?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Calcium phosphate ceramics have been studied as promising materials for biomaterial applications owing to their excellent biocompatibility and osteoconductivity. Herein, we investigated the influence of different mass ratios of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) in a calcium phosphate mixture (CPM) on the microhardness and microstructural properties of a series of xHA-(100-x)β-TCP (x = 0, 30, 50, 70, 90, and 100) mixture samples. The chemical compositions and structural properties of the CPM samples were characterized using X-ray diffraction, Fourier-transform infrared spectroscopy, field emission scanning electron microscopy, and energy-dispersive X-ray spectroscopy. The porosity of the HA/β-TCP composites decreased with increasing β-TCP content and reached a minimum porosity when the mass ratio of HA/β-TCP is 70/30, and then increased with increasing β-TCP content again. The surface microhardness of the CPM composites was measured and found to be inversely proportional to their porosities. Therefore, the CPM of HA/β-TCP with a mass ratio of 70/30 exhibited a maximum surface microhardness of 86.02 MPa.
1. Introduction
Life expectancy for humans has been increasing, and bone tissue engineering using biomaterials for bone tissue defects is gaining traction. Currently, calcium phosphates have attracted attention for bone grafting applications owing to their high osteoconductivity [Citation1–3]. One of the most important advantages of calcium phosphates is their similarity to the mineral phase of the human bone. Calcium phosphates such as hydroxyapatite (HA), α-tricalcium phosphate (α-TCP), biphasic calcium phosphate (BCP), and β-tricalcium phosphate (β-TCP) are widely used as bone-grafting materials for bone regeneration in dental and orthopedic applications. A mixture of HA and β-TCP has been widely used in dental applications, like a mixture of calcium silicates (dicalcium silicate and tricalcium silicate) [Citation4].
Particularly, porous HA has been studied because it aids in generating new bones that can be employed for tissue implantation [Citation5]. Further, it has been used to control the volume of pores [Citation6,Citation7]. However, as an inert implant, HA possesses low biodegradability and poor mechanical stability, and is a high-temperature phase of calcium phosphate, whereas β-TCP is a low-cost resorbable bioceramic [Citation8,Citation9]. Therefore, many studies on enhancing the properties of biphasic calcium phosphate (composite of HA and β-TCP) have focused on different contents of β-TCP based on the HA matrix to increase mechanical stability. Optimum HA/β-TCP mass ratios of 70/30 and 60/40 have often been reported [Citation10–12].
The effects of HA and β-TCP biphasic porous ceramics on the phase content and pore size for biomedical applications have been studied previously [Citation13–15]. However, detailed and systematic research on the effect of the ratio of HA and β-TCP on the calcium phosphate mixture (CPM) in controlling its microstructural and mechanical properties has not been conducted. In ref [Citation10], the microstructural and mechanical properties of various compositions of calcium phosphate scaffolds were investigated. However, the investigated phase compositions include HA, β-TCP, and α-TCP, which are different from our compositions that include only HA and β-TCP. Ref [Citation11] and [Citation12] reported the effect of the ratio of HA and β-TCP on the biocompatibility such as osseointegration, cell proliferation, and biodegradation, not on the mechanical properties. This study compares the microhardness and microstructures of HA/β-TCP mixture samples with different mass ratios. We prepared xHA-(100-x)β-TCP mixtures (x = 0, 30, 50, 70, 90, and 100); hence, this study focused on the influence of the HA content on the microhardness and microstructures of HA/β-TCP mixtures.
