?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A sponge template method was used to synthesize scaffolds from hydroxyapatite (HAp) using varying sintering temperatures and compressive ratios. The HAp scaffolds were fabricated with collagen (COL) or collagen/HAp particles under room temperature coating conditions. The microstructure and mechanical properties of the fabricated biomaterials were analyzed by FE-SEM and EZ Test, respectively. The FE-SEM micrographs showed microporous structure of the fabricated composite scaffolds. Incorporation of COL or COL/HAp into pure HAp scaffolds under coating conditions significantly reduced the porosity and enhanced the mechanical properties. The results demonstrated that the porosity of fabricated scaffolds was reduced by lowering the compressive pressure and increasing the sintering temperature. The maximum estimated compressive moduli at 1000, 1100 and 1200 °C for HAp-COL and HAp-COL/HAp composite scaffolds were 4.36, 2.93, 5.09 MPa and 4.57, 8.19, 5.02 MPa, respectively, at a 50% compressive ratio. It was assumed that porosity was effectively reduced to the maximum level at a 50% compressive ratio. An in vitro stem cell study showed significant cell adhesion and proliferation over the fabricated HAp-COL and HAp-COL/HAp composite scaffolds. These results confirmed that fabrication of pure HAp with COL and COL/HAp materials had advanced effects on the mechanical properties of fabricated porous composite scaffolds.
1. Introduction
Recently, regenerated bone grafts have drawn attention as a promising new bone grafting technology for use in tissue engineering. Regenerated bone grafts are generally constructed using porous scaffolds with bone cells [Citation1]. The scaffolds act as substrates, upon the surface of which cells adhere and grow. The scaffolds also provide structural and mechanical support for cell growth. Providing a temporary home for growth and proliferation of cells is one of the most important features of scaffolds, which allow cells to generate an extracellular matrix (ECM) during the culture period [Citation2]. The porous scaffolds used in bone tissue engineering can be fabricated using both bioactive ceramics and biocompatible polymers. Bioactive ceramics have a chemical composition resembling that of natural bone, allowing osteogenesis and providing bony contact and bonds with host bone [Citation3,Citation4]. Hydroxyapatite (HAp) and β-tricalcium phosphate (β-TCP) are typical bioceramics clinically used as bone substitutes, and they have also been used as scaffold materials in bone tissue engineering owing to their bioactivity, biocompatibility, and osteoconductivity [Citation5–Citation9]. However, the low strength and toughness of HAp have limited its wide application in orthopedic implants [Citation10]. The main reason for these low mechanical properties is thought to be the decomposition of HAp into different calcium phosphate phases, such as tricalcium phosphate (TCP) and even tetra-calcium phosphate (TTCP) [Citation11]. Various pressing and sintering methods, such as underwater shock compaction, hot press sintering, microwave, processing, and spark plasma sintering process have been employed to improve the mechanical properties of HAp [Citation12–Citation16].
Typically, three individual groups of biomaterials, namely bioceramics, synthetic polymers and natural polymers, have been used in the fabrication of scaffolds for bone tissue engineering [Citation17]. Some attempts have been made to introduce bioceramics into polymer-based scaffolds or to combine synthetic polymers with natural polymers to enhance their biological capacity [Citation18]. Collagen (COL), the most common protein in the body, provides strength and structural stability to biological tissues, including skin, blood vessels, tendons, cartilage, and bone. Along with carbonate apatite, type I COL is one of the two major components of bone, making up 89% of its organic matrix and 32% of its volumetric composition [Citation17]. COL has therefore been widely used to fabricate scaffolds, by itself or in combination with other biomaterial groups such as bioceramics or synthetic polymers, to enhance their biological and mechanical properties [Citation19].
Since bioceramic-based scaffolds for bone regeneration are expected to be among the load-bearing components, it is very important to investigate their mechanical properties prior to implantation. It is well known that such microstructural properties of bioceramics as crystallinity, grain size, density, and micro-porosity greatly influence the mechanical performance of bioceramic scaffolds, and those microstructural properties can be controlled by changing the sintering temperature. Lower sintering temperatures can be used to obtain better bioactivity for HAp bone grafts or scaffolds, while higher sintering temperatures and longer sintering times are needed to ensure better mechanical properties [Citation20]. One of the critical factors for porous scaffolds is porosity, which affects the exchange of nutrients, metabolic waste and other elements during cell culture. All the cells present inside the scaffolds must have similar access requirements, which are met by providing a continuous porous structure with an appropriate degree of porosity [Citation2].
In this study, HAp porous scaffolds with different porosities were fabricated using the template method while controlling the porosity and the sintering temperature during the fabrication process. To overcome the brittleness of the HAp scaffolds and improve in the mechanical properties, biopolymer COL was introduced as a coating material on the surface of the HAp scaffolds. As an innovative secondary phase, moreover, COL with distributed HAp powder was also employed as a coating material. Finally, the biocompatibility of the scaffolds was examined in a biological examination using human mesenchymal stem cells (hMSCs).
