ABSTRACT
A titanium aluminum carbide, Ti2AlC, which is classified among MAX phase ceramics, was studied as a potential candidate for various mechanical components used in high-temperature applications. The impact of its chemical composition on its high-temperature oxidation process was determined. Ti2AlC powders with various Al contents and with or without Nb addition were synthesized via a conventional reaction technique followed by 16 h of annealing in vacuum at 1300°C. The synthesized Ti2AlC powders were consolidated by means of 15 min of pulsed electric current sintering at a die temperature of 1300°C in vacuum under a uniaxial pressure of 30 MPa. In the presence of an aluminum reservoir in the form of TiAl3, Ti2AlC has excellent resistance against high-temperature oxidation. The promising results concerning the addition of Nb to TiAl provided the rationale for a similar modification of Ti2AlC. The results of oxidation tests on Nb-doped Ti2AlC likewise showed excellent oxidation resistance. Alloying with Nb can improve the oxidation resistance of Ti2AlC with low Al content, allowing the formation of a protective Al2O3 scale and inhibiting the growth of TiO2.
KEYWORDS:
1. Introduction
MAX phase ceramics are layered ternary transition metal carbides and nitrides represented by the general formula Mn+1AXn, where M stands for a transition metal (Sc, Ti, V, Cr, Mn, Fe, Y, Zr, Nb, Mo, Lu, Hf, Ta or W), A is a group A element (Al, Si, P, S, Cu, Zn, Ga, Ge, As, Pd, Cd, In, Sn, Ir, Au, Tl, Pb or Bi) and X represents C or N [Citation1]. MAX phase ceramics have both metallic and ceramic features because of the presence of both metallic and covalent bonds in the crystal [Citation2]. Since Ti2AlC belongs to this group, it also exhibits both metallic and ceramic properties, including high electrical and thermal conductivity, high thermal shock resistance, lower density than Ni alloys and steels, good high-temperature oxidation resistance and high hardness [Citation3,Citation4]. Ti2AlC has been studied as a potential candidate for application in various mechanical components used at high temperatures as well as room temperature. The mechanical strength of Ti2AlC decreases sharply at around 900°C, at this point, Ti2AlC undergoes the brittle-to-ductile transition (BDT) [Citation5]. The application of Ti2AlC ceramics would therefore be limited at temperatures higher than the BDT temperature.
When it comes to structural elements exposed to high temperatures, one of their most important properties is high-temperature oxidation resistance as well as high-temperature mechanical strength and creep resistance. However, there is no well-established understanding of the oxidation properties of Ti2AlC at high temperatures. Some sources consider Ti2AlC to have improved resistance to oxidizing conditions [Citation6–8], while others do not [Citation9–11]. Certain results indicate linear oxidation behavior occurred with the formation of a non-protective TiO2 scale on the surface of Ti2AlC after long-term oxidation [Citation8]. To promote the applications of Ti2AlC in high-temperature components, the oxidation process of Ti2AlC must be stabilized.
The chemical composition of Ti2AlC may affect its oxidation behavior. Because Ti2AlC is a line phase [Citation12], every commercial Ti2AlC powder contains other phases, such as TiC, Ti3AlC2 or TiAl3 [Citation13]. This makes it impossible to study the oxidation behavior of Ti2AlC using samples with a controlled phase ratio. Because the protective scale that forms on Ti2AlC is Al2O3, its continued growth requires a sufficient Al supply during high-temperature oxidation. Al-rich phases like TiAl3 may therefore improve the oxidation resistance of Ti2AlC.
High-temperature oxidation of TiAl with poor oxidation resistance leads to the formation of oxide scale including the rutile polymorph of TiO2 and Al2O3 [Citation14]. This oxidation behavior is very similar with Ti2AlC with poor oxidation resistance. Improvements of oxidation resistance on TiAl have been studied with several techniques. As one of the common methods for improving the oxidation resistance of TiAl, addition of Nb is applied [Citation15], which would inhibit the growth of the TiO2 scale on the surface of TiAl and subsequently facilitates the formation of a continuous Al2O3 scale. The oxidation resistance of Ti2AlC doped with Nb was studied by Donchev et al. [Citation16], and they reported a decreased oxidation rate, indicating the protective action of a Nb addition. However, the mechanism underlying the beneficial effect of Nb on the oxidation properties of Ti2AlC is unclear in some cases. Adding Nb to Ti2AlC with low Al content may not necessarily improve its oxidation resistance to a very significant degree, since this compound is in itself highly susceptible to oxidation [Citation17].
