?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this experimental study, we synthesized geopolymer materials with different amounts of nano-ZnO (0.3%, 0.5% and 0.7%). The effects of the nano-ZnO concentration on the mechanical, structural and thermal properties were studied in detail. XRD investigation revealed the formation of a glassy phase in all the samples and a small shift of the intense peak, which confirmed the incorporation of nano-ZnO into the geopolymer matrix. FTIR analyses showed an increase in the geopolymerization after the addition of nano-ZnO to geopolymer paste. The morphology of the inorganic polymers was studied by mercury intrusion porosimetry (MIP) and scanning electron microscopy (SEM). The addition of nano-ZnO improved the ultrasonic pulse velocity and increased the compressive strength from 30 to 38 MPa. It was found that the strength reached its maximum value at a critical nano-ZnO concentration of 0.5 at.%. The thermal properties of geopolymer nano-ZnO were also investigated using thermogravimetric analysis (TGA), which showed that the incorporation of nano-ZnO decreased weight loss by the geopolymers. The density and the absorption water were also tested and all the results were discussed.
1. Introduction
Geopolymers are a new class of inorganic polymers synthesized by activation of an aluminosilicate source with an alkali-activated solution. Nowadays, geopolymers have attracted special attention among researchers due to their extraordinary properties: excellent mechanical properties, good chemical resistance, low shrinkage, environmentally friendly nature and long-term durability [Citation1]. These characteristics favor their use in many applications: toxic waste immobilization, sealing, building and high temperature-resistant materials [Citation2]. The increasing demand for these materials is one of the major subject researchers of studies inducted to develop their properties. It is known that many types of admixtures are used to improve the performance of geopolymers [Citation3,Citation4]. In recent decades, their mechanical and structural properties as a function of the filler concentration have been thoroughly studied. Compared with other additives, nanoparticles have many advantages, such as good absorbability, strong coordination bonds and easy dispersal in alkaline solutions. Most notably, their high specific area [Citation5] leads to their use as a filler in geopolymers and also in various polymers [Citation6]. The search for a way to synthesize perfect-quality geopolymers by adding nanoparticles has become a research subject for studying the relationship between the mechanical, structural and thermal properties of geopolymers. Ekinci et al. reported improvement of the compressive strength of mortar containing 2% nano-silica [Citation7], Assaedi et al. [Citation8] showed that the incorporation of nano-SiO2 in geopolymer, based fly ash improved its mechanical, structural and morphological properties. Alomayri et al. studied the effect of nano-Al2O3 on mechanical and microstructure properties; they with showed an improvement in the compressive strength and flexion strength of geopolymers mixed with 2% nano-alumina [Citation9]. Khater et al. studied the structural and mechanical properties of geopolymers having various carbon nano-tube (CNT) content [Citation10], and Duan et al. studied the effect of TiO2 nanoparticles on the carbonation, shrinkage, compressive strength and microstructural properties of geopolymers [Citation11]. All these studies showed that the effect of nanoparticles on geopolymer behavior depends on many factors: the method of dispersion, nanoparticles concentration, and properties of the particles.
The choice of nano-ZnO as an additive is related to its capacity to produce well-compacted geopolymers, its low cost and its wide range of applications as well as its effective influence on improving the properties of many polymers [Citation12,Citation13]. The high heat capacity, low thermal expansion and high melting temperature of ZnO are, moreover, characteristics that are beneficial for ceramic applications.
There are a number of studies in the literate related to the effect of nanoparticles (nano-silica, nano-alumina, nano-clay, nano-TiO2, and carbon nanotubes) on the properties of geopolymers, On the other hand, less attention has been focused on the effect of nano-ZnO. There is missing information about the effects of nano-ZnO on their porosity, density, water absorption, internal structure (UPV) and thermal properties. This study investigated in detail the structural, mechanical and thermal behavior of geopolymers admixed with different concentrations of nano-ZnO: 0.3%, 0.5% and 0.7%.
2. Experimental
2.1. Materials
2.1.1. Natural kaolin
Natural kaolin collected from Mednin (south Tunisia) was dried, crushed and sieved to obtain fine particles with d50 = 8.225 μm tested by laser diffraction. This raw clay was then annealed at 750°C in air for 4 h (Nabertherm electric oven, LH 60/14) in order to transforms the kaolin to metakaolin, an amorphous kaolinite obtained by treatment at between 500°C and 800°C and treatment above 950°C leading to the formation of a material with a crystalline structure of the spinel, cristobalite and mullite type, which is not advantageous for geopolymerization because of its low reactivity in alkaline solutions. The chemical composition of this material as determinate by X-ray Fluorescence (XRF) is shown in .
