?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this work, we proposed to synthesize CuFe2O4/Fe2O3 core-shell materials with different Fe2O3 contents in order to create new and efficient photo-Fenton-like catalysts for the degradation of methylene blue with oxalic acid as a radical-producing source. The catalysts were prepared through two stages: first, CuFe2O4 was prepared by a hydroxide coprecipitation – annealing method and then, Fe2O3 was immobilized on CuFe2O4 surface by a simple impregnation – annealing procedure. According to the experimental results, our CuFe2O4/Fe2O3 core-shell materials exhibit high photo-Fenton-like catalytic activity for the degradation of methylene blue under both UVA light and visible light, as well as good ferromagnetic properties, which allows them to be easily separated from the solution by a magnet. Among them, the catalyst prepared with the molar CuFe2O4/Fe2O3 ratio of 1:2 showed the best catalytic performance with the rate constant of 2.103 h–1 under UVA light and 0.542 h–1 under visible light, which were 2 times higher than CuFe2O4 sample. The enhanced catalytic activities of our core-shell materials can be attributed to the high content of surface Fe3+ species, high specific surface area and the presence of rod-like Fe2O3 particles on their surface.
1. Introduction
The existence of various dye molecules in textile wastewater has been widely considered as one of major environmental problems that our world is facing today. Due to their toxicity, organic dyes have many negative effects not only on human health but also on aquatic life [Berradi et al. Citation2019, Hassan et al. Citation2018, Ito et al. Citation2016, Lellis et al. Citation2019]. Therefore, treatment of textile wastewater containing organic dyes has become an urgent need for the environment protection. Unfortunately, the decomposition of organic dyes is usually ineffective since these organic molecules are remarkably resistant to conventional biological methods such as activated sludge or anaerobic digestion [Katheresan et al. Citation2018]. Over the past decades, homogeneous Fenton processes based on the reactions of Fe3+/Fe2+ ions and H2O2 were commonly known as a potentially better oxidation approach owing to the generation of highly reactive oxygen species like hydroxyl radicals which can completely mineralize most of organic species [Fenton Citation1894; Gligorovski et al. Citation2015, Neamtu et al. Citation2003, Wang and Xu Citation2012]. More specially, it was reported that the production of hydroxyl radicals was greatly improved under UV light [Zepp et al. Citation1992]. However, the homogeneous Fenton and photo-Fenton technologies still present some shortcomings for practical applications. Firstly, Fe3+/Fe2+ ions as homogeneous catalysts are completely dissolved in reaction solution, leading to enormous challenges in catalyst recovery after wastewater treatment. Secondly, for the recovery, these homogeneous catalysts usually require a post-neutralization process, which possibly causes the increase in the treatment cost as well as the formation of ferric sludge as a secondary pollution source [Catrinescu et al. Citation2003, Hanna et al. Citation2008].
In order to overcome these obstacles, several magnetic photo-Fenton-like catalysts were developed in the literature [Heidari et al. Citation2019, Liu et al. Citation2012, Sharma et al. Citation2015, Sharma and Singhal Citation2018] (in general, the photo-Fenton-like reactions are the advanced oxidation processes using oxidants other than H2O2 and transition metals as catalysts other than iron under illumination [Rodríguez-Narváez et al. Citation2019]). These heterogeneous magnetic catalysts relies on the ferrite materials which belong to the spinel structure with the common formula M2+Fe3+2O4 (M = Fe, Mn, Cu, Zn, Ni). Owing to the tunability of their structure and composition, they do not only display promising photo-Fenton catalytic activities but also exhibit highly ferromagnetic properties which help them to be easily separated from the solution after the treatment [Sharma et al. Citation2015]. Nevertheless, the activity of ferrite catalysts is still limited and needs to be further improved for the practical applications. Recently, Guo et al. proved that the addition of tartaric acid into the H2O2-ferrite-photo system could enhance the decolorization of methylene blue from 52% to 92.1% within 80 minutes [Guo et al. Citation2019]. Unfortunately, it was widely recognized that H2O2 is hard to be stocked for a long period because this compound is unstable and easily decomposes to form oxygen gas and water during the storage.
According to our previous reports, the replacement of H2O2 by oxalic acid can extend the storage time and notably ameliorate the photo-Fenton performance of these catalysts [Dinh et al. Citation2017, Ngo TPH and Le TK Citation2018]. In fact, the ferric species on the surface of ferrite catalysts and oxalic acid dissolved in solution seem to be able to create ferrioxalate complexes which can enhance the photoabsorption of UV-visible light to produce more hydroxyl radicals and thus improve the photo-Fenton efficiency [Liu et al. Citation2012]. Beside, our previous works also proved that the amounts of different ions on the surface of ferrite catalysts greatly affect their photo-Fenton performance [Ngo TPH and Le TK Citation2018]. It was observed that the photo-Fenton catalytic activity tends to rise when the surface Fe content of our catalysts gradually increases [Ngo TPH and Le TK Citation2018]. With that in mind, this work aims to prepare new magnetic photo-Fenton catalysts based on CuFe2O4/Fe2O3 core-shell materials with different Fe2O3 contents on their surface in order to enhance the photo-Fenton catalytic activity. Actually, before our study, the combination of Fe2O3 and CuFe2O4 was carried out by Silva et al. who used the modified Pechini method to form new heterogeneous α–Fe2O3/CuFe2O4 catalysts [Silva et al Citation2020]. These new mixed oxides exhibited both magnetic behavior and high photo-Fenton catalytic activity for the degradation of methylene blue under visible light. However, since the modified Pechini method is based on the polyesterification between citrate salts and ethylene glycol followed by a pyrolysis at high temperatures, this technique is not only inappropriate to synthesize the core-shell materials but also complicated, requiring a careful control of polymerization and a long reaction time (24 hours). Therefore, in this study, we proposed to apply a facile impregnation – annealing method to synthesize our CuFe2O4/Fe2O3 core-shell materials with enhanced catalytic activities. Moreover, oxalic acid was used as a stable and effective radical-producing source instead of H2O2 to further improve the photo-Fenton performance. The influences of Fe2O3 contents on the crystal structure, morphology, surface composition and magnetic properties of our catalysts were also discussed in details.