2. Materials and methods
2.1. Synthesis of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP)
HA and β-TCP were prepared by the solid-state reaction of calcium carbonate with ammonium phosphate monobasic. The Ca/P molar ratios of 1.67 and 1.5, which are the desired Ca/P ratios for synthesizing HA and β-TCP, respectively, were maintained. The starting materials, calcium carbonate (CaCO3, 99%, Sigma-Aldrich) and ammonium phosphate monobasic (NH4H2PO4, 99%, Sigma-Aldrich), were weighed and mixed by ball milling using alumina balls with the ball-to-powder weight ratio of 3.86 in acetone solvent for 24 h. Then, the powders were dried at 70°C for 24 h. The dried powders were sintered at 1200°C for 2 h and at 900°C for 10 h for HA and β-TCP, respectively. The synthesized HA/β-TCP lumps were crushed manually using an agate mortar and pestle, and sieved through a 400 mesh sieve. The mass change and thermal behavior of the raw materials were determined by thermogravimetry (TG) and differential thermal analysis (DTA) using a thermogravimetric differential thermal analyzer (TG-DTA, STA409PC2, Netzsch, Germany) at a heating rate of 10°C/min in air from 30°C to 1000°C. The crystalline structure was characterized using X-ray diffraction (XRD, DMAX-2500, Rigaku, Japan) at 40 kV and 100 mA in the 2θ range of 20–60° at a scan speed of 1°/min using Cu Kα1 radiation.
2.2. Fabrication of calcium phosphate mixtures (CPM) and physicochemical characterization
Six types of CPM (a mixture of HA and β-TCP) were prepared by changing the mass ratio of HA/β-TCP (denoted as HA, 90 HA, 70 HA, 50 HA, 30 HA, and TCP). The structure of the CPM was analyzed using Fourier transform infrared (FT-IR) spectroscopy (VERTEX 80 V, Bruker, Germany) with test wavelengths between 500 and 4000 cm−1. The HA/TCP mixtures were uniaxially pressed in a 10-mm-diameter mold at 10 MPa. The CPM green bodies were sintered in furnace at 800°C for 2 h. The surface microstructures of the HA and β-TCP were examined by field emission scanning electron microscopy (FE-SEM, SU8010, Hitachi, Japan). The Ca/P ratios of the CPM were determined using energy-dispersive X-ray spectroscopy (EDX) analysis. The pore size and porosity (density of pores) were quantified by selecting five images from each specimen, and measuring the parameters using an image analysis software (Image-Pro Plus, Version 6.0, Media Cybernetics Inc., USA). The Vickers hardness was determined using a microhardness tester (MVK-H1, Shimadzu Co., Japan) under a load of 1000 gf for a loading time of 15 s. This procedure was repeated seven times, and the average hardness value was recorded for each sample.
3. Results and discussion
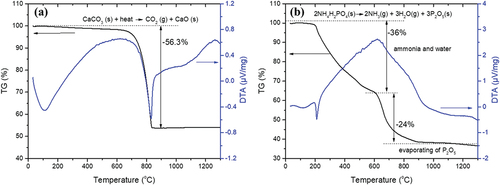
To determine the calcination temperature of calcium carbonate and ammonium phosphate monobasic, TG/DTA curves of the raw materials were obtained and are shown in . The exothermic peak at approximately 800°C and mass loss in the TG curve of CaCO3 indicate the decomposition of calcium carbonate ()). The exothermic peak observed at 208°C in the DTA curve of NH4H2PO4 is attributed to the decomposition temperature of the crystal. The mass loss in the TG curve of NH4H2PO4 until a temperature of approximately 600°C confirms the dissociation of the substance and evaporation of NH3 and H2O. Subsequently, P2O5 evaporates above 660°C. The TG/DTA results show that much of the raw materials (CaCO3 and NH4H2PO4) are decomposed above approximately 800°C. Therefore, the appropriate calcination temperature for synthesizing HA and β-TCP is above 800°C.
Figure 1. Thermogravimetric and differential thermal analyses (TG/DTA) of (a) CaCO3 and (b) NH4H2PO4.
The XRD patterns of the products prepared by the calcination of calcium carbonate and ammonium phosphate monobasic powder at different temperatures are shown in ). The XRD pattern of the sample calcined at 1000°C exhibits two distinct phases: HA (Ca5(PO4)3(OH), hexagonal, JCPDS file No. 00-009-0432) and β-TCP (Ca3(PO4)2, rhombohedral, JCPDS file No. 00-009-0169). On the other hand, the crystalline structure of the sample calcined at 1200°C was identified as the pure HA phase, corresponding to JCPDS file No. 00-009-0432. ) shows the XRD patterns of the β-TCP samples calcined at 700°C, 800°C, and 900°C. The XRD patterns of samples calcined at 800°C and 900°C were indexed to the pure β-TCP phase corresponding to JCPDS file No. 00-009-0169, whereas Ca2P2O7 and β-TCP phases were detected together for the sample calcined at 700°C. shows the lattice parameters a and c of HA and β-TCP calculated from XRD data. The unit cell volume (V) and density (Dx) of HA and β-TCP were also calculated based on the calculated lattice parameters. The calculated Dx of HA and β-TCP were 3.11 and 3.07, respectively. The average crystallite sizes of HA and β-TCP calculated from the XRD data were 64.64 nm and 50.56 nm, respectively.
