?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The flash sintering process has been regarded as a noble method to densify ceramic materials. The distinctive feature of the flash sintering is the reduced sintering time which is caused by the electric field. In this study, flash sintering of the hydroxyapatite bioceramics was carried out at temperatures of 900–1200°C and electric fields of 500–1200 V/cm. Dense sintered sample was prepared in a short time within 10 seconds. Electric field required for flashing became reduced as the furnace temperature increased. Abrupt rise of the sample temperature of up to 120°C was observed when the flash occurred. The incubation time for flashing became shorter with increasing temperature and electric field. The flashing was found to occur at lower temperature and lower electric field in vacuum than in air. Microstructure of the flash sintered sample was not different from that of conventionally sintered sample.
1. Introduction
Dense ceramics are usually produced from powders by sintering method. Normal sintering method requires high temperature and hour-long time for densification. Cologna et al. [Citation1] successfully produced dense zirconia ceramics in just a few seconds by flash sintering method with an aid of electric field at a temperature of 850°C which was much lower than that of normal sintering. In addition to zirconia-based ceramics [Citation2–13], flash sintering was also found effective to consolidate various ceramic materials such as yttria [Citation14,Citation15], alumina [Citation16–20], spinel, titanium oxide, barium titanate, zinc oxide [Citation21–33] and tricalcium phosphate [Citation34,Citation35]. It was suggested that the rapid densification was attributed to the generation of lattice defects and the temperature rise by Joule-heating. Still more additional research is required for the full understanding of flash sintering mechanism [Citation7,Citation36–42].
Hydroxyapatite or Ca10(PO4)6·(OH)2 is a bio-ceramic material which has a similar chemical composition to that of the human bone. This material has excellent bio-friendly properties such as protein adsorption, bone binding, and cell adhesion, so it is widely used as an artificial bone and bio-implant for the human body [Citation43–47]. Hydroxyapatite ceramics are also usually prepared by a sintering method. Muralithran et al. [Citation48] were able to prepare a hydroxyapatite compact by sintering powders for two hours at a temperature of 1250°C or higher. They also reported that sintering for an extended time caused grain growth and the degradation of mechanical properties [Citation49–51]. In addition to normal sintering, studies using hot pressing [Citation52–55] and spark plasma sintering [Citation56,Citation57] have been reported [Citation58–60]. The spark plasma sintering method was found to be effective, but it is an expensive route because it required an individual mold and a pressurizing device on a large scale. Recently, Yun et al. have attempted a flash sintering of hydroxyapatite, and have successfully prepared a dense sample in a short time of 10 seconds without an application of pressure [Citation51,Citation61].
In this study, hydroxyapatite ceramic was sintered using the flash sintering method. The flash onset temperature, voltage, incubation time, flash current and the increase in temperature were investigated. When the flash was observed, the sample temperature increased due to the internal heating of the sample, and a dense sintered body was prepared within a few seconds. The rapid sintering mechanism was discussed.
2. Experiment procedures
Hydroxyapatite powders (Junsei, Japan) were uniaxially pressed to rectangular plate-shaped green sample, with a size of 20 × 5.4 x 1 mm. The scanning electron microscopy showed that the raw powders had a mean particle size of 60 to 100 nm. Two holes having a diameter of 1 mm were drilled onto the pressed green sample in a thickness direction at an interval of 10 mm in the longitudinal direction. The green sample was placed in a furnace and heated up to a constant temperature while applying a voltage through a platinum wire. The sample was hung by a platinum wire hooked in two holes and a platinum paste was applied to the holes to lower the contact resistance. DC power supply (Magna Power, USA) was used for the power source. Current limit was set to 5 mA for the most of the flashing experiments. During flash sintering, voltage, current, video image and sample surface temperatures were monitored and recorded using a digital multi-meter, a CCD and a pyrometer.