2. Materials and methods
2.1. Synthesis of hap porous scaffolds
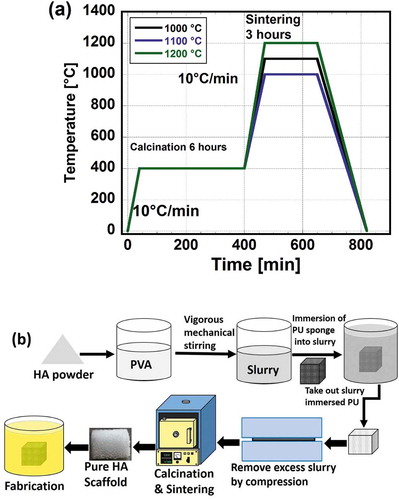
HAp porous scaffolds were prepared from HAp slurry by the template method using polyurethane (PU) foam. The HAp slurry was prepared from HAp nanopowder and a 5 wt% solution of polyvinyl alcohol (PVA) (Wako Pure Chemical Industries, Ltd.). The HAp powder and PVA solution were mixed at a mixing ratio of 1:1 (1 g of HAp to 1 ml of PVA) using a centrifuge mixing machine (Imoto Co, Ltd.) with 10 min kneading and 8 min degassing (removal of gas bubbles) processes. A PU sponge sheet (HR-30, Bridgestone) was cut into cubic templates with dimensions of 10×10×10 mm, and the cubes were immersed in the prepared HAp slurry. Excess slurry was removed by compressing the cubes at compression ratios of 50, 75, 95% to avoid blockage of the pores by the slurry. The cubes were then dried at room temperature for 24 h to remove water and attach the HAp powders to the surface of the sponge framework. The cubes were first calcinated at 400 °C for 6 h firstly and then sintered further at 1000, 1100, or 1200°C for 3 h. The heating rate was set to 10°C/min. The solidified samples were termed “pure HAp scaffolds” thereafter. The sintering process is illustrated schematically in ).
2.2. Fabrication of hap porous scaffolds with COL and HA particles
As-prepared pure HAp scaffolds were dipped into a COL-1 solution (Nippon Meat Packers Inc., Osaka, Japan) and vacuumed for at least 30 min to remove the air. The excess COL solution was removed manually by pipette, and the samples were dried at room temperature for at least 48 h to fabricate HAp scaffolds with COL coating. HAp particles, on the other hand, were mixed thoroughly with the COL-1 solution (1:9) at 30°C using a magnetic stirrer to prepare milk-like slime. The prepared slime was then poured over the pure HAp scaffolds, after which the excess slime was removed and the scaffolds were dried for at least 48 h to fabricate HAp scaffolds with COL-HAp coating. These two different types of composite scaffolds were thereafter referred to as “HAp-COL” and “HAp-COL/HAp particle-coated scaffolds”, respectively. The HAp-COL and HAp-COL/HAp scaffolds fabrication processes are illustrated schematically in ).
3. Characterization and analysis
3.1. Compression testing
The dimensions (length, width and height) of the prepared scaffolds were measure by Digital Vernier Caliper (TDC-150P, TRUSCO PRO TOOL, Japan) prior to the compression testing to examine their shrinkage during the sintering process. The average dimensions of the scaffolds sintered at 1100°C at compressive ratios of 50, 75, and 95% were 10.2 × 8.8 × 7.8 mm3 at a 50% compressive ratio, 10.7 × 7.9 × 7.4 mm3 at a 75% compressing ratio and 10.9 × 9.2 × 7.9 mm3 at a 95% compressive ratio, respectively. Compression tests were then performed at a crosshead speed of 1 mm/min using a compact testing machine (EZTest, Shimadzu Co., Ltd.) equipped with a 500N load cell, and the force-displacement data were recorded. Stress, σ, and strain, ϵ, were calculated by the following formulae:
where F and ∆H are the force and displacement, and L, W, and H are the length, width and height, respectively. Mechanical properties such as the compressive elastic modulus, fracture stress, and strain energy density (SED) were then evaluated from the stress-strain data. The elastic modulus was obtained from the initial slope of the stress-strain curve. For each condition, 6 samples were tested and the average value was recorded.
The porosity of the scaffolds was determined based on the density of pure HAp scaffolds and the volume using the following formula:
3.16 g/cm3 was used as the typical density of HAp [Citation21,Citation22].
The SED (elastic energy stored in the unit volume) of each of the scaffolds under compression was estimated by calculating the area under the corresponding stress-strain curve.
3.2. X-ray diffraction studies
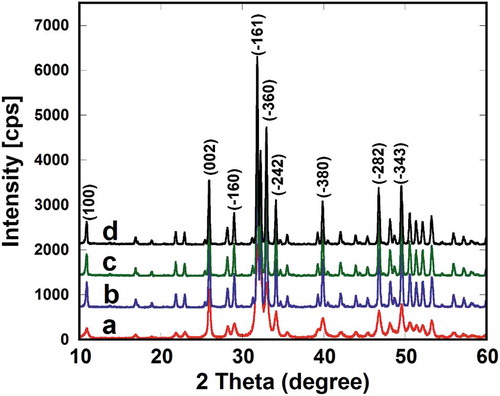
X-ray powder diffraction was employed to determine the effect of the sintering temperature on the phase stability of the scaffolds at various sintering temperatures with CuKα (α = 1.5405 nm) and a Ni filter. Pure raw HA powder and the scaffolds were scanned from 2θ of 10° to 60° in steps of 0.02°. The typical XRD patterns of pure raw HA powder and as-prepared HAp-COL/HAp scaffolds sintered at 1000, 1100, and 1200°C are represented in , respectively.
3.3. Microstructural characterization
The porous microstructures of the scaffolds were characterized using a FE-SEM (Hitachi, Ltd. S-4100) according to Islam et al. [Citation23]. The scaffolds were placed on the specimen holders with a carbon tape and an electro-conductive dotite D-550 (Fujikura Kasei Co, Ltd.) on the bottom surfaces. A vacuum sputter (Hitachi E1030 Ion Sputter) was used to coat the specimens with a thin layer of Pt/Pd-alloy. The scaffolds were then observed with an FE-SEM to characterize the porous microstructures.