Figure 2. XRD patterns of (a) HA and (b) β-TCP synthesized at different temperatures by the reaction of CaCO3 and NH4H2PO4.
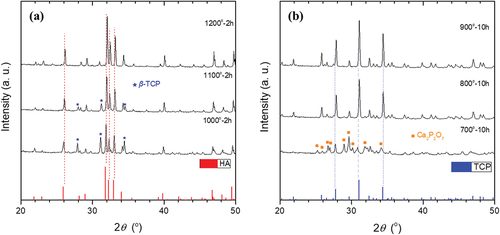
Table 1. Calculated lattice parameters, unit cell volumes, and densities of HA and β-TCP.
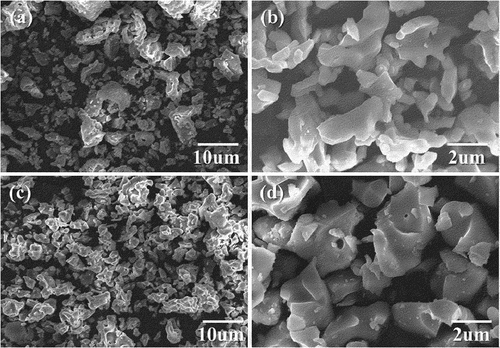
The morphologies and dimensions of the initial HA and β-TCP powders are shown in . ) present typical FE-SEM high-magnification and low-magnification images of the synthesized HA and β-TCP powders after calcination. HA comprises irregularly shaped agglomerated particles and the surface is heterogeneous, with some agglomerations on it. The observed particle sizes of β-TCP powders were approximately 3–5 μm.
Figure 3. FE-SEM morphology of the (a,b) HA and (c,d) β-TCP powders synthesized at 1200 and 800°C, respectively.
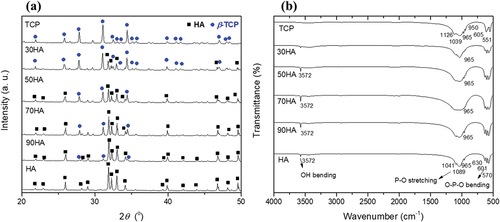
The XRD analysis of CPM with different HA/β-TCP ratios (HA, 90 HA, 70 HA, 50 HA, 30 HA, and TCP) is shown in ). The diffraction peaks of HA gradually decreased with increasing β-TCP content. ) shows the FT-IR spectra of all the CPM samples. The weak band centered at 3572 cm−1 is attributed to the stretching mode of the hydroxyl group corresponding to that of hydroxyapatite [Citation16] and decreased gradually with increasing β-TCP content. A small band at approximately 950 cm−1 is assigned to v1, which is a non-degenerate P-O symmetric stretching mode. The band at 1039 cm−1 is attributed to the v3 symmetric stretching mode. The band with the strongest intensity, which occurs in the range of 1209–946 cm−1, belongs to the stretching modes of the PO3-4 group. For the β-TCP sample, the bands that appear at 605 and 551 cm−1 correspond to the v4 bending mode. The results confirm the presence of HA and β-TCP mixtures.
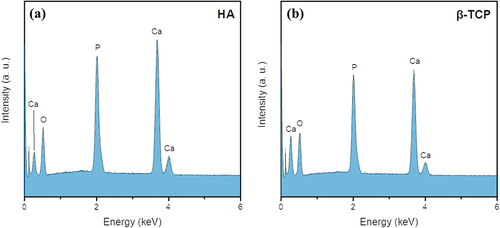
The EDX spectra of HA and β-TCP are shown in ), respectively. The Ca/P ratios were calculated from EDX spectra for CPM with different HA/β-TCP ratios (HA, 90 HA, 70 HA, 50 HA, 30 HA, and TCP) as shown in . The Ca/P ratio decreases with increasing β-TCP content, confirming the mixing ratio of CPM. However, the Ca/P ratios of the samples that are slightly higher than stoichiometric Ca/P ratios suggest the presence of a small amount of CaO in the samples (~1 wt.%). The amount of CaO in the samples was very small; thus, it was not detected in XRD spectra. CaO can originate from thermal decomposition of CaCO3 during solid-state reaction [Citation17,Citation18].