2. Materials and methods
2.1. Sample preparation
In our work, all chemicals were commercially available and used without further purification. The preparation of CuFe2O4/Fe2O3 core-shell materials with different Fe2O3 contents was effectuated through two stages. In the first stage, CuFe2O4 nanoparticles were synthesized by a simple hydroxide coprecipitation – annealing method which used NaOH as the precipitator. Briefly, Cu(NO3)2.3H2O and Fe(NO3)3.9H2O ((>98%, purchased from Sigma Aldrich) in a molar ratio of 1:2 were dissolved in 200 mL distilled water to form a solution containing 0.10 mol.L–1 Cu2+ and 0.20 mol.L–1 Fe3+. Next, 400 mL solution of 0.40 mol.L–1 NaOH was slowly dropped into the above solution under constant stirring to obtain a brown co-precipitate. This co-precipitate was washed with distilled water, dried at 150°C during 2 hours and then annealed in an electric furnace at 800°C for 2 hours to produce magnetic CuFe2O4 nanoparticles.
In the second stage, Fe2O3 was immobilized on the surface of CuFe2O4 nanoparticles via a facile impregnation – annealing process. Firstly, 1.20 g synthesized CuFe2O4 powder was dispersed in 400 mL solution of NaOH (0.225 mol.L–1). The suspension was constantly stirred by a mechanic agitator. In the other hand, a series of aqueous Fe(NO3)3 solutions were prepared with different concentrations (0.05, 0.10, 0.15 mol.L–1). These concentrations were calculated following the desired molar CuFe2O4/Fe2O3 ratios (1:1, 1:2, 1:3, respectively). Then, the aqueous Fe3+ solutions were quickly poured into the suspension containing NaOH and CuFe2O4 to form a slurry solution. This slurry solution was regularly stirred for 30 minutes. After that, the magnetic powders were separated from the solution by a magnet, dried at 150°C for 1 hour and annealed at 500°C for 2 hours. Finally, the products were washed with distilled water, collected by a magnet and dried again at 150°C for 1 hour. All the samples were named as CuFe2O4/Fe2O3-X (X = 0, 1, 2 and 3 corresponding to the molar Fe2O3/CuFe2O4 ratios). Besides, Fe2O3 nanoparticles (without magnetic CuFe2O4 cores) were also prepared from aqueous Fe(NO3)3 and NaOH solutions in the same conditions (annealing at 500°C for 2 hours) in order to compare their photo-Fenton activity with that of our CuFe2O4/Fe2O3 samples.
2.2. Characterization
The structural and phase analyses of CuFe2O4 and CuFe2O4/Fe2O3 samples were performed by powder X-ray diffraction (XRD) using a BRUKER-Binary V3 X-ray diffractometer with monochromatic Cu Kα source (λ = 1.5406 Å) operated at 40 kV and 40 mA. The phase identification and the Rietveld refinement were carried out using Joint Committee on Powder Diffraction Standards database (JCPDS cards) and Fullprof 2009 software, respectively. The surface morphology of our samples were characterized via field emission scanning electron microscopy (FE-SEM) images taken on a HITACHI SU8000 with an accelerating voltage of 5 kV. The specific surface area (SBET) of samples was measured through nitrogen adsorption-desorption isotherms recorded at 77 K using a NOVA 1000e analyzer (Quantachrome Instruments).
The Fourier transform infrared studies (FT-IR) for all our samples were carried out in KBr matrix using Bruker VERTEX 70 spectrometer. The FTIR spectra were recorded at the wavenumber resolution of 4 cm–1 and in the wavenumber range of 4000–400 cm−1. The surface atomic composition of our samples was also investigated by low voltage energy-dispersive X-ray spectroscopy (EDX) on a HITACHI SU8000 instrument operated at 5 kV (corresponding to the penetration depth of 50 nm [Takano Citation2011]).
The magnetic properties of CuFe2O4 and CuFe2O4/Fe2O3-2 samples were measured at room temperature using a vibrating sample magnetometer PPMS6000 (Quantum Design) in the magnetic field range varying from −11 kOe to 11 kOe. The saturation magnetization (MS), the remanent magnetization (MR) and the coercivity (Hc) of each sample were determined from the obtained corresponding hysteresis loops.