3.4. Cell experiments
hMSCs (UE6E7T-3, Riken Bioresource Center, Japan) derived from bonemarrow were cultivated in 5 ml of α-MEM (Wako Pure Chemical Industries, Ltd.) supplemented with 10% of fetal bovine serum (FBS) (Gibco) and 1% of penicillin-streptomycin (Pen-Strep) (Sigma Life Science) [Citation24]. The cells were incubated under standard cell culture conditions at 37°C in an atmosphere of 5% CO2 and 70% humidity. The medium was changed twice per week during the culturing. When the cells reached sub-confluence (80–90%), they were harvested with an Accutase enzyme (Millipore Corporation, Catal. No. SCR005) and sub-cultured again. All the experimental procedures were performed under identical conditions. hMSCs were cultured over the HAp-COL or HAp-COL/HAp coated scaffolds. The cells discussed earlier in item passage 3–4 were used for seeding on the scaffolds. The scaffolds were placed in 24-well plates and exposed to UV for 1 h to sterilize. The scaffolds were then soaked in α-MEM under vacuum for 1 h to remove the air. The soaked scaffolds were incubated at 37°C for 30 min prior to cell seeding and 1 × 104 cells in 100 μl of medium were then seeded onto each scaffold, vacuumed for 1 min and then incubated for another hour to allow the cells to become attached. After incubation for 1 h, 1 ml of the medium, α-MEM supplemented with 10% FBS and 1% penicillin-streptomycin were added and incubated under the standard environment for cell cultures for up to 14 days.
The morphology of the scaffolds with hMSC was observed using FE-SEM. After 7 days of seeding, the scaffolds were washed with PBS several times and the cells were fixed. The hMSCs on the scaffolds were fixed following a procedure of consecutive disinfection with ethanol, distilled water, and t-butyl alcohol (see supplementary material 1). The scaffolds with cells were pre-frozen for at least 1 h at −30°C before the freeze-drying process and then freeze-dried for at least 9 h. Finally, their morphologies were characterized by FE-SEM. The cell growth activity in terms of cell number was evaluated using a spectro-photometric plate reader (2030 ARVO™ X2, Perkin Elmer Co., Yokohama, Japan). The proliferated number of hMSC was estimated using a cell-counting kit (Dojindo Laboratories, Kumamoto, Japan). After 1 to 14 days, the number of cells in the cell culturing was determined on the 7th and 14th days of culturing. For each scaffold with cells, the medium was removed and the scaffold was washed several times with PBS. One thousand (1000) μl of PBS with 100 μl of cell counting kit was then added, followed by incubation at 37°C in a humidified atmosphere of 5% CO2 for 2 h. Subsequently, 1100 μl of the reaction solution extracted from each sample and divided into 10 samples (as 110 μl) and filled into wells of a 96-well plate. Finally, the optical densities were evaluated using a plate reader (Perkin Elmer Arvo X2) at 37°C at a wavelength of 450 nm, and the mean absorbance obtained was converted into a cell number according to the described protocol (Dojindo Laboratories, Kumamoto, Japan).
4. Statistical analysis
All data were presented as means ± standard deviation (SD) and derived from 2–6 independent samples. Analysis of variance (ANOVA) was conducted to evaluate the recorded data using Kaleida Graph (Synergy Software, Reading, PA, USA), and statistical analysis was performed by Fischer’s LSD comparison test, in which any difference was considered statistically significant when the p-value was smaller than 0.05.
5. Results and discussion
5.1. Microstructure and microscopic fracture mechanism
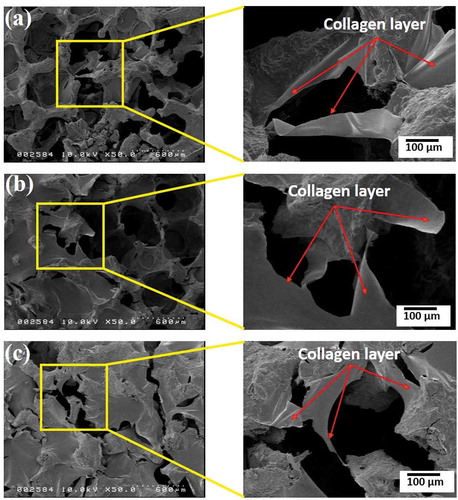
The microstructural morphology of the PU foam was characterized by FE-SEM (see supplementary material 2, ). It was clearly observed that the PU foam comprised macroporous. FE-SEM micrographs of the pure HAp scaffolds, and the composite scaffolds are shown in . It was revealed that all of the scaffolds possessed distinguishable porous structures. The FE-SEM microimages clearly evidenced that the pores of the pure HAp scaffolds were partially blocked and reduced by incorporation of COL or COL/HAp coating at all the sintering temperatures. It was assumed that when the sintering temperature was increased, the porosities of the HAp scaffolds were reduced due to the higher densification of the material. It was reported that at lower temperatures, sintering was incomplete and that the strut structure became highly porous. At 1200°C, moreover, the sintered structure exhibited a much higher degree of densification [Citation2]. The mechanism of porosity reduction and strengthening of the HAp-COL scaffolds with a 95% compressive ratio is shown in .
Figure 3. FE-SEM porous microstructures of pure and fabricated scaffolds at 95% (a), 75% (b) and 50% (c) compressive rate sintering at 1000°C, 1100°C, and 1200°C.