Table 1. Chemical composition of metakaolin (M. K) (wt. %).
2.1.2. Alkaline solution
Alkaline solution and a combination of pure 99%sodium hydroxide and sodium silicate were used as the alkali activator. The sodium silicate solution contained 26.5% SiO2, 10.6% Na2O and 62.9% H2O, and the hydroxide solution was prepared by dissolving the NaOH solid particles in ultrapure water with a constant concentration (10 M).
2.1.3. Nano-ZnO synthesis
For nano-ZnO, a synthesis of ZnO nanoparticles by the sol–gel technique was performed by dissolving 2 g of zinc acetate dehydrate in 14 ml of methanol under magnetic stirring at room temperature until a homogenous solution was obtained. The solution was then dried rapidly in an autoclave under supercritical conditions at a heating rate of 50°C/h.
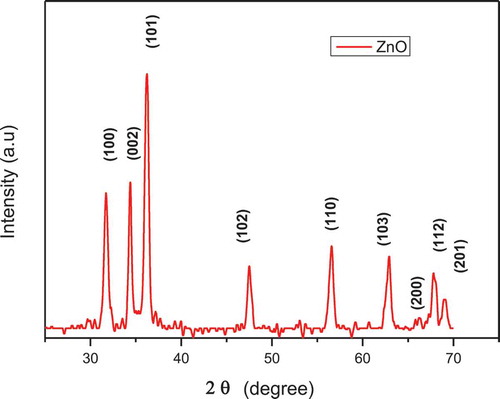
plots the XRD patterns of the nano ZnO. The spectra can be shown in the Wuritzite phase of ZnO according to the JCPDS (36–1451). The crystallite sizes (G, in Å) were calculated from Scherrer's formula [Citation14] using the diffraction intensity peak (101):
where G is the average grain size, λ is the X-ray wavelength (CuKα), β is the full width at half maximum of the (101) peak and θ is the maximum Bragg diffraction peak (in radians).
2.2. Method
The nano-ZnO was added to the metakaolin at loadings of 0.3%, 0.5% and 0.7% by weight. The alkaline solution and nano-ZnO were first stirred for 5 min at low speed and then mixed for another 10 min at high speed until a uniform mixture was achieved .
The metakaolin was added slowly to the solution at a medium speed for 5 min, and then mixed for further another 10 min at high speed until the mixture became homogeneous. The obtained mixtures were then cast into 3*6 cm cylindrical molds. The moalds were vibrated during casting to reduce the air bubbles and improve compaction before they were covered with a plastic film to minimize the loss of the evaporation water and cured for 24 h at room temperature (20 ± 4°C), after which the specimens were demolded and cured at 80°C (). The samples were tested directly for compressive strength, and small crushed samples were then immersed in acetone to stop the geopolymeric reaction and dried for 24 h at 105°C.
Table 2. Mixture proportions of geopolymers: nano-ZnO pastes.
2.3. Characterization
2.3.1. Mechanical properties
To measure the compressive strength, cylindrical specimens with a diameter of 30 mm and a height of 60 mm produced. These samples were stored in a humid place for 28 days and then cured at a temperature of 80°C. The test was carried out in triplicate and average values were obtained. The compressive strength test of each sample was performed with a Tonic-Technik testing machine according to ASTM D1633-00 [Citation15].
Ultrasonic pulse velocity (UPV) tests were performed on concrete samples to investigate the internal structure of the geopolymers with nano-ZnO. The UPV is determined by calculating the ratio of distance to the transit time of ultrasonic pulses between transducers on opposite ends of a given material (direct method):
where d is the distance and t is the transit time
2.3.2. Structural and microstructural characterization
The mineralogical composite and the crystalline phases of the samples were evaluated by X-ray diffraction (XRD) analysis. The characterization was obtained with a Bruker D5005 (Co Ka = 1.78,901 Å radiations) X-ray diffractometer with a 2θ range between 15° and 80°.
Fourier transform infrared spectroscopy (FTIR) was used a optical analysis to examine the structural properties on a nanometric scale. IR spectra were ranging between 400 and 4000 cm−1 were obtained with a Nicolet (Thermo Fisher Scientific 200) infrared spectrometer.