In this study, the effects of varying the Al content and Nb addition on the oxidation behavior of Ti2AlC in air at 800°C was investigated to establish a material design strategy that would optimize the high-temperature oxidation resistance of Ti2AlC ceramics.
2. Experimental procedure
Commercial Ti, Al, and C powders were mixed in Ti:Al:C molar ratios of 2:1:0.9, 2:1.2:0.9, and 2:1.5:0.9, with the corresponding samples receiving the designations Al1, Al1.2 and Al1.5, respectively. Some Al1 and Al1.2 samples had 5% of their Ti content replaced with Nb; these samples are referred to as Al1Nb and Al1.2Nb. The particle size and purity of the raw powders were as follows: 38 μm and 99.9% for Ti, 3 μm and 99.9% for Al, 1–2 μm for C and 45 μm for Nb (Kojundo Chemical Laboratory). The powder mixture was placed in an alumina container and annealed for 16 h in vacuum at 1300°C. The reacted powder underwent phase identification by means of X-ray diffraction (XRD, Rigaku corporation MiniFlex 600, using monochromatic CuKα radiation). Al1 is categorized as Al-poor Ti2AlC, and Al1.2 and Al1.5 are categorized as Al-rich Ti2AlC. shows the symbol and category of each sample investigated in the present study. The synthesized powder was consolidated over 15 min using a pulsed electric current sintering technique with a graphite die at 130°C, in vacuum, and under a uniaxial pressure of 30 MPa. The phases present in the sintered samples were also identified with XRD. The relative density of the samples reached at least 98% of the theoretical one. Samples for oxidation experiments were cut into a small quarter-circle shape with a radius of 7.5 mm and a thickness of 3 mm using a diamond saw. All samples for the oxidation tests were ground with SiC abrasive paper with varying grit (up to 3000), and then subsequently polished with 3 and 1 μm diamond slurry to ensure that the surface of each sample had undergone the same preparation. The samples were heated for times ranging from 2 to 14 d in an electric furnace with laboratory air at 800°C. For thermogravimetric analyses (TGA), the samples were cut into small rectangles with dimensions of 10 × 5 × 1 mm3 using a diamond saw. The oxidation kinetics were evaluated at 800°C in ambient air, over a period of 150 h using a thermogravimetric apparatus which enabled the continuous monitoring of the weight with an accuracy of to 10–6 g (MK2 Vacuum Head Microbalance, CI Electronics Ltd, UK). The phase compositions of the samples were analyzed by means of XRD. The samples were mounted in resin and cut with a diamond cutting wheel for cross-sectional observations by means of scanning electron microscopy (SEM), performed using a FEI Nova NanoSEM 200 microscope coupled with an EDAX Genesis XM2 system required for the analysis of chemical composition via energy-dispersive X-ray spectroscopy (EDS).
Table 1. Sample symbols and categories.
3. Results
shows the XRD patterns of the sintered samples in all the groups. The XRD graphs with the bottom portion enlarged were smoothed to remove noise. The main peaks in these XRD patterns were identified as originating from Ti2AlC. In the case of Al-poor Ti2AlC, peaks attributed to Ti3AlC2, TiAl3 and TiC were also observed. The patterns recorded for the remaining samples had minor peaks originating from Ti3AlC2 and TiAl3. The surface images of Ti2AlC and Nb-doped Ti2AlC samples with different Al molar ratios after oxidation at 800°C for up to 14 d in air are shown in . Yellowish scales had formed on the surface of Al-poor Ti2AlC. After the oxidation test, the surface of the Al1Nb sample differed in color compared to its counterpart without Nb addition. Oxidation time did not affect the appearance of any of the Al-rich and/or Nb-doped samples to a significant degree.
Figure 1. X-ray diffraction patterns recorded for bulk samples: Al1, Al1.2, Al1.5, Al1Nb and Al1.2Nb.