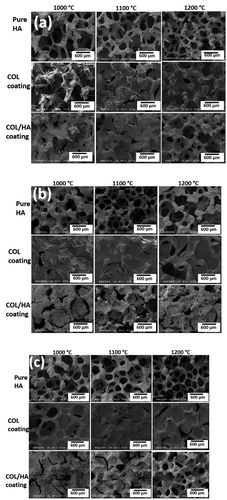
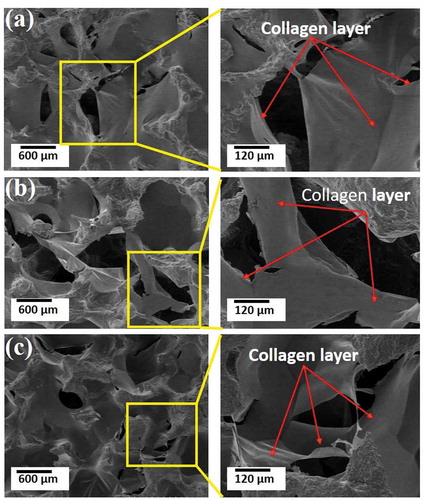
It was observed that COL () or COL/HAp was distributed over the pores of the pure HAp scaffolds. It was anticipated that the introduction of a COL or COL/HAp layer (red arrows) over the composite scaffolds improved the load-bearing ability and reduced the brittleness of the base HAp scaffolds. In our previous study, we developed 2-phase composite scaffolds by introducing a COL or COL/HAp secondary phase into pure HAp scaffolds [Citation25]. It was found that this introduction of a polymer secondary phase resulted in dramatic improvement of the mechanical properties. Moreover, it was found that higher sintering temperatures also enhanced the mechanical properties of the composite scaffolds by reducing porosity. It was therefore anticipated that densification and reduction of porosity () were the primary mechanisms of the mechanical improvement observed in this study.
Table 1. Variations in porosity of fabricated biomaterials at different compressive ratios and sintering temperatures.
Figure 4. Mechanism of reducing porosity and strengthening of HAp scaffolds fabricated by collagen with 95% compressive ratio, sintering at 1000°C (a), 1100°C (b), and 1200°C (c).
It was clearly revealed that the porosity of as-prepared pure HAp scaffolds was remarkably reduced when fabricated with COL or COL/HAp coatings. The effects of sintering temperature on the porosities of the scaffolds were also examined. It was found that increased sintering temperatures led to a notable reduction of porosity. It was also shown that the porosity of the scaffolds could be varied by changing the compressive ratio progressively. For example, in the present study, the porosity of the composite scaffolds varied from 80% to 91%. It was previously reported that a porosity ranging from 40 to 90% encouraged osteointegration on the implant surface and promoted adhesion of implants with bone [Citation26]. The minimum pore size required for a scaffold can be defined as 100 µm, and better osteogenesis occurs at pore sizes larger than 300 µm [Citation10,Citation26,Citation27]. The mean pore size of a scaffold can therefore be one of the critical factors in controlling cell adhesion and migration, tissue formation, and nutrient and oxygen access as well as waste removal [Citation28]. FE-SEM analysis of the HAp-COL scaffolds demonstrated that the average pore size ranged from 200 µm to 300 µm (see supplementary material, ).
Figure 2. Typical XRD patterns of the pure HA powder (a) and HAp-COL/HAp scaffolds sintering at 1000°C (b), 1100°C (c) and 1200°C (d).
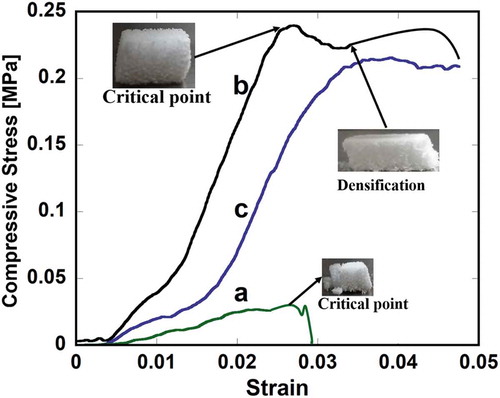
FE-SEM micrographs of typical fracture surfaces of pure HAp, HAp-COL and HAp-COL/HAp scaffolds prepared at a 50% compressive ratio and a sintering temperature of 1100°C are shown in . The stress-strain curve of pure HAp scaffolds ()) shows an abrupt decrease in stress after the critical point due to the typical brittle fracture of ceramics. On the contrary, HAp-COL ()) and HAp-COL/HAp scaffolds ()) show no such brittle fracture of the total structure but instead exhibit a gradual decrease in stress due to localized fracture of the HA struts. In the final stage of the compressive deformation, the composite scaffolds underwent densification corresponding to linear-elastic behavior with a greater elastic modulus than the initial modulus, which indicates a dramatic improvement in fracture resistance. The scaffolds still maintain a well-connected micro-porous structure as compressive deformation progresses because the HAp-COL and HAp-COL/HAp coating layers create ligaments to hold the HA struts, as shown later in . This is considered to be the key factor in enhancing the fracture resistance of the composite scaffolds, in contrast to the abrupt collapse of the pure HAp scaffold. Our previous report evidenced that COL or COL-HA coating was distributed over the pores of the base HA scaffolds, ultimately introducing a layer on the surfaces of the HAp struts which resulted in improvement of their fracture resistance or load-bearing ability [Citation25]. Sultana and Wang observed, moreover that increasing the compressive load induced densification, where the stress started to increase again [Citation29]. FE-SEM images of microscopic fracture mechanisms in composite scaffolds at a 50% compressive ratio and sintered at three different temperatures are shown in . It was clearly perceived that the columns of HAp were strongly connected by COL or COL/HAp secondary coating phases of the HAp columns. These connections (indicated by red arrows) tended to protect the scaffolds from breaking easily. This linkage between the HAp struts and the COL coatings is thought to reduce the stress concentration, resulting in delayed crack initiation and preventing extension of the cracks.