Scanning electron microscopy (Philips XL 30 SEM) was used to evaluate the microstructural properties of each sample. The samples were mounted on stubs of metal with adhesive, coated with 40–60 nm of Palladium and then observed with the microscope.
The thermogravimetric (TGA) technique was used to study the relationship between the material properties and temperatures. The TGA was conducted with a STA6000 thermal analyzer. The weight range of samples tested was 1–2 g with a constant heating rate of 10°C/min in the temperature range from 25°C to 450°C.
2.3.3. Physical properties
Mercury intrusion porosimetry is based on the fact that a liquid not wetting a porous solid will enter its pores only under pressure. When the pores are assumed to be cylindrical, pressure P is required to force the liquid to enter. The porosimeter used was an AUTOPORE IV 9500 from Micromeritics. Pore diameter measurements were performed from 0.9 mm to 5 nm. The MIP is based on the Washburn equation:
γ: surface energy of the liquid (0.485 Nm−1)
θ: contact angle (130◦)
d: pore diameter
Morphological studies:
Water absorption test was carried out in two stages according to ASTM C140: drying of the samples at 105°C for 24 h to obtain the first weight (W1), and immersion of the same samples in water for 1 day to obtain the second weight (W2). The density was determined by measuring weight (W) and volume (V) of the samples [Citation16].
The percentage absorption and density were calculated using the following equations:
where d = the diameter and t = the thickness of the sample.
3. Results and discussion
3.1. Characterizing the crystalline phases (XRD)
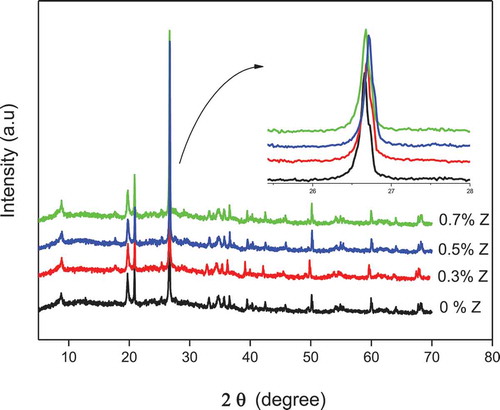
presents the XRD patterns of alkali-activated geopolymers with different amounts of nano-ZnO added. The spectra exhibit intense peaks also at 28° (2θ) showing the formation of a glassy phase in all the samples [Citation17]. We can a observe the effect of nano-ZnO filler addition on the geopolymer samples from the figure which shows a small shift to a higher angle when 0.3 and 0.5 nano-ZnO added, confirming the destructive effect of the filler on the ordered arrangement of the geopolymer side chains, and hence the enhancement of the amorphous phase. This destructive effect may be due to an interaction between the filler surface and the geopolymer. With the addition of 0.7% nano-ZnO, mean while the intense peak is shifted in the opposite direction. This may be due to the agglomeration of nanoparticles [Citation18], which hinders the geopolymeric reaction. The XRD spectra clearly show, therefore that the addition of 0.5% nano-ZnO is the highest level at which the structural properties of metakaolin-based geopolymers are enhanced. Rustan et al. studied the effect of nano-ZnO on geopolymer properties [Citation19]. They showed the existence of nano-ZnO peaks in the XRD spectra when more than 2.5% was added. In this study, we did observe these peaks, which may be related to the low concentration of nano-ZnO used.
3.2. Fourier transform infrared spectroscopy (FTIR)
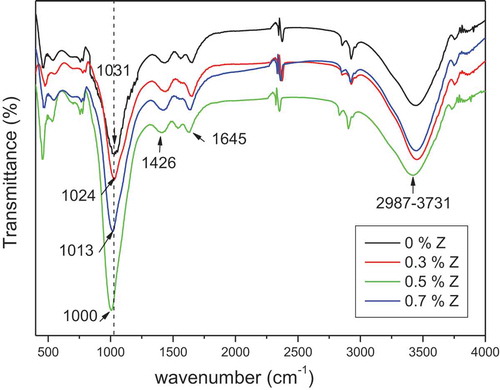
Infrared analysis was conducted to explore the interactions between the geopolymers and the filler. shows FTIR spectra of alkali-activated geopolymers containing different amounts of nano-ZnO. The spectrum of the control sample exhibits the principal bands of a geopolymer: a sharp, a intense band at 1027 cm−1attributed to asymmetric strength of the T-O-Si (T = Si or Al) band, a small band in the region of 1426 cm−1 attributed to the presence of Na2CO3 sodium carbonate, a binding vibration band located in the region of 469 cm−1 related to the Si-O-Si band, and two water molecule bands located at 2987–3731 cm−1 and 1645 cm−1. The figure of the FTIR spectra shows no big changes between the nano-zinc oxide fillers (0.3%, 0.5%, and 0.7% incorporated) possibly due to the low concentration of nano-ZnO. The figure shows an increase in the intensity of the T-O-Si band, indicating an interaction between the polymer and the inorganic nano-filler. Khater et al. obtained the same results when adding nano-SiO2 to geopolymers [Citation16]. The spectrum confirms that no new functional group has appeared after the incorporation of nano-ZnO, indicating that only a physical interaction occurred between the nanoparticles and the geopolymer matrix [Citation20].