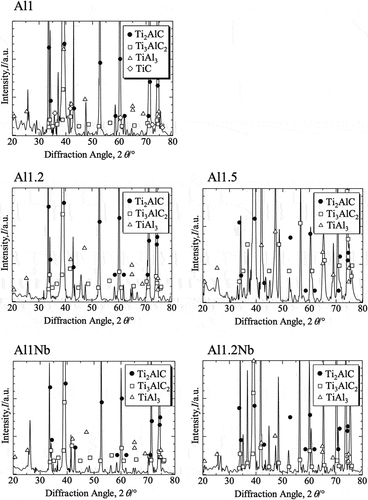
Figure 2. Magnified images of Ti2AlC and Nb-doped Ti2AlC samples with various Al molar ratios after 2 − 14 d of isothermal oxidation in laboratory air at 800°C.
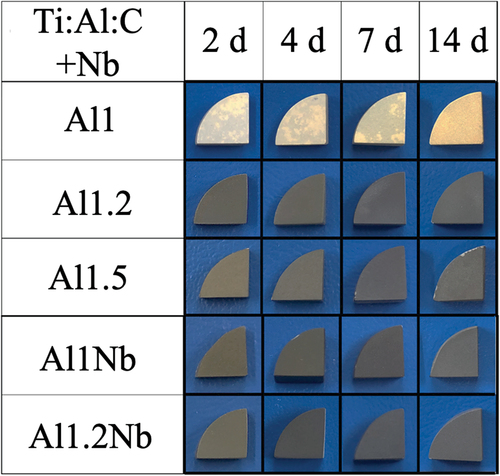
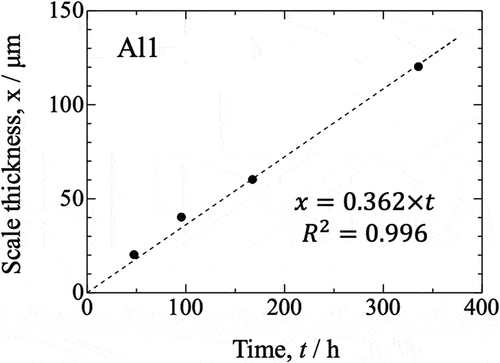
shows the XRD patterns obtained for sintered samples from all groups after 14 d of oxidation. The main peaks in XRD patterns of Al-rich and Nb-doped oxide samples were identified as originating from Ti2AlC. The minor peaks were attributed to rutile-type TiO2 and different polymorphs of Al2O3. Because peaks observed for α-Al2O3, θ-Al2O3, and γ-Al2O3 are similar and they all have low intensity, it was impossible to precisely determine the type of Al2O3. The EDX element mapping of the surface of Al-rich and Nb-doped samples after 14 d of oxidation clearly shows aluminum and oxygen (). The conducted XRD, SEM, and EDX observations indicate that an Al2O3 scale with a thickness of less than 1 μm was formed on these samples. The predominant phase in the oxide scales formed in the case of all Al-rich and Nb-doped samples was Al2O3. shows SEM and EDS images of the surface of Al1.2Nb after 150 h of isothermal oxidation in laboratory air at 800°C. Needle-like growth of Al2O3 was clearly observable only on the surface of Al1.2Nb. shows the cross-sectional SEM and EDX images of the Al-poor sample after oxidation for 14 d. The results of XRD, SEM, and EDX investigations indicate that rutile-type TiO2 formed on the scale’s surface, while Al2O3 and TiO2 had formed underneath it. In the case of this sample, thickness increased linearly with oxidation time, becoming approximately 120 μm after 14 d (). The oxide scale formed on all Al-poor samples was determined to consist of rutile-type TiO2 with poor oxidation resistance.
Figure 3. X-ray diffraction patterns recorded for samples Al1, Al1.2, Al1.5, Al1Nb and Al1.2Nb after 14 d of isothermal oxidation in laboratory air at 800°C.
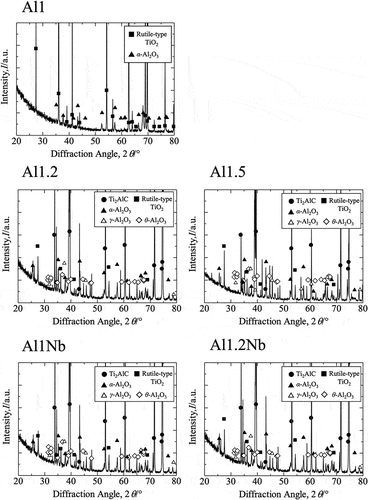
Figure 4. SEM and EDS images of the cross-sections of samples Al1.2, Al1Nb and Al1.2Nb after 14 d of isothermal oxidation in laboratory air at 800°C.