5.2. Phase stability of the scaffolds
It is clearly shown in that all the XRD peak positions and relative intensities of the HAp-COL/HAp scaffolds matched well with those of the base HA powder, indicating that the sintering process employed in this study did not alter the chemical components or structures. No other peaks or peak shifts were observed in the composite scaffolds, suggesting that no chemical reaction occurred. Further, no diffraction peaks from other crystalline forms were detected, which demonstrated that the HAp scaffolds had phase stability and crystallinity. It was previously observed that there were no shifted peaks or new peaks in the HAp/PCL composite scaffolds sintered at 1100°C, which indicated that no chemical reactions arose during the fabrication process [Citation30]. It is well known that sintering temperature plays a key role in determining the mechanical and physical characteristics of scaffolds. It is also worth noting that porosity attenuation enhances the mechanical properties of scaffolds [Citation31]. The phase stability of a scaffold is an important consideration in its selection for use in bone tissue regeneration. A previous report stated that HAp phases remained stable even up to 1200°C with increasing crystallinity shown in all cases. Usually, the phase stability of HAp composites depends on the Ca/P ratio of the starting chemicals, and HAp remains stable even up to 1200°C when the Ca/P ratio is maintained at 1.67 [Citation32]. Werner et al. [Citation33] reported, moreover, that HAp sintered at 1400°C exhibited phase transformation and became α-TCP. It is therefore worth noting that HAp-COL/HAp composite scaffolds sintered at 1000–1200°C remained stable with no alteration of the phase. The findings of the present study are also supported by an earlier report [Citation34].
5.3. Mechanical properties of the scaffolds
Compression tests were carried out on the scaffolds, and the effects of the sintering temperature and compressive ratio on the compressive modulus are shown in . It was clearly observed that increases in the sintering temperature caused increments of the modulus in almost every specimen, but the highest modulus was observed in the HAp-COL/HAp scaffolds sintered at 1100°C ().
Figure 7. Effects of compressive ratio and sintering temperature on the compressive modulus of fabricated biomaterials. Here, (a), (b), and (c) stand for pure HAp, fabricated HAp-COL, fabricated HAp-COL/HAp scaffolds. Each data represented mean ± SD, n = 3, *p < 0.05.
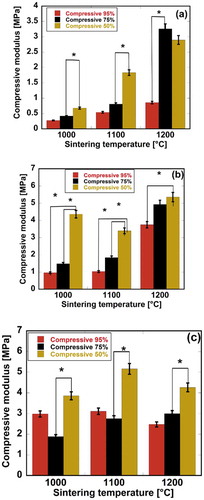
It was also noteworthy that HAp/COL and HAp-COL/HAp scaffolds fabricated with HAp base scaffolds sintered at 1200 and 1100°C demonstrated the best performances. Among the three compressive ratios, a 50% compressive ratio significantly improved the mechanical properties of the composite scaffolds at all sintering temperatures. The maximum compressive moduli at 1000, 1100, and 1200°C were 4.36, 2.93, and 5.09 MPa, respectively, for HAp-COL scaffolds and 4.57, 8.19, and 5.02 MPa, respectively, for HAp-COL/HAp scaffolds at a 50% compressive ratio. It is also shown in that at a 50% compressive ratio, maximum porosity was effectively reduced to lead to improvement of the mechanical properties. Wu et al. reported that scaffolds sintered at 1200°C exhibited the highest compressive strength and that this mechanical strength was due to a higher degree of densification [Citation35]. This densification was due to the disappearance of necks between particles, and the merging of grains, while small micropores remained in the rods sintered at 1200°C. We therefore assumed that higher densification and finer grains produced at a higher sintering temperature and lower compressive ratio increased such compressive mechanical properties as the strength and modulus of the scaffolds. Sabree et al. reported that the mechanical properties of ceramic scaffolds were found to be closely related to their porous structures, e.g. a larger pore size corresponded to lower strength and fracture toughness [Citation2]. Interconnection of the apatite particles during the rapid sintering stage occurring between 1150 and 1250°C yielded the maximum density. Further, it was reported that the densification resulted in increased mechanical strength when scaffolds were annealed at up to 1250°C [Citation35,Citation36]. It is worth noting that in this study, enhanced mechanical properties were observed in the fabricated scaffolds sintered at 1100 and 1200°C.
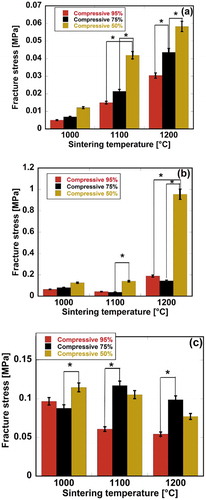
The stress-strain behavior of the scaffolds was also analyzed. A sudden reduction appearing in the stress-strain curve was thought to indicate that the material structure lost its integrity, ensuring stress transfer. The stress at the maximum point was therefore considered to be the fracture stress. The effects of the compressive ratio and sintering temperature on the fracture stress of the scaffolds are presented in . It was found that the maximum fracture stress values for pure HAp, HAp-COL, and HAp-COL/HAp scaffolds were 0.058, 0.96 and 0.12 MPa, respectively. It was therefore certain that the fracture stress was significantly improved by introducing a COL coating phase into the composite scaffold fabrication process. Moreover, the maximum fracture stress values for the scaffolds were found to be at a 50% compressive ratio.