3.3. Chemical composition (RXF)
In order to confirm the incorporation of nano-ZnO in a geopolymers mixture, RXF analysis was performed. The results noted in indicate the presence of ZnO in the geopolymer network with no other impurities. This shows that the samples were homogeneous.
Table 3. Geopolymer pastes with various zinc concentrations measured by the RXF technique.
3.4. Microstructure properties (SEM)
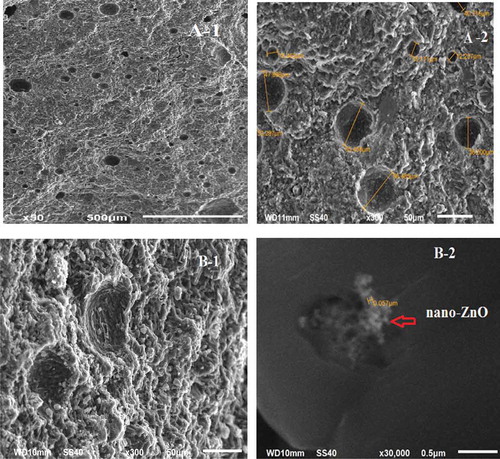
The fracture surfaces of the Metakaolin-geopolymer mixture without and with 0.5% nano-ZnO were examined with a scanning electron microscope (SEM) as shown in . It can be seen that pure geopolymers showed a clear porous structure with non-reacted particles ()). Incorporation of nano-ZnO improved the homogeneity of the geopolymer matrix, moreover, where the network became denser and more compact ()).This may be due to the better interfacial adhesion between the geopolymer matrix and nano-ZnO filler. This enhancement could also be due to the filling of the voids [Citation21]. The same results were observed by Alomyeri et al. and Wang et al. when using nano-Al2O3 and nano-SiO2 as fillers in geopolymers [Citation9,Citation22]
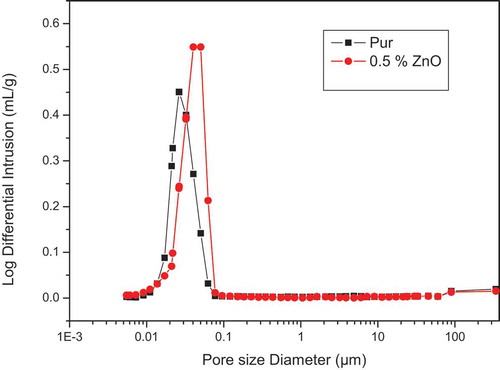
3.5. Mercury intrusion porosimetry (MIP)
exhibits the pore size distribution curve of the geopolymer samples with and without 0.5% nano-zinc. This result shows that the introduction of nanoparticles into geopolymers had different effects on the porosity and pore distribution of the geopolymers. In fact, nano-zinc increased the number of small pores and conserved larger pores. This can be attributed to the absorption of water by the nanoparticles [Citation23]. The percentage of geopolymer porosity is decreased from 32.58 to 31.35 with the incorporation of 0.5% moreover, nano-ZnO, as presented in . This may be due to the filling of the pores with nanoparticles, as already seen in the SEM images.
Table 4. MIP analysis of geopolymers without and with nano-ZnO.
