. Additional XRD characterization was conducted to establish the crystallographic properties of the HAp.
2.3. Fabrication of porous HAp scaffolds
HAp meeting certain molar ratio and crystallographic property criteria was then used to fabricate of porous HAp-based scaffolds. In this study, PVA (MW 145,000, Merck), PEO (MW 400,000, Aldrich), and PVP (MW 10,000, Aldrich) were used as porogens in applying the porogen leaching method to synthesize porous HAp-based scaffolds derived from golden apple snail shells. Scaffolds with optimum porosity were produced by varying the porogen concentration (0%, 5%, 10%, and 15% by wt) to obtain different pore structures. The porogen was leached from the scaffolds while the HAp was molded in 18 mm porcelain molds, densified and recrystallized during the sintering process at 1000°C for 6 h (initial rate of temperature increases was 32°C/min).
The characterization tools used in this study were as follows: scanning electron microscope—energy dispersive X-ray spectroscope (SEM-EDX, Jeol JSM-6510LA); FTIR (ABB MB3000); X-ray diffractometer (XRD, Rigaku Miniflex 600); microhardness tester (Matsuzawa MXT70); and differential thermal/thermogravimetric analyzer (DTA/TGA, Shimadzu DTG-60).
3. Results and discussion
3.1. Decomposition and characterization of golden apple snail shell raw material
The major element present in golden apple snail shell is calcium, with a mass percentage of 62.07 wt %. The presence of this large percentage of calcium suggested that this material had potential as a source of calcium precursors for HAp synthesis. The minor element in the shell is phosphorus, with a percentage of 0.48 wt %. Since the shell contains no dangerous trace elements such as heavy metals, it was determined to be a suitable precursor for synthesis of HAp for biomedical applications.
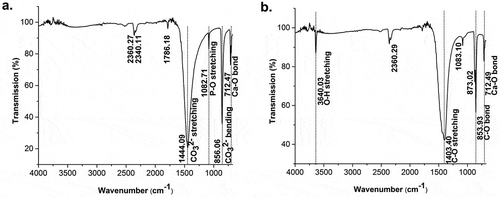
The shell’s molecular functional groups were identified using FTIR. The infrared spectra of the shell powder in shows a vibration mode characteristic of a carbonate functional group. The EDX result of the materials after decomposition at high temperature shows that the phosphate content in the CaO accounted for 0.41 wt %. Characterization by EDX confirmed that 95.69 wt % of the sample content was in the form of CaO and 0.94 wt % in the form of P2O5. Calcined samples were characterized by FTIR to ensure that they contained a functional CaO group. The infrared spectra in show absorbance bands at wavenumbers 1403.40 cm−1 and 853.93 cm−1, indicating the presence of CO vibrations, while the vibration modes at wavenumber 712.49 cm−1 could be identified as Ca-O bonds. The absorbance band at wavenumber 3640.03 cm−1 was identified as a stretching vibration of O-H. The carbonate ions were no longer visible in the infrared spectra.
Figure 1. FTIR spectra of: (a) raw materials of golden apple snail (Pomacea canaliculata) and (b) decomposed materials after calcination at high temperatures.
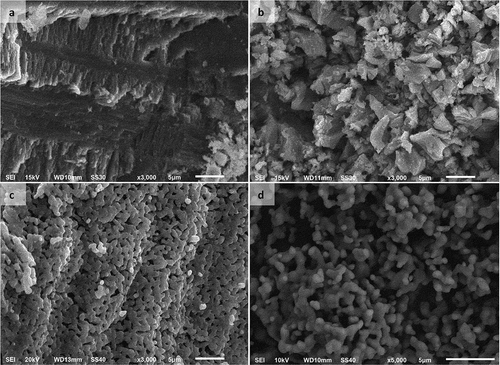
shows the morphological changes in the surface of HAp precursors to form HAp scaffold materials. Morphological characterization of HAp powder by SEM showed interconnection of the HAp particles due to sintering at high temperature. The particle shape of HAp, as seen through SEM scanning, was granular and interconnected.