Figure 8. Effects of compressive ratio and sintering temperature on the fracture stress of fabricated biomaterials. Here, (a), (b), and (c) stand for pure HAp, fabricated HAp-COL, fabricated HAp-COL/HAp scaffolds. Each data represented mean ± SD, n = 3, *p < 0.05.
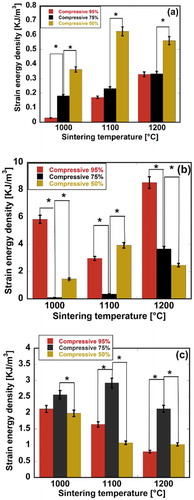
SED was also evaluated for the scaffolds as a mechanical property expressing fracture resistance. SED can be recognized as the elastic energy absorbed by the unit volume of the scaffolds up to the fracture point. Most of the SED was considered to be applied to fractures. SED can be estimated by calculating the area under the stress-strain curve up to the fracture point. Variational behaviors of the SED of the scaffolds are shown in . It was observed that the maximum SED values for pure HAp, HAp-COL, and HAp-COL/HAp scaffolds were 0.63, 8.53 and 2.68 KJ/m3, respectively. It was also apparent that a higher SED was obtained at a 50% compressive ration for pure HAp scaffolds at all the sintering temperatures, while for HAp-COL scaffolds, a higher SED was obtained at a 95% compressive ratio at 1000 and 1200°C. On the other hand, a higher SED was apparent at a 75% compressive ratio for the HAp-COL/HAp scaffolds at all the sintering temperatures. It was also clearly shown that introduction of COL and COL/HAp coating phases significantly improved the SED, suggesting an improvement in fracture resistance. It was observed that excess HAp slurry was removed by the compression process during fabrication to avoid pore blocking during synthesis. A higher compression ratio corresponded to enhanced removal of HAp slurry. It was therefore anticipated that SED, i.e. fracture resistance tended to be reduced with increases in the compressive ratio owing to minimized HAp content in the scaffolds.
5.4. Evaluation of cell adhesion and growth
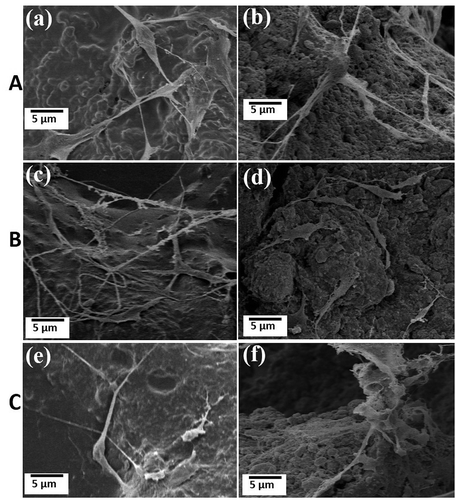
hMSCs were cultured over HAp-COL and HAp-COL/HAp scaffolds. It was confirmed from the evaluation of mechanical properties that the scaffolds exhibited the best performance at a 50% compressive ratio. Scaffolds fabricated at a 50% compressive ratio and sintered at 1000, 1100 and 1200°C were therefore used in the cell adhesion and proliferation experiments. Attachment and spreading of cells within the scaffolds are significant processes occurring of during cell-scaffold interactions [Citation36]. Therefore, biocompatibility is thought to be prerequisite for the development of scaffolds in the tissue engineering field. FE-SEM micrographs of the surface regions of composite scaffolds with cells are shown in . The Adhesion and proliferation behaviors of hMSCs up to 7 days of culturing were observed on the surfaces. FE-SEM micrographs of HAp-COL and HAp-COL/HAp scaffolds are shown in Figs. (a), (c) and (e), and Figs. (b), (d) and (f), respectively. It was clearly apparent that hMSCs were sufficiently attached and proliferated over every scaffold at 7 days of culturing. In Fig. (c), (e) and (f), hMSCs seem to be detached from the scaffold surfaces. One possible reason could be excessive washing or use of a weak fixative such as t-Butyl alcohol. Fusiform-shaped cells were distributed on the top surface of each scaffold. Most cells adhered tightly to the surface and were well disseminated. These results indicated that the composite scaffolds had favorable bioactivity for promotion of cell adhesion and proliferation far excelling of that of pure HAp scaffolds. It was previously reported that the surface properties of scaffolds had great influence on the adhesion and proliferation of cells [Citation37].
Figure 10. FE-SEM micrographs of hMSCs in fabricated HAp-COL and HAp-COL/HAp composite scaffolds at 50% compressive rate and sintering at 1000°C (A), 1100°C (B) and 1200°C (C) up to 7 days of culture. Here, (a), (c), (e), and (b), (d), (f) indicate HAp-COL and HAp-COL/HAp composite scaffolds, respectively.