The furnace was heated up at a rate of 10°C/min and when a temperature reached 900°C voltage began to be applied. When the flash event occurred as the temperature went up, the onset temperature was recorded. Then another sample was heated up at the same heating rate, and when the flash temperature previously recorded was reached, a voltage was applied and the incubation time or the period of time from the instance of voltage application to the flash onset was recorded. Finally, flash sintering was carried out in vacuum (5 torr) and the effect of the atmosphere was investigated. A control sample was prepared using a normal sintering method for comparison.
After sintering, the sample was cut and the section between two electrode holes was taken for the density measurement. Bulk density was measured using Archimedes method. A cross-section of the sample was cut, polished, and thermally etched at 900°C for 30 min in air, and then microstructure was analyzed by scanning electron microscopy (SEM). Transmission electron microscopy (TEM) samples were prepared by a focused ion beam (FIB) technique (Auriga, Carl Zeiss, Germany), and aberration-corrected TEM (JEM-ARM200F, Jeol, Japan) was used to observe grains, grain boundaries, and nanovoids in the sample. Phase analysis was performed using an X-ray diffractometer (Smart Lab, Rigaku, Japan) with Cu Kα radiation source at a scan speed of 0.5°/min and a step scan of 0.02°
3. Results and discussions
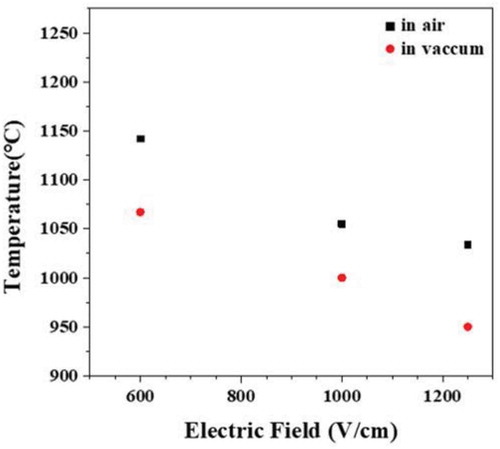
shows the onset temperature and electric field conditions in which the flash occurred in air or in vacuum atmosphere. Regardless of the atmosphere, the flash onset temperature tended to decrease as the electric field increased. The flash onset temperature was 70–80°C lower in vacuum than in air, and the flash onset electric field was about 400 V/cm lower in vacuum than in air for the same temperature, indicating that the flash phenomenon occurs more easily in an atmosphere with a lower gas partial pressure [Citation62,Citation63].
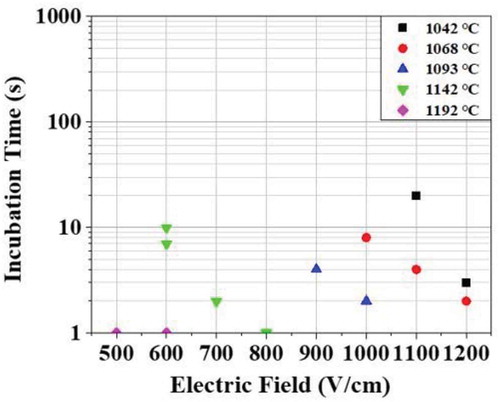
After applying the electric field, the incubation time, or the time taken to generate the flash, was measured and is shown in . The flashing occurred in most of the samples within 30 seconds, but in some samples where the flash condition was not satisfied, the flash did not occur even though it was maintained for 3600 seconds (an hour). In that case, it was judged as “no flash occurrence”. The incubation time became shorter as the electric field or the temperature increased. Flash did not occur in the electric field range of 1200–1400 V/cm at a temperature of 992°C.