3.2. Characterization of bioceramic HAp
The synthesized HAp had a molar Ca/P ratio of 1.67. A molar ratio of calcium phosphate close to 1.67 would further reduce the acidity and solubility of the calcium phosphate material [Citation2].
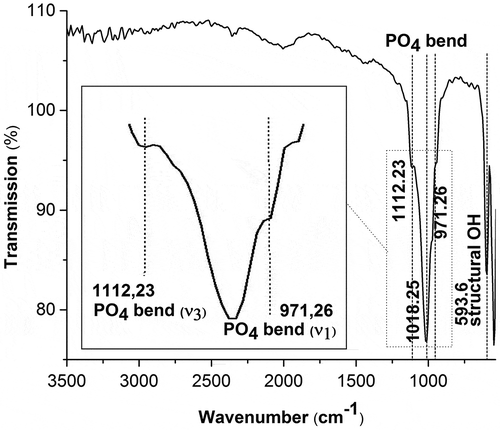
Infrared spectra were used to determine whether the synthesized material exhibited the characteristic spectra of HAp. The infrared spectrum depicted in shows the emergence of a transmittance band at a wavelength of 971.26 cm−1, indicating the occurrence of a bending vibration of PO43-(
1). The peak at wavenumber 1112.23 cm−1 was identified as a bending vibration of PO43- (
3). The peak at wavenumber 593.60 cm−1 was identified as an OH-structure. No carbonate ions were visible in the infrared spectrum.
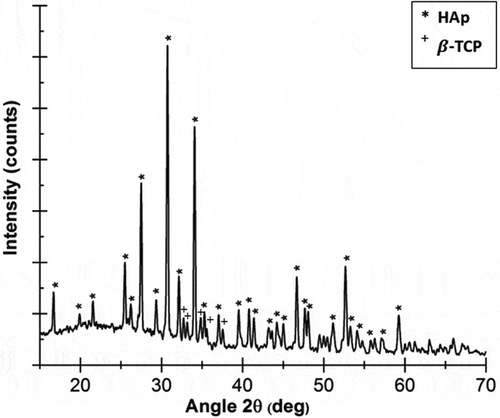
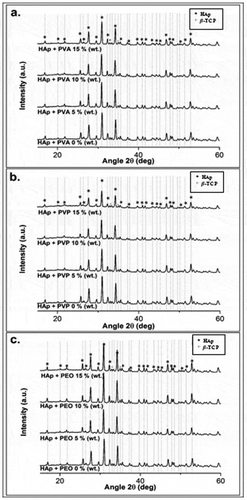
The XRD results depicted in shows a low-intensity β-tricalcium phosphate (TCP) peak in addition to HAp. Tricalcium phosphate (TCP) is itself a tertiary calcium phosphate with high biocompatibility that can promote improvements in bone tissue [Citation7,Citation8].
The crystallite size of the HAp particles was 49 ± 2 nm, with lattice parameters a = 0.954 nm and c = 0.698 nm. The lattice parameters approached those of HAp with a hexagonal crystal structure registering a lattice parameters of 0.942 nm and c lattice parameters of 0.688 nm [9]. The measured HAp particle density was 3.04 g/cm3, which is close to the theoretical HAp density of 3.12 g/cm3 [Citation10]. Fabrication of nanobioceramic HAp is an effective option for improving the mechanical properties of materials, where nanosized ceramics show better mechanical properties when compared to microsized bioceramics [Citation11].