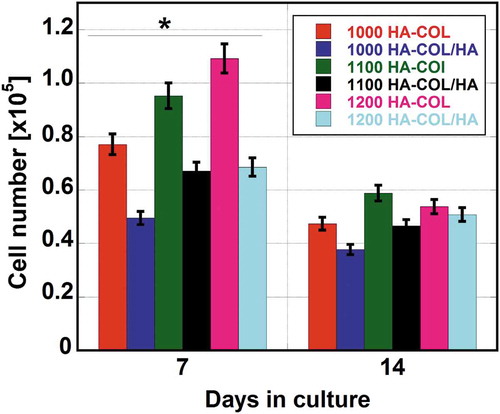
To evaluate the effects of the compressive ratio and sintering temperature on cell-scaffold interactions, a cell proliferation study was performed using a cell-counting kit up to 14 days of culturing. demonstrates the proliferation behavior of hMSCs in the composite scaffolds. Initially, 1 × 104 cells were used to seed the fabricated composite scaffolds and the cell growth performance was recorded at 7 and 14 days of culturing. It was clearly shown that the cell number increased until 7 days of culturing for all types of scaffolds, while cell proliferation weakened as the culturing days proceeded. HAp-COL scaffolds sintered at three different temperatures also exhibited prevalent cell growth performance over their counterparts. The reduction in the cell numbers was quite drastic from 7 to 14 days of culturing. The maximum reduction was noticed for HAp-COL scaffolds sintered at 1200°C. It was assumed that continuous cell proliferation decreased due to degradation of the materials as a result of immersing the scaffolds in the medium over a period of 14 days.
Figure 11. The Proliferation of hMSCs in fabricated HAp-COL and HAp-COL/HAp composite scaffolds at 50% compressive rate and sintering at 1000, 1100, and 1200°C up to 14 days of culture. Each data represent mean ± SD, n = 6, *p < 0.05.
It is worth noting that in our previous study, hMSCs were cultured for up to 28 days and that they proliferated from 14 to 28 days of culturing. It was observed that the number of hMSCs at 28 days was almost twice that at 7 days. The decreasing behavior of cell growth after 7 days of culturing in both types of composite scaffolds was therefore considered to be due to early degradation of the scaffolds rather than to cytotoxic residues eventually degrading out of the scaffolds. It was reported that the proliferation of rMSCs in a COL scaffold became slower after reaching confluence at 14 days because of shrinkage and ductile deformation of the porous structure [Citation38]. It is well known that the mechanical properties of calcium phosphate bioceramics strongly depend on the degree of porosity [Citation39,Citation40], and, furthermore, it has also been shown that the mechanical strength tends to increase with bone ingrowth [Citation41]. The close chemical similarity of HAp to natural bone has led to extensive research efforts to use synthetic HAp as a bone substitute in orthopedic applications [Citation42]. Composite biomaterials such as synthesized HAp mixed with polymers or metals have been developed and used for orthopedic implants to achieve both better mechanical integration and bioactivity and to provide an excellent platform for the existing bone tissue engineering [Citation43–Citation45]. Therefore, in this study, porous HAp scaffolds with COL coatings as well as with HA particles introduced to improve both their mechanical and biological properties were fabricated.
Ideally, a scaffold should have mechanical properties consistent with the anatomical site into which it is to be implanted and, from a practical perspective, must be strong enough to allow surgical manipulation during implantation [Citation17]. Development of scaffolds with adequate mechanical properties is therefore one of the great challenges in bone tissue engineering. Implanted scaffolds must have sufficient mechanical integrity to function from the time of implantation to the completion of the remodeling process [Citation46].
The range of mechanical properties of the composite scaffolds fabricated in this study was sufficiently close to that of natural bone. Hence, these scaffolds could be candidates for use in the application of bone regenerative medicine. It should be noted that hMSCs growth response over the composite scaffolds was also remarkable and affirmative. Detailed investigations of the comparative biocompatibility and osteoblastic cellular responses of the composite scaffolds are currently under consideration, and the results obtained will be published soon.
6. Conclusions
The present study focused on the preparation and fabrication of porous HAp scaffolds with COL or COL/HAp coatings. Efforts were made to control their mechanical properties by altering the compressive ratios and sintering temperatures. The effects of three different ratios and three different sintering temperatures on both the mechanical and biological properties of the composite scaffolds were evaluated. The composite scaffolds fabricated from pure HAp scaffolds sintered at 1200 or 1100°C and with COL or COL/HAp coatings demonstrated better performances than the others. On the contrary, among the 95, 75 and 50% compressive ratios, the 50% compressive ratio significantly improved the mechanical properties of the composite scaffolds at all sintering temperatures. The in vitro hMSC culturing study showed significant cell adhesion and proliferation in the HAp-COL or HAp-COL/HAp composite scaffolds at a 50% compressive ratio. It may therefore be concluded that these composite scaffolds could be relevant to applications in bone tissue engineering.
Supplemental Material
Download Zip (649.8 KB)Acknowledgments
The present work was supported by a Grant-in-Aid for Scientific Research (No. 24•02380) and a Grant-in-Aid for JSPS Fellows (No. P14362) from the Japan Society for the Promotion of Science (JSPS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Wang H, Li Y, Zuo Y, et al. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28:3338–3348.
- Sabree I, Gough JE, Derby B. Mechanical properties of porous ceramic scaffolds: influence of internal dimensions. Ceram Int. 2015;41:8425–8432.
- Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981;157:259–278.
- Hench LL, Wilson J. Surface-active biomaterials. Science. 1984;226:630–706.
- Li Z, Ramay RH, Hauch KD. Xiao D and Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919–3928.
- Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74:1487–1510.
- Kitsugi TLT, Yamamuro T. Nakamura T and Oka M. Transmission electron microscopy observations at the interface of bone and four types of calcium phosphate ceramics with different calcium/phosphorus molar ratios. Biomaterials. 1995;16:1101–1107.
- Posner AS, Betts F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc Chem Res. 1975;8:273–281.
- Legeros RZ. Apatites in biological systems. Prog Crys Growth Ch. 1981;4:1–45.
- Hulbert SF, Young FA, Mathews RS, et al. Talbert CD and Stelling FH. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res A. 1970;4:433–456.