Table 2. Results of calculation of Ca/P ratio based on EDX analysis.
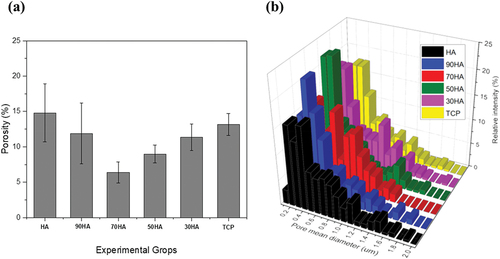
Cross-sections of the HA/β-TCP composites sintered at 800°C were observed by FE-SEM, as shown in . The pore sizes ranged from 0.4 to 0.5 μm, and partially isolated HA/β-TCP particles were observed. ) shows the porosities quantitatively calculated using an image analysis technique. The porosities of pure HA and β-TCP exhibited a similar value and were reduced in the mixture samples (90 HA, 70 HA, 50 HA, and 30 HA). The porosity decreased with β-TCP content until it reached a minimum value of 6.39% for 70 HA and then increased again. The porosity of a binary mixture of irregularly shaped particles is closely related to the equivalent packing diameter, dp [Citation19]:
Figure 6. FE-SEM cross-sectional images of (a) HA, (b) 90 HA, (c) 70 HA, (d) 50 HA, (e) 30 HA, and (f) TCP sintered at 800°C for 2 h.
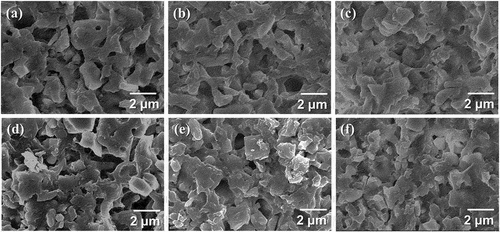
Figure 7. (a) Porosity and (b) pore size distribution depending on the surface microhardness of xHA-(100-x)β-TCP (x = 0, 30, 50, 70, 90, and 100) CPM.
where dv and Ψ are the equivalent volume diameter and sphericity, respectively. dp can be defined as the diameter of a sphere that has original specific volume of a particle. HA and β-TCP have different morphologies, which can be influenced by various factors such as different crystal structures. As shown in , HA and β-TCP have different lattice parameters, unit cell volumes, and densities, which can cause differences in radii and sphericity that are the required input parameters for Equationequation (1)(1)
(1) . The mixture of two particles with different morphologies can be considered as a model for binary mixtures of two different sizes of spheres with diameters of dp. In such a model, the relationship between porosity and volume fraction exhibits a “V-shape” trend, in which the mixture has its minimum porosity (maximum packing density) at a critical volume fraction [Citation20,Citation21]. Therefore, 70 HA is the nearest to the critical volume fraction for the minimum porosity. The pore size distributions of the different HA contents are shown in ). The narrowest pore size distribution was observed for 70 HA, which is consistent with a previous report [Citation22].