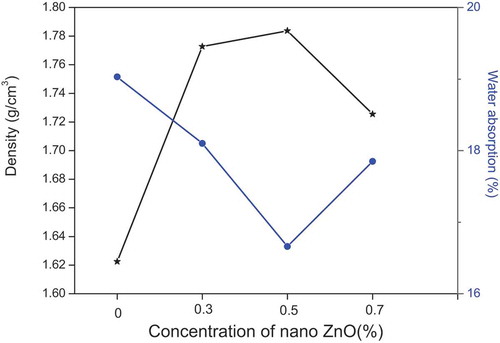
3.6. Density and water absorption
The density and the absorption of alkali-activated geopolymers with different amounts of nano-ZnO are presented in this curve (). The results show that the increasing the percentage of nano-ZnO in the mixes by up to 0.5% increased the density of the geopolymers. This is due to a decrease in the number of the pores as indicated by the SEM images [Citation24]. A reverse negative result is reported for 0.7% nano-ZnO. This may be attributable to the agglomeration of nanoparticles shown in the FTIR and XRD investigations, which can cause the appearance of micro-cracks. Indeed, the curve shows the evolution of water absorption according to the percentage of nano-ZnO. It presents the same density behavior in which the addition 0.5% nano-ZnO exhibits the optimum content to decrease water absorption from 19% to 16%. Similar observations were reported by Assaedi et al. [Citation25]. The test confirmed increasing the improvement by nano-ZnO of the structural properties of geopolymers.
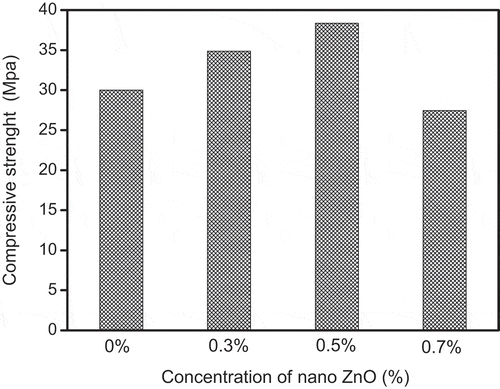
3.7. Compressive strength
To study the mechanical properties of geopolymer mortar with different nano-zinc contents (0.3–0.7%), tests of compressive strength were carried out (). Geopolymers with nano-ZnO added had higher mechanical strength than pure geopolymers. The compressive strength of composites increased with the increases in the concentration of nano-ZnO and then decreased. It reached its highest value (38 MPa) when the dosage of nano-ZnO was 0.5%. That was due to the interfacial adhesion between the nano-ZnO particles and geopolymers, which decreases the interfacial transition zone (ITZ) [Citation26]. When the nano-ZnO dosage was 0.7%, however the compressive strength decreased because of agglomeration of nanoparticles that were not uniformly dispersed [Citation27], according to a study by Rustan et al. [Citation19]. Many research reports present the effect of nanoparticles on the mechanical properties of geopolymers. Lo et al. studied the influence of a low concentration (0.5%) of nano-silica on geopolymer properties, and reported observing no significant change in mechanical strength and a 2.1% enhancement of compressive strength, but in this work, we noted an increase of about 16% in compressive strength.with 0.3% nano-ZnO. Phoo-Ngernkham et al. reported, moreover that the incorporation of 2% nano-SiO2 and 2% nano-alumina, as the best amounts, improved compressive strength by 7% and 25%, respectively [Citation28], and Asseadi et al. showed that the addition of 2% nano-clay improved compressive strength by 23% [Citation29]. In the present study, meanwhile the incorporation of 0.5% nano-ZnO increased compressive strength by 26%.
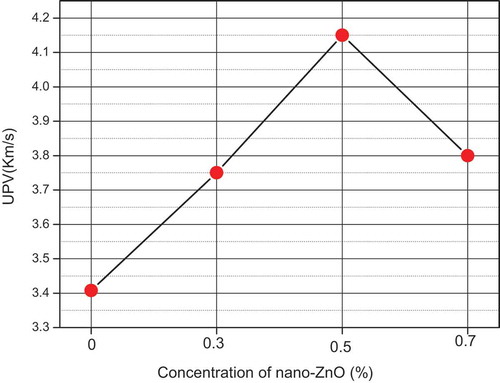
3.8. Ultrasonic pulse velocity (UPV)
To control the effects of nano-ZnO on the internal structure of the geopolymer matrix, UPV measurements of the samples containing different nano-ZnO contents are plotted in As can be clearly observed from the curve, as the percentage of nano-ZnO increased from 0.3% to 0.7%, the velocities of direct UPV increased by 8.82%, 22.05% and 11.76% for Z0.3, Z0.5 and Z0.7 respectively. The highest UPV value achieved of 4.14 Km/s for Z0.5 indicates the higher quality of the geopolymers. In fact, this resultshows that the nanoparticles led to changes in the materiel from a mesoporous structure (<3.5 Km/s) to a compact structure (>4 Km/s) [Citation30], which was attributed to the enhancement of the density of the matrix as illustrated in the previous analysis. Furthermore, it can be noted that the reduction of the UPV value for the sample Z0.7 as compared to the optimum values indicates the existence of defects and internal cracks in the concrete. These UPV results support the findings obtained from the strength measurements after incorporation of the nano-ZnO, where the compressive strength values improved with their addition of ZnO up 0.5% and then decreased with the addition of 0.7%. The same behavior was obtained by Diab et al. [Citation31].