- Mobasherpour I, Hashjin MS, Toosi SSR, et al. Effect of the addition ZrO2-Al2O3 on nanocrystalline hydroxyapatite bending strength and fracture toughness. Ceram Int. 2009;35:1569–1574.
- Chiba A, Kimura S, Raghukandan K, et al. Effect of alumina addition on hydroxyapatite biocomposites fabricated by underwater-shock compaction. Mater Sci Eng A. 2003;350:179–183.
- Xihua Z, Changxia L, Musen L, et al. Fabrication of hydroxyapatite/diopside/alumina composites by hot-press sintering process. Ceram Int. 2008;35:1969–1973.
- Curran DJ, Fleming TJ, Towler MR, et al. Mechanical parameters of strontium doped hydroxyapatite sintered using micro-wave and conventional methods. J Mechan Behav Biomed Mater. 2011;4:2063–2073.
- Que W, Khor KA, Xu JL, et al. Hydroxyapatite/titaniananocomposites derived by combining high-energy ball milling with spark plasma sintering processes. J Eur Ceram Soc. 2008;28:3083–3090.
- Chaudhry AA, Yan H, Gong K, et al. High-strength nanograined and translucent hydroxyapatite monoliths via continuous hydrothermal synthesis and optimized spark plasma sintering. Acta Biomater. 2011;7:791–799.
- O’Brien FJ. Biomaterials & scaffolds for tissue engineering (Review). Mater Today. 2011;4:88–95.
- Kim -S-S, Sun Park M, Jeon O, et al. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–1409.
- Friess W, Schlap M. Sterilization of gentamicin containing collagen/PLGA microparticle composites. Eur J Pharm Biopharm. 2006;63:176–187.
- Legeros RZ, Lin S, Rohanizadeh R, et al. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater M. 2003;14:201–209.
- Landi E, Tampieri A. Celotti G and Sprio S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J Eur Ceram Soc. 2000;20:2377–2387.
- Munar ML, Udoh KI, Ishikawa K. Matsuya S and Nakagawa M. Effects of sintering temperature over 1300 °C on the physical and compositional properties of porous hydroxyapatite foam. Dent Mater J. 2006;25:51–58.
- Islam MS, Kusumoto Y, Abdulla-Al-Mamun M. Novel rose-type magnetic (Fe3O4, γ-Fe2O3 and α-Fe2O3) nanoplates synthesized by simple hydrothermal decomposition. Mater Lett. 2011;66:165–167.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147.
- Islam MS, Todo M. Effects of sintering temperature on the compressive mechanical properties of collagen/hydroxyapatite composite scaffolds for bone tissue engineering. Mater Lett. 2016;173:231–234.
- Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491.
- Lien SM, Ko LY, Huang TJ. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5:670–679.
- Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng B. 2013;19:485–502.
- Sultana N, Wang M. Fabrication of HA/PHBV composite scaffolds through the emulsion freezing/freeze-drying process and characterization of the scaffolds. J Mater Sci Mater M. 2008;19:2555–2561.
- Phanny Y, Development TM. Characterization of Poly (ε-caprolactone) Reinforced Porous Hydroxyapatite for Bone Tissue Engineering. Key Eng Mater. 2013;529–530:447–452.
- Zamaniana A, et al. The effect of sintering temperature on the microstructural and mechanical characteristics of hydroxyapatite macroporous scaffolds prepared via freeze-casting. Key Eng Mater. 2012;529–530:133–137.
- Rajendran A, Barik RC, Natarajan D, et al. Synthesis, phase stability of hydroxyapatite–silver composite with antimicrobial activity and cytocompatability. Ceram Int. 2014;40:10831–10838.
- Werner J, Linner-Krčmar B, Friess W, et al. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials. 2002;23:4285–4294.
- Ramesh S, Aw KL, Tolouei R, et al. Sintering properties of hydroxyapatite powders prepared using different methods. Ceram Int. 2013;39:111–119.
- Wu Q, Zhang X, Wu B, et al. Effects of microwave sintering on the properties of porous hydroxyapatite scaffolds. Ceram Int. 2013;39:2389–2395.
- Mostafa NY. Characterization, thermal stability and sintering of hydroxyapatite powders prepared by different routes. Mater Chem Phys. 2005;94:333–341.
- Aminzare M, Eskandari A, Barooniand MH, et al. Hydroxyapatite nanocomposites: synthesis, sintering and mechanical properties. Ceram Int. 2013;39:2197–2206.
- Arahira T, Todo M. Effects of proliferation and differentiation of mesenchymal stem cells on compressive mechanical behavior of collagen/β-TCP composite scaffold. J Mechan Behav Biomed Mater. 2014;39:218–230.
- Sous M, Bareille R, Rouais F, et al. Cellular biocompatibility and compression of macroporous b-tricalcium phosphate ceramics. Biomaterials. 1998;19:2147–2153.
- Shors EC, Holmes RE. Porous hydroxyapatite. In: Hench LL, Wilson J, editors. An introduction to bioceramics. Singapore: World Scientific Publ.; 1993. p. 181–198.
- Bouler JM, Trecant M, Delacin J, et al. Macroporous biphasic calcium phosphate ceramics: influence of five synthesis parameters on compressive strength. J Biomed Mater Res. 1996;32:603–609.
- Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769–2781.
- Ramakrisna S, Mayer J, Wintermantel E, et al. Biomedical application of polymer composite materials: a review. Compos Sci Technol. 2001;61:1189–1224.
- Pattanayak DK. Apatite wollastonite-poly methyl methacrylate biocomposites. J Mater Sci Eng C. 2009;29:709–714.
- Agrawal CM, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 2001;55:141–150.
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543.