Figure 2. Change of the incubation time for the occurrence of flash with a change of temperature and electric field
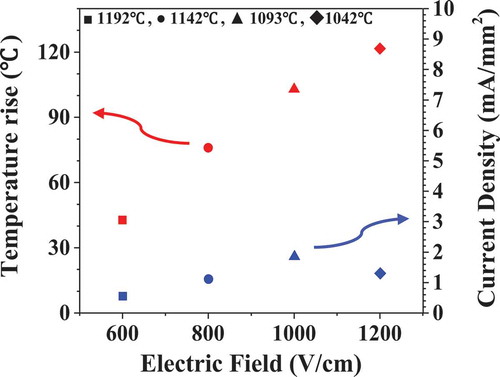
Electric current flowing and the temperature rise of the sample during flash are shown in . When the flash occurred, the electric current increased from zero to 1–10 mA which corresponds to 0.5–1.85 mA/mm2 of current density. The sample temperature measured by pyrometer abruptly rose to a maximum by 42–120°C in stage II and decreased. The amount of temperature rise was found to increase with the increasing electric field and current value.
Figure 3. Electric current density and temperature rise of the sample during flashing under the various electric field
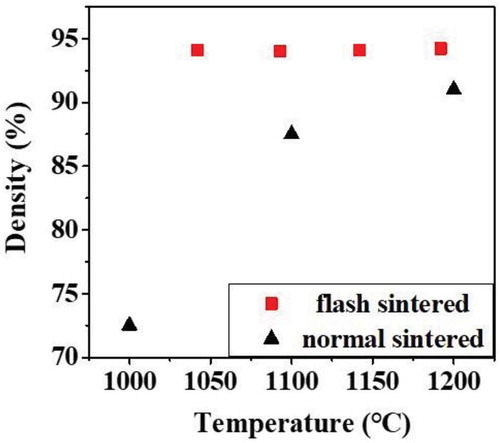
shows the densities of the sample flash-sintered for 10 seconds and of the sample normal sintered for 5 minutes. Densities of the flash sintered samples remained same with varying furnace temperature. And the densities of normal-sintered samples were significantly lower compared to flash sintered samples, indicating that flash sintering was effective for low-temperature sintering.
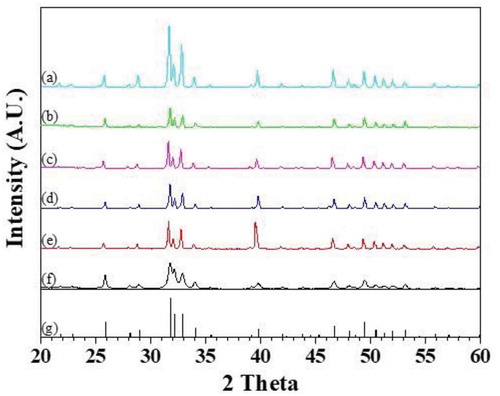
X-ray diffractometry (XRD) pattern of flash sintered sample showed only peaks of hydroxyapatite without any traces of tricalcium phosphate (TCP) or tetracalcium phosphate (TTCP) phase indicating that no phase transformation occurred during flash sintering ().