3.9. Thermal analysis
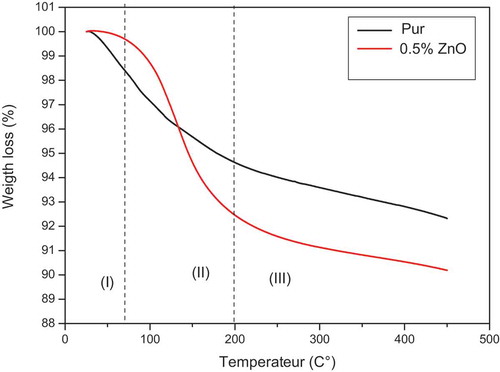
The influence of nano-zinc on the thermal properties of geopolymers is reported in this section. The TG analysis was performed only at between 25°C and 450°C to control the start of the sample decomposition precisely, and to determine accurate temperature values. The spectra were divided into three different ranges, as shown in . The first range of between 25°C and 70°C shows that the control sample lost almost 2% of its initial mass while the other sample remained invariable, this indicating that the addition of nanoparticles to a geopolymer makes it possible to store water. In the second range from 70°C to 200°C, at there were very clear changes, as the pure geopolymer curve showed a decrease in weight at a slow pace and the nano-ZnO/geopolymer curve showed a large weight loss at a very fast pace. There changes can be attributed to the absorption of free water by the nanoparticles. This may be due to the large surface area of these nanoparticles [Citation32]. In the third range (200–450°C) the weight loss continued at the same rate. This range represents the chemical band of water [Citation32], due to the large amount of water and gel enhancing the development of the structure. This can be further supported by comparative studies between nano-ZnO/polymers and pure polymers which have been reported by Masoud et al. [Citation33]. They showed that using nano-ZnO as filler in polymers significantly improved their thermal properties.
4. Conclusions
The effect of nano-ZnO on the structural, mechanical and thermal properties of geopolymer-based metakaolin was studied. The XRD patterns showed that adding nano-ZnO improved the interaction between the nanoparticles and the geopolymer, as indicated by a shift of the geopolymers peak. The content of the gel geopolymers developed with the introduction of from 0.3% to 0.5% nano-ZnO as shown by increases in the T-O-Si band attributed to the enhancement of the geopolymeric process, which had a positive effect on the microstructural properties of the geopolymers as observed in SEM images. Furthermore, mechanical tests showed a significant increase in velocity up to 4.4% and in compressive strength up to 38 MPa. However, the incorporation of 0.7% nano-ZnO decreased the overall properties of the geopolymers. The MIP and tests of density and water absorption also confirmed these results. In fact, 0.5%, was considered the optimal amount of nano-ZnO to improve the structural and mechanical properties of geopolymers. TG/DTA analysis showed the thermal stability of the geopolymers and indicate that the incorporation of nano-ZnO improved the thermal properties of geopolymer. This study showed a strong relationship between mechanical, structural and thermal properties of geopolymers. Moreover, all the investigations presented in this report show that the addition of a small amount of nano-ZnO (0.5%) to geopolymer used in the construction field is appropriate because the resulting composites have a compressive strength almost equal the compressive strength of Portland cement (38 ≈ 40 MPa).
Lists of changes
* The title of this manuscript should define any chemical symbols or structures by the names, so I suggest that authors change the title to “Study the effect of nano zinc oxide on structural, thermal, and mechanical properties of geopolymer-based metakaolin”.
“Study the effect of nano-ZnO on structural, mechanical and thermal properties of geopolymer-based metakaolin”
→ Effect of nano-ZnO on mechanical and thermal properties of geopolymer
Abstract
* Please, authors, clarify and rephrase the concept of the sentences in lines 20 and 21.