3.3. Characterization of HAp-based scaffolds
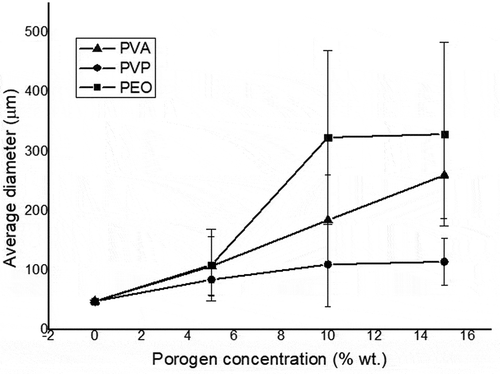
The HAp particles in scaffolds fabricated without a porogen had a tightly packed, interconnected particle structure that formed a solid surface and variations in particle size below 3 μm. The addition of a porogen to the mixture slowed down the particle densification process and caused a less complete interconnection of the particles as the porogen concentration was increased in the mixture. shows the general trend toward increasing pore size with increases in the porogen concentration. The trend shown is for identical weight percentages of porogen, with average pore size increases in the order of PEO > PVP > PVA.
Figure 5. Increases in the pore size of HAp scaffolds as the porogen (PVA, PVP, or PEO) concentration is increased.
SEM images showed that even HAp scaffolds synthesized without using a porogen had a macropore structure, but that their pore distribution was smaller than that seen in scaffolds fabricated using PVA (), PVP (), or PEO (). Dispersal of larger amounts of the porogen in HAp suspensions resulted in the formation of a greater number of pores on the scaffold surface.
Figure 6. Macropore morphologies of hydroxyapatite (HAp) scaffolds fabricated using polyvinyl alcohol (PVA) as a porogen in the following concentrations: (a) 0 wt %; (b) 5 wt %; (c) 10 wt %; and (d) 15 wt % (Insets: HAp-based scaffolds magnified 3000 times).
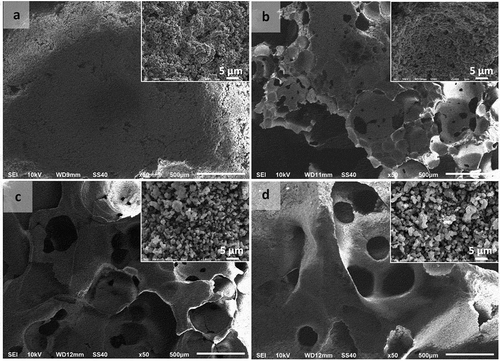
Figure 7. Macropore morphologies of hydroxyapatite (HAp) scaffolds fabricated using polyvinylpyrrolidone (PVP) as a porogen in the following concentrations: (a) 0 wt %; (b) 5 wt %; (c) 10 wt %; and (d) 15 wt % (Insets: HAp-based scaffolds magnified 3000 times).

Figure 8. Macropore morphologies of hydroxyapatite (HAp) scaffolds fabricated using polyethylene oxide (PEO) as a porogen in the following concentrations: (a) 0 wt %; (b) 5 wt %; (c) 10 wt %; and (d) 15 wt % (Insets: HAp-based scaffolds magnified 3000 times).
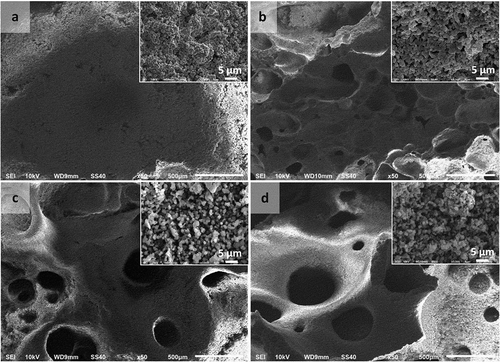
The separation of impurities from materials affects the boundary mobility of the particles [Citation12]. The porogens used in the HAp scaffold fabrication process had the same effect as impurities in the HAp particles.
The pores on the scaffolds were expected to function as sites for nutrient supply to the bone tissue. The SEM results show an increase in agglomeration of the particles with increases in the concentration of porogens used in the scaffold fabrication process.