Figure 5. X-ray diffractometry pattern of the samples produced by flash sintering in the condition of (a) 1192 °C 0 V/cm, (b) 1192 °C 600 V/cm, (c) 1142 °C 800 V/cm, (d) 1093 °C 1000 V/cm, (e) 1042 °C 1200 V/cm, and of (f) hydroxyapatite powders, and (g) JCPDS file (9-432) of hydroxyapatite
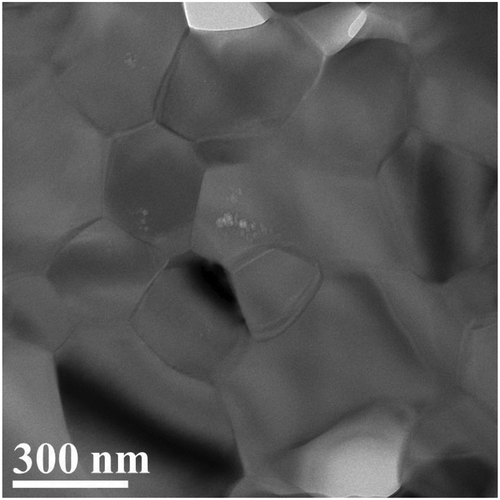
Scanning electron microscopic observation of the cross-section of the sample flash-sintered at 1051°C for 10 seconds showed that it was similar to that of the sample normal-sintered for one hour at 1192°C as shown in even though duration of time was quite different. Transmission electron microscopic image shows that grains are of a size of 100–300 nm, and voids are of a size of 5–10 nm located in grains ()
Figure 6. SEM image of the cross-sectional microstructure of the samples prepared by (a) flash sintering at 1051 °C 1000 V/cm for 10 seconds, and (b) normal sintering at 1192°C for one hour
Figure 7. Transmission electron microscope image of hydroxyapatite sample prepared by flash sintering
As shown in , it was found that some samples partially melted. Melt trace was formed on the surface with the shape of a narrow band between two electrode holes. Recrystallized grains were observed at the melt trace by SEM. Melt trace was formed on a front surface near an electrode hole but connected to another one on the back near the other hole, showing that melting was done not only on the surface but also extended to the inside of the sample. An instantaneous temperature rise of 400–700°C was recorded during flashing in the sample with a partial melting in contrast to 120°C of the sample without. The current measured was 42 mA in the sample with a partially melting which was very high compared to 5 mA in the sample without. Even though the trace shows non-uniform melting in a route, flashing seems to occur uniformly because the cross-sectional microstructure was dense and uniform. Once the local temperature exceeds melting point, sample begins to melt locally. Since the resistivity of liquid melt is much lower than that of solid phase [Citation64], electric current preferentially flows through melt, and further melting would be accelerated, finally forming a melt trace. It is easier for the surface to melt than inside because the sample surface has a higher defect concentration and no restriction on volume expansion during melting. However, the melting was regarded as a non-uniform erroneous densification and was avoided by limiting the maximum current to 5 mA in a current control mode of power supply.
Figure 8. A sample with partial melting during flash sintering, (a) Front and back surface images of the flash sintered sample under the condition of 1000 V/cm, One can see band-like melt trace between the two electrode holes. (b) A cross-sectional microstructure under the melt trace (c) low magnification(x50) image of the melt trace. One can see splash

It has been known that the flash phenomenon is caused by thermal runaway and the generation and movement of defects in the material. Generally defect concentration of the oxide ceramics is a function of the oxygen partial pressure, and the flash occurrence temperature changes with a redox reaction depending on the oxygen partial pressure [Citation7,Citation36–40]. In case of zirconia ceramics oxygen vacancy is a major defect [Citation65], and it has been reported for example that the addition of yttrium oxide with trivalent cations to zirconia with tetravalent cations, increases the oxygen vacancy concentration, and significantly lowers the flashing temperature [Citation66,Citation67]. However, in case of hydroxyapatite ceramics, hydroxide and hydrogen ions are major defects contributing to electrical conduction [Citation68–71] while the oxygen-type defects rarely contribute [Citation69]. When dehydration reaction occurs and hydroxyl groups are decomposed at high temperatures, lattice defects of hydroxyl and hydrogen vacancies and water vapor are generated as shown in EquationEquation (1)(1)
(1) with a square sign for OH vacancy and EquationEquation (2)
(2)
(2) with Kroger-Vink notation [Citation51,Citation59,Citation71,Citation72]. Although the claims of previous researchers are not in agreement and unclear as to which of these two defects is more important in terms of their effect on the electrical conductivity at high temperatures, at least it is obvious that the dehydration reaction is an important defect-generating reaction for electrical conduction in hydroxyapatite [Citation51,Citation71].