2.3. Catalytic tests
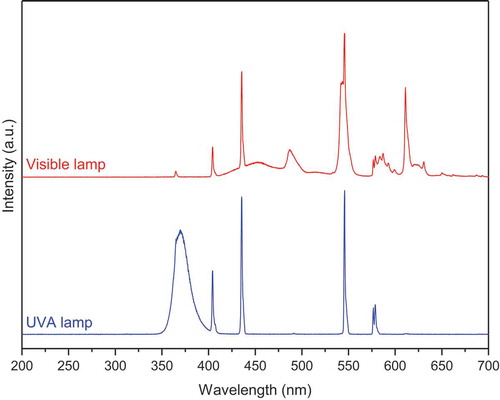
The photo-Fenton-like catalytic activities of CuFe2O4, Fe2O3 and CuFe2O4/Fe2O3 samples with H2C2O4 as a radical-producing source were evaluated through the degradation of methylene blue (MB, purchased from Merck) under UVA light and visible light illumination. The typical experiments were conducted in a batch-type reactor based on a glass beaker containing a solution of MB (2 × 10−5 mol.L−1) and H2C2O4 (10−3 mol.L−1). Firstly, 0.125 g of catalytic powder was dispersed into this solution, which was then constantly stirred by a mechanic agitator in the dark for 60 minutes until the MB adsorption-desorption equilibrium was established. The solution temperature was maintained at about 30°C by a circulation system of water. Next, for photo-Fenton-like reactions, the suspension was irradiated by a visible light (VIS) lamp (9 W Osram Dulux S with the visible-light intensity of 12.5 W.m–2) or an ultraviolet A (UVA) light lamp (9 W Radium 78 with the UVA-light intensity of 33.0 W.m–2) fixed 10 cm above the solution surface. The light intensity of both lamps was measured by an Ocean Optics USB4000 spectrometer. It should be noted that MB has the maximum absorption at 664 nm whereas the light spectra of both our UVA and visible lamps (, measured by a StellarNet spectrometer USB4C00211) does not show any peak around this wavelength. Furthermore, the capacity of our lamps is only 9 W, much lower than that of other works [Liu et al. Citation2012, Sharma et al. Citation2018], which allows us to avoid the self-sensitization of MB molecules. At given time intervals, aliquots (5 mL) of solution were collected, followed by the separation of catalytic powder from the solution by a magnet. Finally, the concentrations of remaining dye were analyzed using a Helios Omega UV – VIS spectrophotometer (Thermo Fisher Scientific, USA) at 664 nm. The total organic carbon (TOC) of the solution was also determined by Shimadzu TOC–VCPH analyzed. The calibration curve was prepared using potassium hydrogen phthalate (99.99%, purchased from Merck).
3. Results
3.1. Crystal structure and phase composition
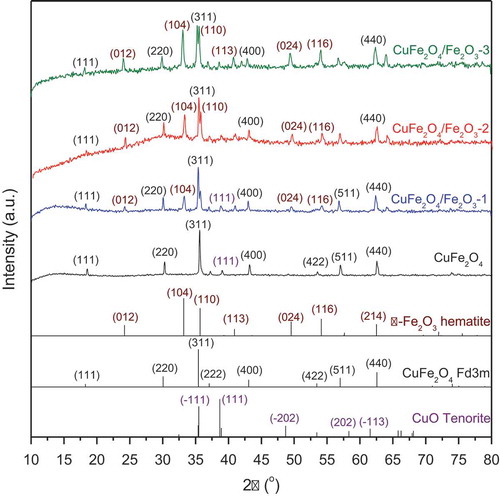
XRD characterization was used to determine the crystal structure of our magnetic CuFe2O4 powder and CuFe2O4/Fe2O3 core-shell materials (). From their patterns, the quantitative analysis of phase composition was carried out by using Fullprof 2009 program and the results are given in . Accordingly, the pattern of CuFe2O4 sample shows the characteristic diffraction peaks at 18.2°, 30.0°, 35.3°, 36.9°, 43.0°, 53.2°, 56.8° and 62.2°, corresponding to the crystallographic planes of CuFe2O4 cubic spinel phase (space group Fd3m, JCPDS No.34–0425) [Gupta et al. Citation2020]. These peaks were found to be symmetric and intense, indicating the high crystallinity and high content of CuFe2O4 spinel phase (92.0%). A weak peak was also detected at 38.7°, which can be indexed to the CuO tenorite phase (space group C2/c, JCPDS No. 05–0661) as the minor impurity phase (8.0%). When different contents of Fe2O3 were applied on the surface of CuFe2O4 nanoparticles, a new series of diffraction peaks was observed at 24.1°, 33.1°, 40.9°, 49.5°, 54.1° and 64.1°. These peaks can be attributed to the α-Fe2O3 hematite phase (space group R-3 c, JCPDS No. 86–0550) [Ghaffari et al. Citation2020, Gupta et al. Citation2020], suggesting the successful combination of two components, CuFe2O4 and Fe2O3, in our core-shell materials. Moreover, as shown in , the amount of hematite phase gradually increases with the content of Fe2O3 used in the preparation of CuFe2O4/Fe2O3 samples.
Table 1. Phase composition of CuFe2O4, CuFe2O4/Fe2O3-1, CuFe2O4/Fe2O3-2 and CuFe2O4/Fe2O3-3 samples
3.2. Morphology and surface specific area
The FE-SEM images of CuFe2O4 and CuFe2O4/Fe2O3 core-shell samples are represented in . It can be seen that the CuFe2O4 sample is composed of agglomerated cubic particles with their size range between 80 and 200 nm (). In contrast, all CuFe2O4/Fe2O3 core-shell materials show the formation of rod-like particles which nearly covered the entire surface of cubic CuFe2O4 particles. The rod-like particles are likely to be assigned to Fe2O3 nanoparticles since they were grown on the surface of magnetic core during the synthesis process. Interestingly, the FE-SEM observation (with magnification of 100 K) also showed the evolution of particle size when the content of Fe2O3 increased. For CuFe2O4/Fe2O3-1 sample, the rod-like particles are about 100 nm in length and 40 nm in diameter (). This length tends to increase to 150 nm whereas the diameter tends to decrease to 25 nm for the core-shell material prepared with the molar CuFe2O4/Fe2O3 ratio of 1:2 (). However, when the molar CuFe2O4/Fe2O3 ratio was up to 1:3 (), the rod-like particles were strongly shortened (60–70 nm in length). More specially, in this sample, some rod-like particles seems to be transformed to spherical particles with the size of about 60 nm. This result suggests that loading a very large quantity of Fe3+ ions on the surface of CuFe2O4 may reduce the available surface area for the development of Fe2O3 seeds, as a consequence, hindering the growth of rod-like Fe2O3 nanoparticles.