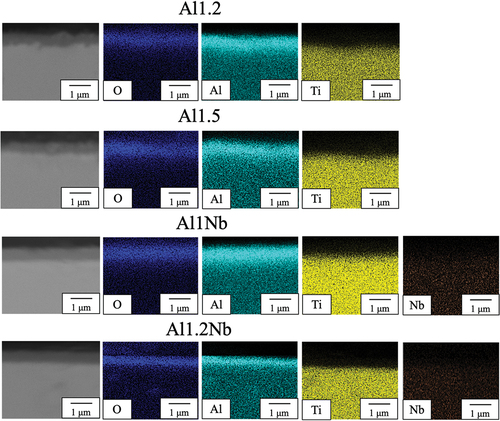
Figure 5. SEM and EDS images of the surface of Al1.2Nb after 14 d of isothermal oxidation in laboratory air at 800°C.

Figure 6. SEM and EDS images of the cross-sections of Al1 after 14 d of isothermal oxidation in laboratory air at 800°C.

shows the kinetics of isothermal oxidation for all of the sintered samples in a linear plot representing mass gain per unit area in a function of time. The data show that the oxidation kinetics of the samples approximately obeys the parabolic rate law. The slowest constituent process that determines the oxidation rate is therefore the diffusion of reagents in the oxidation product. The largest increase in the mass of the corrosion product was observed for the Al1 sample, while the Al1.2Nb sample was characterized by the smallest increase. The parabolic oxidation rate constants (kp) of the samples determined using the Pilling-Bedworth equation are listed in [Citation18]. As can be seen, the parabolic rate constant of oxidation decreases with increasing aluminum content. The oxidation rate constants of the Al1 and Al1.5 samples are different by as much as one order of magnitude. On the other hand, the addition of niobium reduced the kp values by another order of magnitude, further improving the oxidation resistance of the Al1Nb and Al1.2Nb samples.
Figure 8. Oxidation kinetics for all investigated samples over 150 h of isothermal oxidation in laboratory air at 800°C, presented in a linear plot.
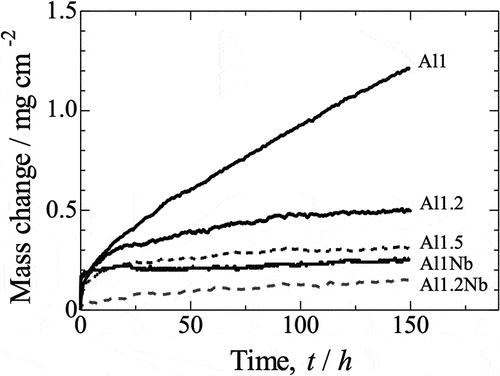
Table 2. Parabolic rate constants of oxidation (kp) of all samples determined after 150 h of isothermal oxidation in laboratory air at 800°C.
4. Discussion
The Al2O3 oxide scale formed on the surface of Al-rich Ti2AlC such as Al1.2 and Al1.5 and Nb-doped one can be considered a protective layer, even though its thickness was below 1 μm even after 14 d of oxidation. This is demonstrated by the lower oxidation resistance of Al-poor Ti2AlC, which had a main TiO2 scale on its surface and a non-continuous Al2O3 scale beneath it. It is the evidence that the dispersion of an Al-rich phase and doping with Nb are effective at improving the oxidation resistance of Ti2AlC ceramics.