The decomposition of hydroxyl groups starts at or above 800°C [Citation59]. As temperature increases, defect concentration increases by enhanced dehydration. Defect mobility also and finally electrical conductivity increases with temperature. Indeed, it was found in this study that as the temperature increased, the flash occurred more easily. In addition, the dehydration reaction is accelerated in vacuum, increasing the defect concentration and making the flash easier, being consistent with the result of this study.
Temperature can be more sensitive factor for the flash occurrence than the voltage in the sense that defect concentration is more sensitive to the former than the latter. In fact 40% higher voltage was required when temperature decreases by 8% as shown in . As the temperature increases, the defect concentration increases, and when the defect concentration exceeds a threshold, current begins to flow, causing heat generation inside the sample, and again making the temperature rise. An increase in the temperature further increases the defect concentration, resulting in a synergistic effect of increasing heat generation. Such a chain reaction makes a flash. If the chain reaction is not properly controlled, the temperature of the sample rises rapidly, causing the sample to melt as we observed.
There are two reasons why the densification occurs very rapidly during flash sintering: an increase in the diffusion rate and a change in the sintering mechanism. Since the diffusion rate is a function of temperature, it is clear that a high diffusion rate at high temperatures will contribute to rapid densification. However, Raj [Citation36] and Qin et al. [Citation8] reasoned that the increase in sintering rate cannot be explained only by temperature effect and there must be a fundamental change during flash sintering. Frenkel defects model was proposed for the high conductivity and diffusion rate [Citation36] but its validity was suspicious because the electric field for their formation was much higher than the reported one for the flash sintering experiments. Grain boundary may be an effective path for the current and diffusional flow and plays a role of a heat source resulting in a rapid densification. Further study is on the way.
4. Conclusions
The dense sample of hydroxyapatite ceramics was successfully prepared by flash sintering. Flash occurred in the conditions of an electric field of 500–1200 V/cm and a temperature of 925°C–1200°C. Flash occurred more easily in vacuum than in air. As the temperature increased, the flash occurrence electric field decreased, while the flash generation temperature decreased as the electric field increased. The incubation time required for a flash occurrence was shortened with increasing temperature or electric field. With the occurrence of flash, the sample temperature rose abruptly over the furnace temperature with an amount of up to 120°C. The microstructure of the flash sintered sample was not different from that of the normal sintered sample despite of the rapid sintering. No phase transformation was found during flash sintering. The flash phenomenon was due to a joule heating induced by a defect generation at high temperatures and an increase in current flow. Hydroxyl or hydrogen type of defects generated by dehydration reaction attributed to the increase in the electric current.
Acknowledgments
Authors are grateful to Dr. S. Y. Kim (Yeungnam University, Korea) for his technical advice and Mr. Jisoo Kim (Gumi Electronics & Information Technology Research Institute, Korea) for his technical assistance in transmission electron microscopy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Cologna M, Rashkova B, Raj R. Flash sintering of nanograin zirconia in < 5 s at 850°C. J Am Ceram Soc. 2010;93:3556–3559.
- Francis JSC, Cologna M, Raj R. Particle size effects in flash sintering. J Eur Ceram Soc. 2012;32:3129–3136.
- Francis JSC, Raj R. Flash-sinter-forging of nanograin zirconia: field assisted sintering and super plasticity. J Am Ceram Soc. 2012;95:138–146.
- Downs JA, Sglavo VM. Electric field assisted sintering of cubic zirconia at 390°C. J Am Ceram Soc. 2013;96:1342–1344.
- Muccillo R, Muccillo ENS. Electric field-assisted flash sintering of tin dioxide. J Eur Ceram Soc. 2014;34:3871–3877.
- Naik KS, Sglavo VM, Raj R. Flash sintering as a nucleation phenomenon and a model thereof. J Eur Ceram Soc. 2014;34:2435–2442.
- Todd RI, Zapata-Solvas E, Bonilla RS, et al. Electrical characteristics of flash sintering: thermal runaway of Joule heating. J Eur Ceram Soc. 2015;35:1865–1877.