Furthermore, infrared specters showed an increase of geopolymer gel content with the addition of nano-ZnO
→ FTIR analyses showed an increase in the geopolymerization after the addition of nano-ZnO to the geopolymer paste.
* The sentences in the lines 25 and 26 need rephrasing and English grammar check, please.
→ The thermal properties were analyzed using the thermogravimetric technique (TG/DTA)
The thermal properties of geopolymer nano-ZnO are also investigated using thermogravimetric analysis (TGA).
Experimental section
* Authors should number “Alkaline solution” as subheading (For example 2.2. Alkaline solution). The same with the rest (2.3. Nano-ZnO synthesis).
→ OK
* The Equation (1) in line 53, the symbol G needs to be defined, please.
→ G is the average grain size
* Section 2.2. Method, the word should change to Nano-ZnO/Metakaolin composite preparation because the section “Method” does not make sense. This section needs more rephrasing, clarification and checks the English grammar styles.
The nano-ZnO was added to the metakaolin at the loadings of 0.3%, 0.5% and 0.7% by weight. The alkaline solution and nano-ZnO were first stirred for 5 min at a low speed and then mixed for another 10 min at high speed until a uniform mixture was achieved .
The metakaolin was then added slowly to the solution at a medium speed for 5 min, and then further mixed for another 10 min on high speed until the mix became homogeneous. The obtained mixtures were then casted into 3*6 cm cylindrical molds. The molds were then vibrated during casting to reduce air bubbles and improve the compaction before they were covered with a plastic film to minimize the loss of the evaporation water and cured for 24 h under room temperature (20 ± 4°C), then the specimens are demolded and cured at 80°C (). The samples are directly tested for compressive strength then small-crushed samples are immersed in acetone to stopping the geopolymeric reaction and then dried 24 h at 105°C.
* Section 2.3, authors should capitalize the first word.
→ OK
* Line 101, authors should sperate this long paragraph to sections. For example, authors should start with 2.3. Characterizations and then list the measurements separately.
→ OK
Discussion section
* The discussion of and FTIR spectra of (0%Z, 0.5%Z, and 0.7%Z) are still not satisfied, and authors should re-test FTIR again.
→ OK
References
- Ali I, Naje A, Al-Zubaidi H, et al. Performance evaluation of fly ash-based geopolymer concrete incorporating nano slag. Glob NEST J. 2019;21:70–75.
- Rashad AM, Ouda AS. Thermal resistance of alkali-activated metakaolin pastes containing nano-silica particles. J Therm Anal Calorim. 2019;136:609–620.
- Su Z, Guo L, Zhang Z, et al. Influence of different fibers on properties of thermal insulation composites based on geopolymer blended with glazed hollow bead. Constr Build Mater. 2019;203:525–540.
- Xie J, Wang J, Rao R, et al. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos Part B Eng. 2019;164:179–190.
- Ismail A, Menazea A, Kabary HA, et al. The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by sol–gel method. J Mol Struct. 2019;1196:332–337.
- Ning Y, Fielding LA, Nutter J, et al. Spatially controlled occlusion of polymer‐stabilized gold nanoparticles within ZnO. Angew Chem. 2019;131:4346–4351.
- Ekinci E, Türkmen İ, Kantarci F, et al. The improvement of mechanical, physical and durability characteristics of volcanic tuff based geopolymer concrete by using nano silica, micro silica and styrene-butadiene latex additives at different ratios. Constr Build Mater. 2019;201:257–267.
- Assaedi H, Alomayri T, Shaikh F, et al. Influence of nano silica particles on durability of flax fabric reinforced geopolymer composites. Materials. 2019;12:1459.
- Alomayri T. Experimental study of the microstructural and mechanical properties of geopolymer paste with nano material (Al2O3). J Build Eng. 2019;25:100788.
- Khater H, El Gawaad HA. Characterization of alkali activated geopolymer mortar doped with MWCNT. Constr Build Mater. 2016;102:329–337.
- Duan P, Yan C, Luo W, et al. Effects of adding nano-TiO2 on compressive strength, drying shrinkage, carbonation and microstructure of fluidized bed fly ash based geopolymer paste. Constr Build Mater. 2016;106:115–125.