Figure 3. FE-SEM micrographs (with magnification of 100 K) of (a) CuFe2O4, (b) CuFe2O4/Fe2O3-1, (c) CuFe2O4/Fe2O3-2 and (d) CuFe2O4/Fe2O3-3 samples; FE-SEM micrographs (with magnification of 200 K) of (e) CuFe2O4 and (f) CuFe2O4/Fe2O3-2 samples
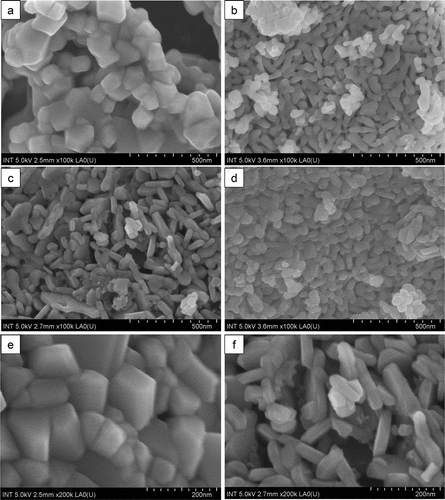
Besides, we also noticed the difference in surface texture between CuFe2O4 and CuFe2O4/Fe2O3-2 samples via FE-SEM images with higher magnification (×200 K). Owing to the presence of randomly distributed rod-like particles, the surface of CuFe2O4/Fe2O3-2 sample () becomes roughness with more porosity than CuFe2O4 surface (), likely resulting in higher surface area for core-shell materials. In order to better investigate the effect of loading Fe2O3 on the surface morphology of CuFe2O4, the nitrogen adsorption-desorption isotherms analysis was carried out. For CuFe2O4/Fe2O3-2 sample, the BET specific surface area was found to be 11.498 m2.g–1, which is about ten times higher than that of CuFe2O4 sample (only 1.159 m2.g–1). The enhanced surface area was also observed for ZnO/Fe2O4 hollow nanospheres [Li et al. Citation2014] and MgFe2O4@SiO2 core shell nanocomposites [Tiwari and Kaur Citation2020]. These FE-SEM and BET results reinforce the fact that Fe2O3 nanoparticles were successfully bound to the surface of CuFe2O4, making the material surface rougher, thereby increasing the specific surface area.
3.3. Surface functional groups
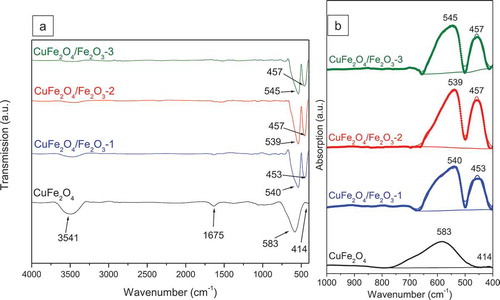
reveals the FTIR spectra of CuFe2O4 and CuFe2O4/Fe2O3 core-shell materials. The spectrum of CuFe2O4 sample contains a large band at around 3541 cm–1 and a weak peak at 1675 cm–1, which can be attributed to the stretching and bending vibrations of surface hydroxyl groups, respectively [Huang et al. Citation2007]. The intensities of these peaks were dramatically reduced for CuFe2O4/Fe2O3 samples, indicating the impacts of Fe2O3 immobilization upon the surface of magnetic core. Furthermore, since CuFe2O4 exhibits an inverse spinel-type structure in which Cu2+ ions only occupy octahedral sites whereas Fe3+ ions can occupy both tetrahedral and octahedral sites, the FTIR spectrum of our CuFe2O4 sample also shows two peaks in the fingerprint region, corresponding to the metal-oxygen bonds in tetrahedral sites (Mtetra–O, 596 cm–1) and the metal-oxygen bonds in octahedral sites (Mocta–O, 436 cm–1) [Aliyan et al. Citation2017, Zaki and Dawoud Citation2010]. However, different from the high intensity of Mtetra–O peak, the Mocta–O peak is extremely low. Only when Fe2O3 nanoparticles were introduced, the intensity of the Mocta–O peak was greatly improved. According to the FTIR absorption spectra (), among different samples, the CuFe2O4/Fe2O3-2 sample shows the most intense Mocta-O peak, suggesting the highest content of octahedral metal ions on the surface. In addition, when combining Fe2O3 and CuFe2O4, it appears that the Mocta–O peak shifts to higher wavenumbers (453–457 cm–1) whereas the Mtetra – O peak shifts to lower wavenumbers (539–545 cm–1). The results demonstrates that the coating of Fe2O3 on CuFe2O4 can strongly affect the surface functional groups of our materials.