- Qin W, Majidi H, Yun J, et al. Electrode effects on microstructure formation during FLASH sintering of yttrium-stabilized zirconia. J Am Ceram Soc. 2016;99:2253–2259.
- Qin W, Yun J, Thron AM, et al. Temperature gradient and microstructure evolution in AC flash sintering of 3 mol% yttria stabilized zirconia. Mater Manuf Proc. 2017;42:549–556.
- Liu D, Gao Y, Liu J, et al. Effect of holding time on the microstructure and properties of flash-sintered Y2O3-doped ZrO2. Ceram Int. 2016;42:17442–17446.
- Liu D, Gao Y, Liu J, et al. SiC whisker reinforced ZrO2composites prepared by flash-sintering. J Eur Ceram Soc. 2016;36:2051–2055.
- Liu D, Liu J, Gao Y, et al. Effect of the applied electric field on the microstructure and electrical properties of flash-sintered 3YSZ ceramics. Ceram Int. 2016;42:19066–19070.
- Du Y, Stevenson AJ, Vernat D, et al. Estimating Joule heating and ionic conductivity during flash sintering of 8YSZ. J Eur Ceram Soc. 2016;36:749–759.
- Yoshida H, Sakka Y, Yamamoto T, et al. Densification behaviour and microstructural development in undoped yttria prepared by flash-sintering. J Eur Ceram Soc. 2014;34:991–1000.
- Yoshida H, Morita K, Kim HN, et al. Reduction in sintering temperature for flash-sintering of yttria by nickel cation-doping. Acta Mater. 2016;106:344–352.
- Cologna M, Francis JSC, Raj R. Field assisted and flash sintering of alumina and its relationship to conductivity and MgO-doping. J Eur Ceram Soc. 2011;31:2827–2837.
- Biesuz M, Luchi P, Quaranta A, et al. Theoretical and phenomenological analogies between flash sintering and dielectric breakdown in a-alumina. J Appl Phys. 2016;120:145107.
- Biesuz M, Sglavo VM. Flash sintering of alumina: effect of different operating conditions on densification. J Eur Ceram Soc. 2016;36:2535–2542.
- Biesuz M, Luchi P, Quaranta A, et al. Photoemission during flash sintering: an interpretation based on thermal radiation. J Eur Ceram Soc. 2017;37:3125–3130.
- Biesuz M, Sglavo VM. Current-induced abnormal and oriented grain growth in corundum upon flash sintering. Scr Mater. 2018;150:82–86.
- Yoshida H, Uehashi A, Tokunaga T, et al. Formation of grain boundary second phase in BaTiO3 polycrystal under a high DC electric field at elevated temperatures. J Ceram Soc Japan. 2016;124:388–392.
- Yoshida H, Biswas P, Johnson R, et al. Flash-sintering of magnesium aluminate spinel (MgAl2O4)Ceramics. J Am Ceram Soc. 2017;100:554–562.
- Zhang Y, Nie J, Chan JM, et al. Probing the densification mechanisms during flash sintering of ZnO. Acta Mater. 2017;125:465–475.
- Yoon BL, Yadav D, Raj R, et al. Measurement of O and Ti atom displacements in TiO2 during flash sintering experiments. J Am Ceram Soc. 2017;101:1811–1817.
- González EG, Perejon A, Sánchéz-Jimenez PE, et al. Phase-Pure BiFeO3 produced by reaction flash sintering of Bi2O3 and Fe2O3 and Fe2O3. J Mater Chem A. 2018;6:5356–5366.
- Charalambous H, Jha SK, Lay RT, et al. Investigation of temperature approximation methods during flash sintering of ZnO. Ceram Int. 2018;44:6162–6169.
- Luo J. The scientific questions and technological opportunities of flash sintering: from a case study of ZnO to other ceramics. Scr Mater. 2018;146:260–266.