Brumm and Grabke stated that the oxidation rate depends on the oxide phase formed on the alloy; γ-Al2O3, θ-Al2O3, and α-Al2O3 have been observed, and the growth of each of these phases was associated with different kp values [Citation19]. shows the kp values of Ti2AlC determined in the present study compared with those of NiAl reported by Brumm and Grabke [Citation19]. The kp value for Al2O3 growth on Ti2AlC in the cited study is similar to those for γ- or θ-Al2O3. The kp of Al1.2 was slightly higher than that of other Al-rich and Nb-doped samples because of partial non-protective oxidation of the Al1.2 sample’s corners. Non-protective oxidation tends to occur at the corners of Ti2AlC samples [Citation10]. The supply of Al is limited at this location because of the small area of the lower part. When a continuous Al2O3 scale is formed on a protruding part, such as a corner, the Al2O3 scale may crack due to growth stress [Citation13]. Needle-like Al2O3 was observed on the surface of Al1.2Nb oxidized at 800°C for 150 hours. The needlelike Al2O3 were sparsely present in some places and did not form dense colonies. Mechanism of the formation of needlelike shape is explained by the stress generated by the transformation from γ- to θ-Al2O3. When γ-Al2O3 transforms into θ-Al2O3, the sample volume decreases by 1.7% [Citation20]. The reduced volume causes tensile stress in the θ-Al2O3 layer and compressive stress in the γ-Al2O3 layer formed on top. Ando et al. reported that a possible mechanism is that the <111> plane shifts in the <112> direction to form twins that relieve compressive stress generated in the γ-Al2O3 grain near the transition interface from γ- to θ-Al2O3 [Citation20]. The twin boundary serves as a preferential diffusion path for Al, and the twinned part preferentially grows outward, resulting in a needle shape.
Figure 9. Arrhenius plot showing the parabolic rate constants determined for the oxidation of Ni50AI50 [Citation19] and the kp values determined in the present experiment ().
![Figure 9. Arrhenius plot showing the parabolic rate constants determined for the oxidation of Ni50AI50 [Citation19] and the kp values determined in the present experiment (Table 2).](/cms/asset/eb32d78e-33ec-4e21-8f0f-77cc90878c5d/tace_a_2140497_f0009_b.gif)
Needle-like crystals of γ- or θ-Al2O3 may form on the surface when Al2O3-forming materials are oxidized at low temperatures of 500–800°C as well [Citation21–23]. The shape of the Al2O3 grains and the comparison of kp indicates that the Al2O3 scale formed on Ti2AlC at 800°C should be regarded as being γ- or θ-Al2O3.
When γ-Al2O3 transforms into θ-Al2O3, the sample volume decreases by 1.7%; the transformation from θ-Al2O3 to α-Al2O3 is associated with a decrease of 12.3% [Citation20]. The volume reduction caused by the change of the Al2O3 phase causes tensile stress and leads to the cracking of the scale. This phenomenon may cause decrease in the oxidation resistance of Ti2AlC. The formation mechanism of Al2O3 should therefore be considered when designing Ti2AlC with excellent oxidation resistance in mind.
The following tendencies can be observed during the oxidation of Al-rich Ti2AlC. A continuous Al2O3 scale forms under a non-continuous TiO2 scale as the material starts to oxidize. Since the diffusion coefficient of Al in Ti2AlC is much faster than that of Ti, the Al2O3 scale on top of Ti2AlC becomes thin and non-continuous [Citation24]. When continuous Al2O3 does not form in the initial oxidation stage, its subsequent formation is accompanied by the formation of TiO2 [Citation25]. The rate at which this non-continuous TiO2 scale grows is faster than the growth rate of Al2O3 [Citation26], and its underlying mechanism is the outward diffusion of Ti through the grain boundaries of the discontinuous Al2O3 scale, made possible because the diffusion coefficient of O in the TiO2 particles is smaller than that of Ti [Citation27]. TiO2 forms on the surface, and Al2O3 forms underneath it. In order for the Al2O3 scale to be continuous, the supply of Al from Ti2AlC needs to be sufficient. The formation of a continuous Al2O3 scale suppresses the formation of the TiO2 scale.
The oxidation of Al-poor Ti2AlC takes a different course, similar to that observed for Al-poor TiAl. The oxide scale that forms in this case consists mostly of rutile-type TiO2 and Al2O3 located underneath it, and it does not provide significant protection against oxidation [Citation14]. The supply of Al to the surface is insufficient to allow the formation of the Al2O3 scale. Ti is oxidized into TiO2 and its volume increases, which destroys the Al2O3 scale. In order for the oxidation resistance of Ti2AlC to be improved, it is therefore necessary to ensure that the supply of Al is sufficient and that the growth of the TiO2 layer is inhibited.