3.4. Magnetic properties
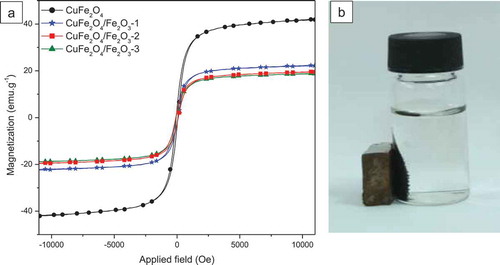
The magnetic hysteresis curves of our samples are depicted in . From these curves, the magnetic parameters such as saturation magnetization, remanent magnetization and coercivity are determined and shown in . All samples were found to follow the soft ferromagnetic behavior at room temperature with the coercivity values inferior to 100 Oe. Among them, CuFe2O4 sample presents the highest saturation magnetization (42.0 emu.g–1) but the lowest coercivity (68.4 Oe). In contrast, CuFe2O4/Fe2O3 core-shell materials exhibit lower MS values and higher HC values, which can be attributed to the presence of antiferromangetic α-Fe2O3 [Bhowmik and Saravanan Citation2010] on the surface of CuFe2O4. However, the magnetic properties of our core-shell materials are still good enough to make them easily be separated from the solution by a magnet ().
Table 2. Magnetic parameters of CuFe2O4, CuFe2O4/Fe2O3-1, CuFe2O4/Fe2O3-2 and CuFe2O4/Fe2O3-3 samples
3.5. Photo-Fenton catalytic activity
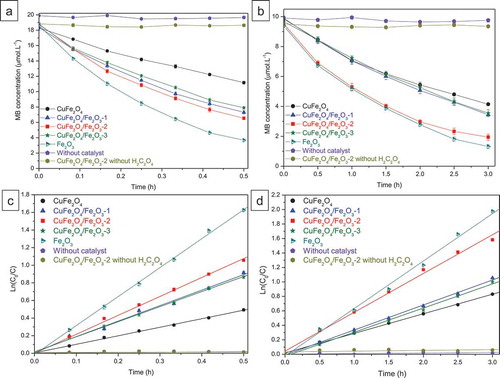
The photo-Fenton catalytic performance of CuFe2O4, Fe2O3 and CuFe2O4/Fe2O3 core-shell materials was evaluated through the MB degradation under UVA light and visible light (, respectively). The apparent rate constant (k) for each sample is calculated using the pseudo-first-order Langmuir-Hinshelwood kinetic model (). Without our catalysts, the MB concentration remains almost unchanged up to 60 minutes of UVA irradiation and 3 hours of visible-light irradiation, confirming that MB is resistant to self-photolysis by UVA light and visible light. Additionally, the photocatalytic test without using oxalic acid (only using CuFe2O4/Fe2O3-2 sample) also demonstrated the stability of MB under both UVA light and visible light despite the presence of our catalyst, which suggests that our sample can not act as an effective photocatalyst in the experimental conditions. The photo-Fenton degradation of MB was only observed when our catalysts were combined with oxalic acid. In fact, as shown in , with oxalic acid as the radical-producing source, CuFe2O4 sample exhibited the rate constants of 0.962 h–1 under UVA light and 0.271 h–1 under visible light. When CuFe2O4 was replaced by CuFe2O4/Fe2O3 catalysts, the rate constants greatly increased and reached the best values for the CuFe2O4/Fe2O3-2 sample (k = 2.103 h–1 and 0.542 h–1 under UVA and visible light, respectively). However, when the Fe2O3 content was further enhanced, the rate of MB degradation tended to decrease. These results prove that the photo-Fenton-like catalytic activity of our samples is able to be controlled by the CuFe2O4/Fe2O3 ratios used in catalyst preparation. Moreover, the UV-light-induced catalytic activity is always higher than the visible-light-induced catalytic activity for all catalysts, indicating that the light energy is also a factor affecting their catalytic performance.
Table 3. Comparison of MB degradation rate constants on different catalysts under UVA light and visible light in the presence of oxalic acid
Figure 6. Comparison of MB degradation on different catalysts: MB degradation curves as a function of time under UVA light (a) and visible light (b), Ln (C0/C) versus time plot of MB degradation under UVA light (c) and visible light (d). C is the MB concentration (mol.L−1) at time t and C0 is the initial MB concentration (mol.L−1)
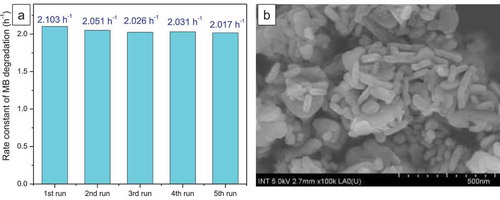
In order to verify the stability and the reproducibility of our catalysts, the leaching test and the reuse tests for the CuFe2O4/Fe2O3-2 sample were carried out as follows: after the first photo-Fenton catalytic run with our CuFe2O4/Fe2O3-2 sample and oxalic acid under UVA light or visible light, the catalyst was removed from the solution by a magnet, washed with distilled water and dried at 150°C for 1 hour. Then, the catalyst was reused for four next consecutive runs in the same conditions. Besides, the iron concentration in the solution of the first run was evaluated by atomic absorption spectrometry (AAS) using Shimadzu AA-6300 spectrometer. Within 0.5 hour under UVA light or 3 hours under visible light, the catalytic performance of the CuFe2O4/Fe2O3-2 sample was only slightly reduced after four-time reuses (). The FE-SEM image also displays that rod-like and spherical Fe2O3 nanoparticles still cover nearly the entire surface of this sample after 5 consecutive catalytic tests (), indicating the reproducibility and the potential of this catalyst for practical applications. Furthermore, the AAS result shows a very low concentration of leached Fe3+ ions (1.56 mg.L–1), suggesting that our heterogeneous catalyst is stable in the experimental conditions and the dissolution of iron species by oxalic acid can be neglected.