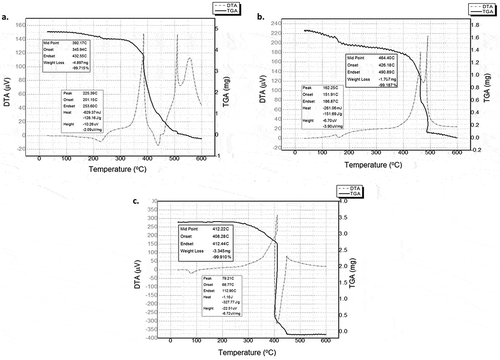
DTA/TGA analysis of PVA, PVP, and PEO scaffolds showed the occurrence of multistage degradation during the sintering process (). The DTA curve showed drastic degradation of PVA at 345.94–432.55°C with a loss of 99.7%. Mass degradation of the porogen (TGA) was associated with an exothermic event. This process was followed by an endothermic event, indicating that the sample in this process decreased in enthalpy to form a more stable structure. A drastic decrease in the weight of the PVP porogenic sample occurred at 426.18–490.89°C with a loss of 99.2%. The PEO sample weight was drastically reduced at 408.28–412.44°C with a loss of 99.9% of the mass. The results of the DTA/TGA characterization of the porogens confirmed that all the three porogens used as agents in pore scaffold structure engineering processes for HAp would be completely degraded at the HAp sintering temperature. The sintering step can therefore be used to leach out porogens from scaffolds.
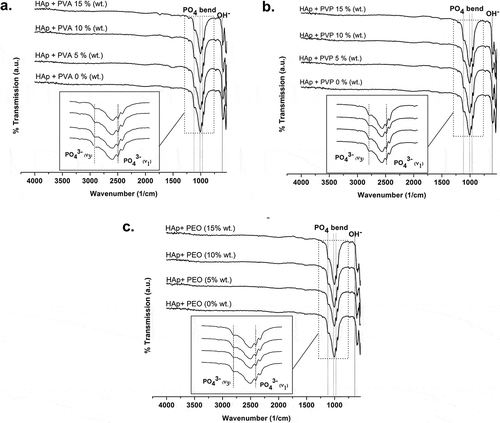
FTIR spectroscopy was also used to view the phase changes in the chemical groups of the material before and after scaffold fabrication. The fabricated scaffolds were expected to exhibit HAp spectra that would confirm that the synthesized HAp scaffolds no longer contained a porogenic agent. The infrared spectra were therefore used to determine whether the synthesized material exhibited the characteristic spectrum of HAp.
The FTIR spectra showed that scaffolds fabricated using PVA, PVP, or PEO porogens exhibited the characteristic absorptions expected of HAp functional groups (). The emerging absorbance bands can be identified as a bending PO43− vibration, a stretching PO43− vibration, and an OH vibration. No carbonate ions are visible in the infrared spectra.
Figure 10. FTIR spectra of HAp-based scaffolds fabricated using porogens: (a) PVA; (b) PVP; and (c) PEO.
Fabricated HAp scaffolds still have β-TCP peaks of low intensity (). However, the relatively small intensity and the distribution of X-ray diffraction peaks of β-TCP indicate that HAp dominates the X-ray diffraction pattern. In any case, β-TCP itself is highly biocompatible and can promote the repair of bone tissue. Several other studies have also used HAp/TCP composite materials for bone tissue engineering applications, such as TCP-HAp-based scaffold fabrication [Citation13], HAp/α-TCP composite fabrication for scaffold applications [Citation14], and HAp/β-TCP composite fabrication for bone cement applications [Citation15].
Figure 11. X-ray diffraction patterns of HAp-based scaffolds fabricated using (a) PVA; (b) PVP; and (c) PEO.