Al-rich Ti2AlC had high oxidation resistance owing to the development of an Al2O3 scale. The Al-rich phase (TiAl3) supplied the needed Al, allowing the continuous Al2O3 scale to form and improve the material’s resistance to oxidation. In addition to increasing the amount of Al, the supply of Al for Ti2AlC can also be improved by shortening the Al supply path. Al atoms in Ti2AlC can migrate more easily in the outward direction along grain boundaries and the base planes than other atoms during high-temperature oxidation [Citation24]. Yu et al. reported that obtaining finer Ti2AlC grains facilitates Al diffusion and thus the development of the Al2O3 scale, which is yet another way in which the oxidation resistance of Ti2AlC can be improved [Citation28]. There are various ways to reduce grain size in Ti2AlC, including the use of a finer starting powder or pinning with fine impurity particles. In addition to using a finer starting powder, the preparation process can also be optimized by applying a lower sintering temperature, which yields Ti2AlC with a high concentration of Al.
Some results concerning the oxidation of Ti2AlC show that linear kinetics lead to poor oxidation resistance after long-term oxidation [Citation8]. In such conditions, Al-depletion zones in Ti2AlC develop as the Al is drained by the formation of the continuous Al2O3 scale, and after a sufficiently long time of oxidation or if cycles are involved Al-rich Ti2AlC may be affected in much the same way as the material with low Al content. An additional method to prevent non-protective oxidation of Ti2AlC is required for long-term use.
This was precisely the rationale for the addition of Nb. When Nb5+, which has a higher valence than Ti4+, is added to TiO2, the number of oxygen vacancies in TiO2 decreases [Citation29], and the growth of TiO2 is suppressed. After the solid dissolution of Nb2O5 in TiO2, the Nb concentration at which the oxygen ion vacancies in TiO2 disappear is estimated to be Ti:Nb = 1:0.04 [Citation15]. For the Nb-doped sample in this experiment, Nb was added in an amount equal to 5% of Ti, an amount considered sufficient to fill the oxygen ion vacancies in TiO2. The cause of non-protective oxidation was the destruction of the Al2O3 scale due to the growth of TiO2. Inhibiting TiO2 growth by adding Nb is one of the factors that improve oxidation resistance.
Adding more Nb (10%) to TiAl than the solid solution limit in TiO2 may further improve oxidation resistance [Citation15,Citation30]. However, when Nb is added to TiAl in large amounts (15% or more), Nb2O5 and TiNb2O7 scales are formed, and oxidation resistance is reduced [Citation15,Citation30]. The oxidation resistance of Ti2AlC may also be further improved by increasing the amount of Nb added; the optimal amount of Nb is yet to be determined.
When combined, the change in the amount of Al and the addition of Nb have a pronounced effect on the oxidation resistance of the Ti2AlC ceramics, which is a property of fundamental importance from the perspective of its eventual application. Such modifications are therefore necessary for the stable operation of components manufactured from this material in conditions that involve high temperatures.
5. Conclusions
The oxidation behavior of Ti2AlC with various Al concentrations and with or without Nb addition was investigated in air at 800°C in periods ranging from 2 to 14 d. Ti2AlC powder samples were synthesized for different molar ratios of Al and amounts of added Nb. Al-poor Ti2AlC was characterized by low oxidation resistance due to the formation of a TiO2 scale that did not reduce the oxidation rate. Nb-doped Ti2AlC and samples with higher Al contents exhibited far superior resistance to oxidation owing to the formation of a protective Al2O3 scale. The addition of Nb improved oxidation resistance even in the case of the Ti2AlC sample with the lowest Al content.
Acknowledgments
This work was supported partly by the Osawa Scientific Studies Grants Foundation.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Gonzalez-Julian J. Processing of MAX phases: from synthesis to applications. J Am Ceram Soc. 2021;104(2):659–690.
- Barsoum M. The MN+1AXN phases: a new class of solids; thermodynamically stable nanolaminates. Prog Solid State Ch. 2000;28(1–4):201–281
- Radovic M, Barsoum M. MAX Phases: bridging the gap between metals and ceramics. Bull Am Ceram Soc. 2013;92(3):20–27.
- Wang X, Zhou Y. Layered machinable and electrically conductive Ti2AlC and Ti3AlC2 ceramics: a review. J Mater Sci Technol. 2010;26(5):385–416.
- Bai Y, He X, Wang R, et al. High temperature physical and mechanical properties of large-scale Ti2AlC bulk synthesized by self-propagating high temperature combustion synthesis with pseudo hot isostatic pressing. J Eur Ceram Soc. 2013;33(13–14):2435–2445.