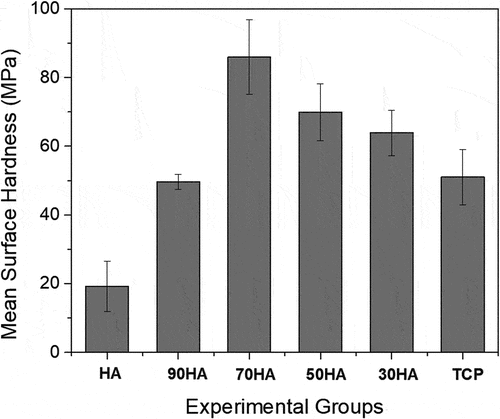
The mean surface hardness versus the HA/β-TCP ratio is shown in . The mean surface hardness of HA and β-TCP are 19.19 MPa and 51.00 MPa, respectively. The measured surface hardness for HA was very low compared to the typical surface hardness for HA [Citation23,Citation24], which is attributable to relatively high porosity (6–15%) of the samples. Generally, surface hardness decreases with an increase in porosity of materials primarily due to the pores within the material collapsing under load [Citation25–27]. The mixture samples (90 HA, 70 HA, 50 HA, and 30 HA) exhibited higher surface hardness than either HA or TCP. Compared to HA, the low content of β-TCP in HA enhanced its mechanical properties. The surface hardness increases until it reaches a maximum hardness value of 86.02 MPa (when the mass ratio of HA/β-TCP is 70/30), and then decreases with increasing β-TCP content. This result is consistent with the porosity trend, considering the inverse proportion to hardness of porosity. In addition, it is in accordance with the previous research reported by Shuai et al., in which a scaffold made of a HA/β-TCP mixture with a mass ratio of 70/30 exhibited optimum fracture toughness and compressive strength [Citation28]. They elucidated their result by using the ceramic–ceramic composite approach [Citation28–30]. When the β-TCP content in HA is low, it acts as a reinforcement dispersed in the HA matrix to improve the surface hardness. This approach can also be taken to explain that an increase in the hardness for 70 HA compared to HA was significantly large (approximately 4.5 times).
4. Conclusion
Hydroxyapatite (HA) and beta-tricalcium phosphate (β-TCP) powders were synthesized by the solid-state reaction of NH4H2PO4 with CaCO3 in Ca/P molar ratios of 1.67 and 1.5, respectively. The optimum heat treatment conditions were calcination for 2 h at 1200°C and 10 h at 900°C for HA and β-TCP, respectively. The chemical compositions and structural properties of the CPM samples were investigated by XRD, FT-IR spectroscopy, FE-SEM, and EDX analysis. The porosity of the HA/β-TCP composites decreased with β-TCP content and reached a minimum porosity when the mass ratio of HA/β-TCP is 70/30, and then increased with β-TCP content again. The measured surface microhardness of the CPM composites followed the same trend in porosity. Therefore, the maximum surface microhardness was achieved for the HA/β-TCP mixture with a mass ratio of 70/30 (70 HA) sample. This study confirmed the possibility of controlling the mechanical and structural properties of the HA/β-TCP composites by adjusting the HA/β-TCP ratio. In the case of block-type bone grafting materials used for bone reconstruction, such as onlay and inlay grafting, the mechanical properties are significant because they are required to withstand heavy loads. Therefore, desirable mechanical properties of block-type bone grafting materials can be achieved by appropriate tuning of HA/β-TCP ratio.
Acknowledgments
This work was supported by the Technology development Program (S3319649) funded by the Ministry of SMEs and Startups (MSS, Korea).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the authors.
References
- Orlovskii VP, Komlev VS, Barinov SM. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg Mater. 2002;38(10):973–984.
- Ogose A, Hotta T, Kawashima H, et al. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J Biomed Mater Res Part B. 2005;72(1):94–101.
- Xu S, Liu J, Zhang L, et al. Effects of HAp and TCP in constructing tissue engineering scaffolds for bone repair. J Mater Chem B. 2017;5(30):6110–6118.
- Kim Y, Lee SW, Rho HT, et al. Hydration and microhardness of mineral trioxide aggregate depending on ratio between di- and tricalcium silicates. Inter J Appl Ceram Technol. 2021; 10.1111/ijac.13946
- Yuan H, Blitterswijk CAV, Groot KD, et al. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J Biomed Mater Res A. 2006;78(1):139–147.
- Impens S, Schelstraete R, Luyten J, et al. Production and characterisation of porous calcium phosphate structures with controllable hydroxyapatite/ β -tricalcium phosphate ratios. Adv Appl Ceram. 2009;108(8):494–500.
- Vani R, Girija EK, Elayaraja K, et al. Hydrothermal synthesis of porous triphasic hydroxyapatite/(a and b) tricalcium phosphate. J Mater Sci Mater Med. 2009;20:S43–S48.