An increase in crystallite size should occur during the sintering process because this heating process causes grain growth of the HAp crystals due to particle surface diffusion. The decrease in crystallite size that occurred in this study was due to the use of a suspension rather than a dry sample in the sintering process. Fluid in the mixture caused the formation of dihedral angles between the particles [Citation16] and created repulsive forces between the particles that inhibited the grain growth of the HAp particles.
The HAp scaffolds showed increases in the values of lattice parameters a and c when compared to HAp powder. The variations in the lattice parameters could be due to the sintering process performed during the HAp densification process. The changes indicate a lattice contraction in the HAp. The highest peak position (211) appears to represent a shift in position from the interplanar distance standard value (211) for HAp. This shift is caused by changes in the d-spacing values and local distortions occurring in crystals. A uniform strain occurring in the crystals causes isotropical changes in the dimensions of the cell units as well as changes in the lattice parameters and peak shifts. The difference in the density of HAp scaffolds with each variation in the porogen concentration does not appear to have statistical significance. When compared to the density of synthesized HAp powder, the density of the scaffolds did seem to be significantly reduced.
The presence of a porogen during the scaffold fabrication process affects the densification process, and creates pores between the grains of particles to form interstitial spaces in the material [Citation17]. This causes the lattice volume of HAp particles to expand, and the expansion in the volume of the HAp scaffold grating causes the HAp density to decrease in comparison with the initial density of HAp before scaffold fabrication.
Another marker of the heterogeneity of crystalline apatite compositions is microstrain. Heterogeneity here refers to dimensional variations in the cellular units that affect the diffraction peaks by widening to form of misleading peaks due to a decrease or increase in crystallite size. A change in the microstrain on a scaffold indicates that the porogen used affects the distribution of HAp particle ions in the crystal lattice spaces.
Solid HAp can have mechanical strength values ranging from 100 to 900 MPa [Citation10]. These variations in mechanical strength depend on the densification method used in HAp fabrication. The compression strengths of the scaffolds created in the present study from golden apple snail shell range from 118 to 543 MPa. The microhardness of the scaffolds in this study tended to decrease with the addition of a porogen () and was inversely proportional to the increase in the mean pore size of the scaffold obtained by increasing the porogen concentration in the fabrication process. The average pore size increased with increases in porosity. Thus, the material’s mechanical strength is inversely proportional to its porosity.
Figure 12. Effects of porogen concentration on HAp scaffold microhardness: (a) PVA; (b) PVP; and (c) PEO.
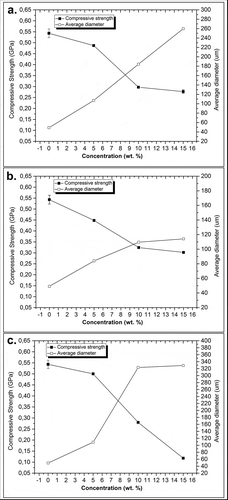
Scaffolds fabricated using 5–15 wt % of PVA and PVP achieved mechanical strength above 200 MPa, while fabricated scaffold using 15 wt % of PEO seemed to have low mechanical strength of less than 200 MPa. The mechanical strength of the porous scaffolds engineered from fabricated HAp in this study require further improvement due to the degradation of the HAp material that could occur after a certain period leading to a decrease in mechanical strength of the material.
4. Conclusions and suggestions
This report presents a successful synthesis of HAp from golden apple snail (Pomacea canaliculata) shell. Pore engineering using polymeric porogens (PVA, PVP, and PEO) achieved successful fabrication of porous HAp scaffolds, and the porosity increased with increases in the porogen concentration, with the average pore size increases described as PEO > PVP > PVA for identical weight percentages of porogen. The presence of pores gives scaffolds the potential for use as implants that can support the formation of bone tissue by enhancing the stability of bone and scaffold integration. On the other hand, the increasing porosity of the scaffolds with the addition of a porogen decreases their mechanical strength.