- Nie J, Zhang Y, Chan JM, et al. Water-assisted flash sintering: flashing ZnO at room temperature to achieve ~98% density in seconds. Scr Mater. 2018;142:79–82.
- Nakagawa Y, Yoshida H, Uehashi A, et al. Electric-current-controlled synthesis of BaTiO3. J. Ceram Soc Japan. 2017;100:3843–3850.
- Bicer H, Beyoglu B, Ozdemir TE, et al. Direct in situ observation of electric field assisted densification of ZnO by energy dispersive X-ray diffraction. Ceram Int. 2019;45:7614–7618.
- Francis JSC, Cologna M, Montinaro D, et al. Flash sintering of anode–electrolyte multilayers for SOFC applications. J Am Ceram Soc. 2013;96:1352–1354.
- Zhang J, Wang Z, Jiang T, et al. Densification of 8 mol% yttria-stabilized zirconia at low temperature by flash sintering technique for solid oxide fuel cells. Ceram Int. 2017;43:4037–14043.
- Shi R, Pu Y, Wang W, et al. Flash sintering of barium titanate. Ceram Int. 2019;45:7085–7089.
- Frasnelli M, Sglavo VM. Flash sintering of tricalcium phosphate(TCP) bioceramics. J Eur Ceram Soc. 2018;38:279–285.
- Frasnelli M, Pedranz A, Biesuz M, et al. Flash sintering of Mg-doped tricalcium phosphate (TCP) nanopowders. J Eur Ceram Soc. 2019;39:3883–3892.
- Raj R. Joule heating during flash-sintering. J Eur Ceram Soc. 2012;32:2293–2301.
- Holland TB, Anselmi-Tamburini U, Quach DV, et al. Effects of local Joule heating during the field assisted sintering of ionic ceramics. J Eur Ceram Soc. 2012;32:3667–3674.
- Lebrun JM, Raj R, First A. Report of photoemission in experiments related to flash sintering. J Am Ceram Soc. 2014;97:2427–2430.
- Naik KS, Sglavo VM, Raj R. Flash sintering as a nucleation phenomenon and a model thereof. J Eur Ceram Soc. 2014;34:4063–4067.
- Terauds K, Lebrun JM, Lee HH, et al. Electroluminescence and the measurement of temperature during Stage III of flash sintering experiments. J Eur Ceram Soc. 2015;35:3195–3199.
- Morisaki N, Yoshida H, Tokunaga T, et al. Consolidation of undoped, monoclinic zirconia polycrystals by flash sintering. J Am Ceram Soc. 2017;100:3851–3857.
- Yoshida H, Sasaki Y. Low temperature and high strain rate superplastic flow in structural ceramics induced by strong electric-field. Scr Mater. 2018;146:173–177.
- Pasteris JD. A mineralogical view of apatitic biomaterials. Am Mineral. 2016;101:2594–2610.
- Valentim RMB, Andrade SMC, dos-Santos MEM, et al. Composite based on biphasic calcium phosphate (HA/β-TCP) and nanocellulose from the açaí tegument. Materials. 2018;11:2213.
- Al-Sanabani JS, Madfa AA, AL-Sanabani FA. Application of calcium phosphate materials in dentistry. Int J Biomater. 2013;2013:1–12.
- Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed. 2002;44:3130–3146.
- Eliaz N, Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 2017;10:334.
- Muralithran G, Ramesh S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram Int. 2000;26:221–230.
- Champion E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013;9:5855–5875.
- Carrodeguas RG, Aza SD. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 2011;7:3536–3546.
- Bajpai I, Han YH, Yun J, et al. Preliminary investigation of hydroxyapatite microstructures prepared by flash sintering. Adv Appl Ceram. 2016;115:276–281.
- Halouani R, Bernache-Assolant D, Champion E, et al. Microstructure and related mechanical properties of hot pressed hydroxyapatite ceramics. J Mater Sci Mater Med. 1994;5:563–568.