Figure 7. (a) Degradation of methylene blue on CuFe2O4/Fe2O3-2 catalyst in five consecutive experiments, (b) FE-SEM image of CuFe2O4/Fe2O3-2 sample after five consecutive catalytic tests
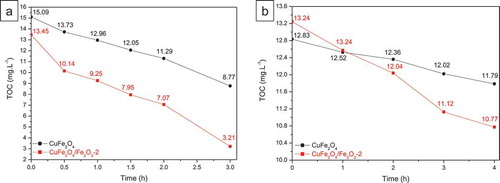
Finally, compares the TOC evolution versus irradiation time of CuFe2O4 and our core-shell CuFe2O4/Fe2O3-2 sample. It was observed that CuFe2O4/Fe2O3-2 sample always showed the better TOC removal than CuFe2O4 under UVA light and visible light. Nevertheless, for both samples, the TOC removal is slower than the discoloration of MB solution, which indicates that most MB molecules were effective broken by the highly reactive oxygen species but small organic intermediates produced from the MB degradation are difficult to be removed from the solution.
4. Discussion
As shown in and , Fe2O3 nanoparticles (without magnetic CuFe2O4 cores) exhibited great photo-Fenton catalytic activities for MB degradation (k = 3.285 h–1 under UVA light and k = 0.655 h–1 under visible light). However, since these nanoparticles are antiferromagnetic and well dispersed in the solution, they are difficult to be recovered and reused. In contrast, although CuFe2O4 and other ferrite materials can be easily recovered owing to their ferromagnetic properties, their catalytic performance was not high enough for practical applications due to the limited iron species on their surface. Therefore, the combination of Fe2O3 and CuFe2O4 can be a promising solution for the improvement of magnetic photo-Fenton catalysts. On the other hand, it should be reminded that our catalysts did not display photocatalytic activities in the experimental conditions (using a 9 W Osram Dulux S lamp as visible light source and a 9 W Radium 78 lamp as UVA light source) although in some studies, the authors reported the photocatalytic activity of CuFe2O4 materials. For these studies, the authors usually used a 500 W xenon lamp [Zhu et al. Citation2013] or a 150 W xenon arc lamp [Ismael et al. Citation2020]. Their capacity is much higher than that of our lamps (only 9 W). There are also some works on CuFe2O4 photocatalysts using a 8 W lamp, but this lamp emits the light in the UVC region [Gupta et al. Citation2020] whereas our study only uses the UVA lamp and visible lamp. Hence, the photocatalytic activity of our samples can be excluded from this study.
According to the experimental results, via the immobilization of Fe2O3 onto CuFe2O4 particles, we really improved the photo-Fenton-like catalytic activity of CuFe2O4 for the degradation of MB under both UVA light and visible light. This enhancement of activity should be explained by various factors including the change in phase composition, the evolution of morphology and the variations of functional groups on the surface of our catalysts. In fact, the growth of Fe2O3 nanoparticles on the surface of magnetic CuFe2O4 cores did not only increase the hematite phase in the structure of samples but also modified the distribution of metallic ions on their surface. As displayed in the FTIR spectra, the magnetic CuFe2O4 powder shows an extremely weak Mocta–O peak, indicating a very limited presence of surface octahedral metal ions (Cu2+ and Fe3+). In contrast, when the surface of CuFe2O4 was coated by Fe2O3 nanoparticles, the intensity of this peak remarkably increased, which can be associated with the fact that our Fe2O3 nanoparticles crystallize in the corundum structure and contain all Fe3+ ions in octahedral sites [Kraushofer et al. Citation2018, Li et al. Citation2016]. Interestingly, the Mtetra–O peak of these CuFe2O4/Fe2O3 core-shell materials is still intense, even more intense than that of CuFe2O4 sample. These results demonstrate that our CuFe2O4/Fe2O3 core-shell catalysts contain the high amounts of both tetrahedral and octahedral Fe3+ ions on their surface, which can be considered as the main reason for the improvement of photo-Fenton-like catalytic performance. Among all our catalysts, the CuFe2O4/Fe2O3-2 sample seems to show the highest content of surface Fe3+ ions owing to the most intense Mocta–O and Mtetra–O peaks in its FTIR spectrum. The enhanced Fe content on the surface of our core-shell catalysts is also supported by the EDX study. In fact, shows the surface elemental composition of CuFe2O4 and CuFe2O4/Fe2O3-2 samples taken by EDX spectra recorded at 5 kV. The Fe quantity of CuFe2O4 is found to be only 16.60 at% while the Fe content of CuFe2O4/Fe2O3-2 reaches 23.05 at%. Moreover, when Fe2O3 was immobilized on CuFe2O4 surface, the surface Cu content was dramatically reduced, from 12.06 at% for CuFe2O4 to 0.09 at% for CuFe2O4/Fe2O3-2. Since all our samples still show the ferromagnetic properties of CuFe2O4 particles, we believe that these results support the core-shell structure of our CuFe2O4/Fe2O3 catalysts.