The HAp scaffolds’ lattice parameters expanded due to the presence of polymeric porogens during the sintering process. The agglomeration of HAp particles in the scaffolds tended to increase with increases in the porogen concentration. The FTIR spectra of the scaffolds showed absorption bands characteristic of the functional groups associated with HAp. The XRD results showed the presence of impurities in the form of low-intensity β-TCP peaks. The similarity of the chemical groups comprising the scaffold materials and natural bone can improve the biocompatibility of the scaffolds in anticipation of biological resistance that could occur with their implantation.
Acknowledgments
The authors wish to express their profound gratitude to the Indonesia Endowment Fund for Education (LPDP). They are also immensely grateful to the Indonesian Ministry of Research, Technology, and Higher Education for its financial support in this research through a PTUPT Grant. We also wish to thank LPPT UGM for its technical assistance.
Disclosure statement
The authors certify that they have no affiliation with nor involvement in any organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Funding
References
- Aksakal B, Yildirim OS, Gul H. Metallurgical failure analysis of various implant materials used in orthopedic applications. J Fail Anal Prev. 2004;4:17–23.
- Vallet-Regi M, Gonzalez-Calbet JM. Calcium phosphate as substitution of bone tissues. Prog Solid State Chem. 2004;32:1–31.
- Tripathi G, Basu B. A porous hydroxyapatite scaffold for bone tissue engineering: physico-mechanical and biological evaluations. Ceram Int. 2012;38:341–349.
- Leelatawonchai P, Laonapakul T. Preparation and characterization of calcium sources from golden apple snail shell for naturally based biomaterials. Adv Mater Res. 2014;931–932:370–374.
- Udomkan N, Limsuwan P. Temperature effects on freshwater snail shells: Pomacea canaliculata Lamarck as investigated by XRD, EDX, SEM, and FTIR techniques. Mater Sci Eng C. 2007;28:316–319.
- Minton J Design, fabrication, and analysis of polymer scaffolds for use in bone tissue engineering [master’s thesis]. Ohio: Miami University; 2013.
- Angela NMH, Aminuddin BS, Tan KK, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Ortho Sci. 2005;10:192–199.
- Tan KK, Shamsul BS, Aminuddin BS, et al. Bone graft substitute hydroxyapatite scaffold seeded with tissue engineered autologous osteoprogenitor cells in spinal fusion: early result in sheep as a model. Med J Malaysia. 2005;60:53–58.
- LeGeros RZ, Ben-Nissan B. Introduction to synthetic and biologic apatitites. In: Ben-Nissan B, editor. Advance in calcium phosphate biomaterials. Heidelberg: Springer; 2014. p. 1–17.
- Ratner BD. Biomaterials Science: an introduction to materials in medicine. San Diego: Elsevier Academic Press; 2004.
- Wang J, Shaw LL. Nanocrytalline hydroxyapatite with simultaneous enhacements in hardness and toughness. Biomaterials. 2009;30:6565–6572.
- Brook RJ. Pore-grain boundary interactions and grain growth. J Am Ceram Soc. 1969;52:56–57.
- Sulaiman SB, Keong TK, Cheng CH, et al. Tricalcium phosphate/hydroxyapatite (TCP-HA) bone scaffold as potential candidate for the formation of tissue engineered bone. Indian J Med Res. 2013;137:1093–1101.
- Asaoka T, Ohtake S, Furukawa KS, et al. Development of bioactive porous -TCP/HAp beads for bone tissue engineering. J Biomed Mater Res. 2013;101:1552–4965.
- Gallineti S, Canal C, Ginebra M, et al. Development and characterization of biphasic hydroxyapatite/β-TCP cements. J Am Ceram Soc. 2014;97:1065–1073.
- De Jonghe LC, Rahaman MN. Sintering of ceramics. In: Somiya S editor. Handbook of advance ceramics. California: Academic Press; 2003. p. 187–264.
- Kang SJL. Sintering: densification, graingrowth, and microstructure. Amsterdam: Elsevier; 2005.