- Ioku K, Yamamoto K, Yanagisawa K, et al. Low temperature sintering of hydroxyapatite by hydrothermal hot-pressing. Phosphorus Res Bull. 1994;4:65–70.
- Suchanek W, Yashima M, Kakihana M, et al. Processing and mechanical properties of hydroxyapatite reinforced with hydroxyapatite whiskers. Biomater. 1996;17:1715–1723.
- Raynaud S, Champion E, Lafon JP, et al. Calcium phosphate apatites with variable Ca/P atomic ratio III.Mechanical properties and degradation in solution of hot pressed Ceramics. Biomater. 2002;23:1081–1089.
- Guo X, Xiao P. Fabrication of nanostructured hydroxyapatite via hydrothermal synthesis and spark plasma sintering. J Am Ceram Soc. 2005;88:1026–1029.
- Chaudhry AA, Yan H, Gong K, et al. High-strength nanograined and translucent hydroxyapatite monoliths via continuous hydrothermal synthesis and optimized spark plasma sintering. Acta Biomater. 2011;7:791–799.
- Eriksson M, Liu Y, Hu J, et al. Transparent hydroxyapatite ceramics with nanograin structure prepared by high pressure spark plasma sintering at the minimized sintering temperature. J Eur Ceram Soc. 2011;31:1533–1540.
- Liu Y, Shen Z. Dehydroxylation of hydroxyapatite in dense bulk ceramics sintered by spark plasma sintering. J Eur Ceram Soc. 2012;32:2691–2696.
- Prakasam M, Locs J, Salma-Ancane K, et al. Fabrication, properties and applications of dense hydroxyapatite: a review. J Funct Biomater. 2015;6:1099–1140.
- Yun J, Qin W, Benthem KV, et al. Nanovoids in dense hydroxyapatite ceramics after electric field assisted sintering. Adv Appl Ceram. 2018;117:376–382.
- Zhang Y, Luo J. Promoting the flash sintering of ZnO in reduced atmospheres to achieve nearly full densities at furnace temperature of <120°C. Scr Mater. 2015;106:26–29.
- Caliman LB, Bichaud E, Soudant P, et al. A sample flash sintering setup under applied mechanical stress and controlled atmosphere. Methods X. 2015;2:392–398.
- Pozniak I, Pechenkov A, Shatunov A. Electrical conductivity measurement of oxides melts. Int Sci Colloq. 2006:43:155–160.
- Morisaki N, Yoshida H, Kobayashi T, et al. Intergranular amorphous films formed by DC electric field in pure zirconia. J Am Ceram Soc. 2018;101:3282–3287.
- Grasso S, Sakka Y, Rendtorff N, et al. Modeling of the temperature distribution of flash sintered zirconia. J Ceram Soc Japan. 2011;119:144–146.
- Biesuz M, Pinter L, Saunders T, et al. Investigation of electrochemical, optical and thermal effects during flash sintering of 8YSZ. Materials. 2018;11:1214.1–1214.15.
- Yamashita K, Kitagaki K, Umegaki T. Thermal instability and proton conductivity of ceramic hydroxyapatite at high temperatures. J Am Ceram Soc. 1995;78:1191–1197.
- Yamashita K, Owada H, Nakagawa H, et al. Trivalent‐cation‐substituted calcium oxyhydroxyapatite. J Am Ceram. 1986;69:590–594.
- Takahashi T, Tanase S, Yamamoto O. Electrical conductivity of some hydroxyapatites. Electrochim Acta. 1978;23:369–373.
- Gittings JP, Bowen CR, Dent ACE, et al. Electrical characterization of hydroxyapatite-based bioceramics. Acta Biomater. 2009;5:743–754.
- Ou SF, Chiou SY, Ou KL. Phase transformation on hydroxyapatite decomposition. Ceram Int. 2013;39:3809–3816.