Table 4. Surface elemental composition of CuFe2O4 and CuFe2O4/Fe2O3-2 samples
Generally, the enhanced content of Fe3+ ions on the surface of our CuFe2O4/Fe2O3 catalysts can promote the formation of surface ferrioxalate complexes via the reaction between surface ≡Fe3+ ions and H2C2O4 [Jeong and Yoon Citation2005, Liu et al. Citation2012, Ngo TPH and Le TK Citation2018]. Then, due to light irradiation, these ferrioxalate complexes will be excited to produce numerous radicals such as C2O4●–, O2● – and ●OH (Equationeq. 1(1)
(1) –Equation4
(4)
(4) ) [Liu et al. Citation2012, Mulazzani et al. Citation1986], which are both highly reactive and thus able to degrade effectively MB molecules in solution. Therefore, the best performance of CuFe2O4/Fe2O3-2 catalyst is likely attributed to the highest surface Fe3+ content of this sample.
Secondly, when Fe2O3 nanoparticles were combined with CuFe2O4, the specific surface area of our catalysts was strongly enhanced (about ten times higher than that of CuFe2O4). This can increase the reactive sites on their surface, leading to the enhancement of their photo-Fenton-like activity. Moreover, we also noticed a sound correlation between the shape of Fe2O3 particles and the catalytic activity. Depending on the molar CuFe2O4/Fe2O3 ratios, the Fe2O3 particles could be transformed between the spherical shape and the rod-like morphology. It seems that the rod-like Fe2O3 particles in CuFe2O4/Fe2O3-1 and CuFe2O4/Fe2O3-2 samples showed the better performances than the spherical Fe2O3 particles in CuFe2O4/Fe2O3-3 catalyst. Although the reason for the enhanced activities of rod-like particles is still unclear and needs to be further studied, this phenomena was also observed in some previous works [Chaudhari et al. Citation2012, Liang et al. Citation2015]. Chaudhari et al. compared the peroxidase mimic activity of hematite iron oxides with different nanostructures, including hexagonal prism, cube-like and rod-like particles, and found that the rod-like particles showed the best activity [Chaudhari et al. Citation2012]. Likewise, Liang et al. reported that the photocatalytic degradation of rhodamine B can be improved when using X-shaped α-Fe2O3 nanocrystals which are composed of two rod-like particles crossing each other [Liang et al. Citation2015]. These results indicate that the particle shape also plays an important role in catalytic performance. As a result, by managing the molar CuFe2O4/Fe2O3 ratios, we can control the particle shape of Fe2O3 and consequently the photo-Fenton-like activity of our materials.
5. Conclusion
In summary, we successfully developed a new and effective magnetic photo-Fenton-like catalysts based on the immobilization of Fe2O3 nanoparticles on the surface of CuFe2O4 particles. These core-shell materials exhibited excellent activities for the degradation of methylene blue in the presence of oxalic acid under both UVA light and visible light. Among them, the CuFe2O4/Fe2O3-2 catalyst show the best catalytic performance, which can be assigned not only to the highest surface Fe3+ content of this sample but also to the high specific surface area and the presence of Fe2O3 rod-like particles immobilized on the surface of its magnetic CuFe2O4 cores. Moreover, owing to the presence of magnetic CuFe2O4 cores, our core-shell catalysts can be easily separated from the solution and recovered by using a magnet, making them suitable for practical applications.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
All authors are individually mentioned in the author contribution statement. Thu Uyen Tran Thi prepared the samples and the manuscript. Van Hung Phan estimated the catalytic activity of samples. Huu Thinh Pham Nguyen analyzed the FTIR spectra and FESEM images of samples. The Luan Nguyen analyzed the XRD results and interpreted the data. An Nang Vu measured the magnetic properties of samples. Tien Khoa Le developed the ideas and was a major contributor in organizing the manuscript. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aliyan N, Mirkazemi SM, Masoudpanah SM, et al. The effect of post-calcination on cation distributions and magnetic properties of the coprecipitated MgFe2O4 nanoparticles. Appl Phys A. 2017;123(6):446.
- Berradi M, Hsissou R, Khudhair M, et al. Textile finishing dyes and their impact on aquatic environs. Heliyon. 2019;5(11):e02711.
- Bhowmik RN, Saravanan A. Surface magnetism, Morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3 (hematite) nanograins. J Appl Phys. 2010;107(5):053916.
- Catrinescu C, Teodosiu C, Macoveanu M, et al. Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res. 2003;37(5):1154.
- Chaudhari KN, Chaudharia NK, Yu JS. Peroxidase mimic activity of hematiteiron oxides (α-Fe2 O3) with different nanostructures. Catal Sci Technol. 2012;2(1):119–124.
- Dinh TT, Nguyen TQ, Quan GC, et al. Starch-assisted sol–gel synthesis of magnetic CuFe2O4 powder as photo-Fenton catalysts in the presence of oxalic acid. Int J Environ Sci Technol. 2017;14(12):2613–2622.
- Fenton HJH. Oxidation of tartaric acid in presence of iron. J Chem Soc. 1894;65:899–910.
- Ghaffari Y, Gupta NK, Bae J, et al. One-step fabrication of Fe2O3/Mn2O3 nanocomposite for rapid photodegradation of organic dyes at neutral pH. J Mol Liq. 2020;315:113691.
- Gligorovski S, Strekowski R, Barbati S, et al. Environmental implications of hydroxyl radicals (•OH). Chem Rev. 2015;115(24):13051–13092.
- Guo X, Wang K, Xu Y. Tartaric acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of methylene blue. Mater Sci Eng B. 2019;245:75–84.
- Gupta NK, Ghaffari Y, Kim S, et al. Photocatalytic degradation of organic pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) nanoparticles at neutral pH. Sci Rep. 2020;10(1):4942.
- Gupta NK, Ghaffari Y, Bae J, et al. Synthesis of coral-like α-Fe2O3 nanoparticles for dye degradation at neutral pH. J Mol Liq. 2020;301:112473.