- Kohri M, Miki K, Waite DE, et al. In vitro stability of biphasic calcium phosphate ceramics. Biomaterials. 1993;14(4):299–304.
- Dorozhkin SV. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012;8(3):963–977.
- Wu S, Hsu H, Hsu S, et al. Preparation and characterization of four different compositions of calcium phosphate scaffolds for bone tissue engineering. Mater Charact. 2011;62(5):526–534.
- Frayssinet P, Trouillet JL, Rouquet N, et al. Osseointegration of macroporous calcium phosphate ceramics having a different chemical composition. Biomaterials. 1993;14(6):423–429.
- Tanimoto Y, Shibata Y, Murakami A, et al. Effect of varying HAP/TCP ratios in tape-cast biphasic calcium phosphate ceramics on response in vitro. J Hard Tissue Biol. 2009;18(2):71–76
- Webler GD, Zapata MJM, Agra LC, et al. Characterization and evaluation of cytotoxicity of biphasic calcium phosphate synthesized by a solid state reaction route. Curr Appl Phys. 2014;14(6):876–880.
- Jongprateep O, Nueangjumnong C, Palomas J. Effect of solids loadings, sintering temperatures and sintering periods on microstructure of hydroxyapatite. Key Eng Mater. 2017;751:629–635.
- Ramadas M, Mabrouk KE, Ballamurugan AM. Apatite derived three dimensional (3D) porous scaffolds for tissue engineering applications. Mater Chem Phys. 2020;24:122456.
- Chandrasekar A, Sagadevan S, Dakshnamoorthy A. Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int J Phys Sci. 2013;8:1639–1645.
- Wang H, Lee J-K, Moursi A, et al. Ca/P ratio effects on the degradation of hydroxyapatitein vitro. J Biomed Mater Res A. 2003;67(2):599–608.
- Khiri MZA, Matori KA, Zaid MHM, et al. Crystallization behavior of low-cost biphasic hydroxyapatite/β-tricalcium phosphate ceramic at high sintering temperatures derived from high potential calcium waste sources. Results Phys. 2019;12:638–644.
- Yu A-B, Standish N, McLean A. Porosity Calculation of Binary Mixtures of Nonspherical Particles. J Am Ceram Soc. 1993;76(11):2813–2816.
- El-Husseiny A. Unified Packing Model for Improved Prediction of Porosity and Hydraulic Conductivity of Binary Mixed Soils. Water. 2021;13(4):455.
- El-Husseiny A, Vanorio T, Mavko G. Predicting porosity of binary mixtures made out of irregular nonspherical particles: application to natural sediments. Adv Powder Technol. 2019;30(8):1558–1566.
- Niakana A, Ramesh S, Ganesan P, et al. Teng, Sintering behavior of natural porous hydroxyapatite derived from bovine bone. Ceram. 2015;41:3024–3029.
- Hoepfner TP, Case ED. The influence of the microstructure on the hardness of sintered hydroxyapatite. Ceram Int. 2003;29(6):699–706.
- Pramanik S, Agarwal AK, Rai KN. Development of high strength hydroxyapatite for hard tissue replacement. Trends Biomater Artif Organs. 2005;19:45–49.
- Cherry JA, Davies HM, Mehmood S, et al. Investigation into the Effect of process parameters on microstructural and physical properties of 316L stainless steel parts by selective laser melting. J Adv Manuf Technol. 2015;76(5–8):869–879.
- Luo J, Stevens R. Porosity-dependence of elastic moduli and hardness of 3Y-TZP ceramics. Ceram Int. 1999;25(3):281–286.
- Jang B-K, Matsubara H. Influence of porosity on hardness and Young’s modulus of nanoporous EB-PVD TBCs by nanoindentation. Mater Lett. 2005;59(27):3462–3466.
- Shuai C, Li P, Liu J, et al. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater Charact. 2013;77:23–31.
- Rice RW. Mechanisms of toughening in ceramic matrix composites. Ceram Eng Sci Proc. 1981;2:661–701.
- Raynaud S, Champion E, Lafon JP, et al. Calcium phosphate apatites with variable Ca/P atomic ratio III. Ceram Eng Sci Proc. 1981;2:661–701.