- Zheng Z, Mounsamy M, Lauth-de Viguerie N, et al. Luminescent zinc oxide nanoparticles: from stabilization to slow digestion depending on the nature of polymer coating. Polym Chem. 2019;10:145–154.
- Ponnamma D, Cabibihan -J-J, Rajan M, et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater Sci Eng C. 2019;98:1210–1240.
- Omri K, Bettaibi A, Khirouni K, et al. The optoelectronic properties and role of Cu concentration on the structural and electrical properties of Cu doped ZnO nanoparticles. Phys B Condens Matter. 2018;537:167–175.
- ASTM, A. D1633–00 (Reapproved 2007). Stand Test Methods Compressive Strength Molded Soil-Cem Cylind. 2007.
- Khater HM. Effect of nano-silica on microstructure formation of low-cost geopolymer binder. Nanocomposites. 2016;2:84–97.
- Khater HMM. Physicomechanical properties of nano-silica effect on geopolymer composites. J Build Mater Struct. 2016;3:1–14.
- Adak D, Sarkar M, Mandal S. Effect of nano-silica on strength and durability of fly ash based geopolymer mortar. Constr Build Mater. 2014;70:453–459.
- Rustan M, Junaedi S, Irhamsyah A. Studi Tentang Pengaruh Nanopartikel Zno (Seng Oksida) Terhadap Kuat Tekan Geopolimer Berbahan Dasar Metakaolin. J Sains Dan Pendidik Fis. 2015;11:286–291.
- Singh S, Arora N, Paul K, et al. FTIR and rheological studies of PMMA-based nano-dispersed gel polymer electrolytes incorporated with LiBF 4 and SiO 2. Ionics. 2019;25:1495–1503.
- Rostami MR, Abbassi-Sourki F, Bouhendi H. Synergistic effect of branched polymer/nano silica on the microstructures of cement paste and their rheological behaviors. Constr Build Mater. 2019;201:159–170.
- Wang J, Du P, Zhou Z, et al. Effect of nano-silica on hydration, microstructure of alkali-activated slag. Constr Build Mater. 2019;220:110–118.
- Nuaklong P, Sata V, Wongsa A, et al. Recycled aggregate high calcium fly ash geopolymer concrete with inclusion of OPC and nano-SiO2. Constr Build Mater. 2018;174:244–252.
- Mohseni E, Kazemi MJ, Koushkbaghi M, et al. Evaluation of mechanical and durability properties of fiber-reinforced lightweight geopolymer composites based on rice husk ash and nano-alumina. Constr Build Mater. 2019;209:532–540.
- Assaedi H, Shaikh FUA, Low IM. Effect of nano-clay on mechanical and thermal properties of geopolymer. J Asian Ceram Soc. 2016;4:19–28.
- Gao X, Yu QL, Brouwers HJH. Characterization of alkali activated slag–fly ash blends containing nano-silica. Constr Build Mater. 2015;98:397–406.
- Assaedi H, Shaikh FUA, Low IM. Influence of mixing methods of nano silica on the microstructural and mechanical properties of flax fabric reinforced geopolymer composites. Constr Build Mater. 2016;123:541–552.
- Phoo-ngernkham T, Chindaprasirt P, Sata V, et al. The effect of adding nano-SiO2 and nano-Al2O3 on properties of high calcium fly ash geopolymer cured at ambient temperature. Mater Des. 2014;55:58–65.
- Assaedi H, Shaikh FUA, Low IM. Effect of nanoclay on durability and mechanical properties of flax fabric reinforced geopolymer composites. J Asian Ceram Soc. 2017;5:62–70.
- Colangelo F, Roviello G, Ricciotti L, et al. Mechanical and thermal properties of lightweight geopolymer composites. Cem Concr Compos. 2018;86:266–272.
- Diab AM, Elyamany HE, Elmoaty AEMA, et al. Effect of nanomaterials additives on performance of concrete resistance against magnesium sulfate and acids. Constr Build Mater. 2019;210:210–231.
- Zaharaki D, Komnitsas K, Perdikatsis V. Use of analytical techniques for identification of inorganic polymer gel composition. J Mater Sci. 2010;45:2715–2724.
- Masoud EM, Hassan ME, Wahdaan SE, et al. (VdF/HFP)/PVAc/lithium hexafluorophosphate composite electrolyte containing nano ZnO filler for lithium ion batteries application: effect of nano filler concentration on structure, thermal stability and transport properties. Polym Test. 2016;56:277–286.