- Hanna K, Kone T, Medjahdi G. Synthesis of the mixed oxides of iron and quartz and their catalytic activities for the Fenton-like oxidation. Catal Commun. 2008;9(5):955.
- Hassan MM, Carr CM. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere. 2018;209:201–219.
- Heidari MR, Varma RS, Ahmadian M, et al. Photo-Fenton like catalyst system: activated carbon/CoFe2O4 nanocomposite for reactive dye removal from textile wastewater. Appl Sci. 2019;9(5):963.
- Huang D, Liao S, Quan S, et al. Preparation of anatase F doped TiO2 sol and its performance for photodegradation of formaldehyde. J Mater Sci. 2007;42(19):8193–8202.
- Ismael AM, El-Shazly AN, Gaber SE, et al. Novel TiO2/GO/CuFe2 O4 nanocomposite: a magnetic, reusable and visible-light-driven photocatalyst for efficient photocatalytic removal of chlorinated pesticides from wastewater. RSC Adv. 2020;10(57):34806.
- Ito T, Adachi Y, Yamanashi Y, et al. Long-term natural remediation process in textile dye-polluted river sediment driven by bacterial community changes. Water Res. 2016;100:458–465.
- Jeong J, Yoon J. pH effect on OH radical production in photo/ferrioxalate system. Water Res. 2005;39(13):2893–2900.
- Katheresan V, Kansedo J, Lau SY. Efficiency of various recent wastewater dye removal methods: a review. J Environ Chem. 2018;6(4):4676.
- Kraushofer F, Jakub Z, Bichler M, et al. Atomic-scale structure of the hematite α-Fe2 O3 (11̅02) “R-Cut” surface. J Phys Chem C. 2018;122(3):1657–1669.
- Lellis B, Fávaro-Polonio CZ, Pamphile JA, et al. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol Res Innov. 2019;3(2):275–290.
- Li J, Liu Z, Zhu Z. Magnetically separable ZnFe2 O4, Fe2 O3/ZnFe2 O4 and ZnO/ZnFe2 O4 hollow nanospheres with enhanced visible photocatalytic properties. RSC Adv. 2014;4(93):51302–51308.
- Li W, Liang X, An P, et al. Mechanisms on the morphology variation of hematite crystals by Al substitution: the modification of Fe and O reticular densities. Sci Rep. 2016;6(1):35960.
- Liang H, Chen W, Wang R, et al. X-shaped hollow α-FeOOH penetration twins and their conversion to α-Fe2O3 nanocrystals bound by high-index facets with enhanced photocatalytic activity. Chem Eng J. 2015;274:224–230.
- Liu SQ, Feng LR, Xu N, et al. Magnetic nickel ferrite as a heterogeneous photo-Fenton catalyst for the degradation of rhodamine B in the presence of oxalic acid. Chem Eng J. 2012;203:432–439.
- Mulazzani QG, D’Angelantonio M, Venturi M, et al. Interaction of formate and oxalate ions with radiation-generated radicals in aqueous solution Methylviologen as a mechanistic probe. J Phys Chem. 1986;90(21):5347–5352.
- Neamtu M, Yediler A, Siminiceanu I, et al. Oxidation of commercial reactive azo dye aqueous solutions by the photo-Fenton and Fenton-like processes. J Photochem Photobio A. 2003;161:87–93.
- Ngo TPH, Le TK. Polyethylene glycol-assisted sol-gel synthesis of magnetic CoFe2O4 powder as photo-Fenton catalysts in the presence of oxalic acid. J Sol-Gel Sci Technol. 2018;88:211–219.
- Rodríguez-Narváez OM, Pérez LS, Yee NG, et al. Comparison between Fenton and Fenton‑like reactions for ʟ‑proline degradation. Int J Environ Sci Technol. 2019;16:1515–1526.
- Sharma R, Bansal S, Singhal S. Tailoring the photo-Fenton activity of spinel ferrites (MFe2O4) by incorporating different cations (M=Cu, Zn, Ni and Co) in the structure. RSC Adv. 2015;5:6006–6018.
- Sharma R, Singhal S. Spinel ferrite mediated photo-Fenton degradation of phenolic analogues: a detailed study employing two distinct inorganic oxidants. Clean-Soil Air Water. 2018;46:1700605.
- Silva EN, Brasileiro ILQ, Madeira VS, et al. Reusable CuFe2O4–Fe2O3 catalyst synthesis and application for the heterogeneous photo-Fenton degradation of methylene blue in visible light. J Environ Chem Eng. 2020;8:104132.
- Takano M. Evaluation of the Ni diffusion to the surface of Au plating for soldering process control. J Surf Anal. 2011;18:2–6.
- Tiwari S, Kaur M. Mechanistic insight into structural and adsorptive properties of core shell reversal nanocomposites of rice husk silica and magnesium ferrite. Adv Powder Technol. 2020;31:2315–2326.
- Wang JL, Xu LJ. Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol. 2012;42:251–325.
- Zaki HM, Dawoud HA. Far-infrared spectra for copper–zinc mixed ferrites. Phys B: Condens Matter. 2010;405:4476–4479.
- Zepp RG, Faust BC, Hoigne J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron (II) with hydrogen peroxide: the photo-Fenton reaction. J Environ Sci Technol. 1992;26:313–319.
- Zhu Z, Li X, Zhao Q, et al. Photocatalyt ic performances and activities of Ag-doped CuFe2O4 nanoparticles. Mater Res Bull. 2013;48:2927–2932.