- Haftani M, Saeedi Heydari M, Baharvandi H, et al. Studying the oxidation of Ti2AlC MAX phase in atmosphere: a review. Int J Refract Hard Met. 2016;61:51–60.
- Basu S, Obando N, Gowdy A, et al. Long-Term oxidation of Ti2AlC in air and water vapor at 1000-1300°C temperature range. J Electrochem Soc. 2012;159(2):90–96.
- Tallman D, Anasori B, Barsoum M, et al. A critical review of the oxidation of Ti2AlC, Ti3AlC2 and Cr2AlC in air. Mater Res Lett. 2013;1(3):115–125.
- Barsoum M, Tzenov N, Procopio A, et al. Oxidation of Tin+1AlXn(n = 1-3 and X = C,N) II. Experimental result. J.ElectrochemSoc. 2001;148(8):551–562.
- Badie S, Sebold D, Vaßen R, et al. Mechanism for breakaway oxidation of the Ti2AlC MAX phase. Acta Mater. 2021;215:117025.
- Lin Z, Li M, Wang J, et al. Influence of water vapor on the oxidation behavior of Ti3AlC2 and Ti2AlC. Scr Mater. 2008;58(1):29–32.
- Witusiewicz V, Hallstedt B, Bondar A, et al. Thermodynamic description of the Al-C-Ti system. J Alloys Compd. 2015;623:480–496.
- Badie S, Dash A, Sohn Y, et al. Synthesis, sintering, and effect of surface roughness on oxidation of submicron Ti2AlC ceramics. J Am Ceram Soc. 2021;104(4):1669–1688.
- Reddy R, Wen X, Divakar M. Isothermal oxidation of TiAl alloy. Metall Mater Trans A. 2001;32(9):2357–2361.
- Yoshihara M, Miura K. Effects of Nb addition on oxidation behavior of TiAl. Intermetallics. 1995;3(5):357–363.
- Donchev A, Schütze M, Ström E, et al. Oxidation behaviour of the MAX-phases Ti2AlC and (Ti, Nb)2AlC at elevated temperatures with and without fluorine treatment. J Eur Ceram Soc. 2019;39(15):4595–4601.
- Li X, Xie X, Gonzalez-Julian J, et al. Mechanical and oxidation behavior of textured Ti2AlC and Ti3AlC2 MAX phase materials. J Eur Ceram Soc. 2020;40(15):5258–5271.
- Mrowec S, Werber T. Modern scaling-resistant materials. Washington DC: National of Standards and National Science Foundation; 1982.
- Brumm M, Grabke H. The oxidation behaviour of NiAl-I. Phase transformations in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros Sci. 1992;33(11):1677–1690.
- Andoh A, Taniguchi S, Shibata T. TEM observation of Al2O3 scales formed on Al-deposited Fe-Cr-Al alloy foil. Tetsu-to-Hagane. 1998;84(4):43–48.
- Yamaguchi N, Kakeyama T, Yoshioka T, et al. Effect of the third elements on high temperature oxidation resistance of TiAl3 intermetallic compounds, mater. Trans. 2002;43(12):3211–3216.
- Eliassi A, Ranjbar M. Application of novel gamma alumina nano structure for preparation of dimethyl ether from methanol. Int J Nanosci Nanotechnol. 2014;10(1):13–26.
- Abdollahifar M, Zamani M, Beiygie E, et al. Synthesis of micro-mesopores flowerlike γ-Al2O3 nano-architectures. J Serbian Chem Soc. 2014;79(8):1007–1017.
- Wang J, Zhou Y, Liao T, et al. A first-principles investigation of the phase stability of Ti2AlC with Al vacancies. Scr Mater. 2008;58(3):227–230.
- Wang X, Zhou Y. High-Temperature oxidation behavior of Ti2AlC in Air. Oxid Met. 2003;59(3–4):303–320.
- Smialek J. Kinetic aspects of Ti2AlC MAX phase oxidation. Oxid Met. 2015;83(3–4):351–366.
- Yang H, Pei Y, Rao J, et al. High temperature healing of Ti2AlC: on the origin of inhomogeneous oxide scale. Scr Mater. 2011;65(2):135–138.
- Yu W, Vallet M, Levraut B, et al. Oxidation mechanisms in bulk Ti2AlC: influence of the grain size. J Eur Ceram Soc. 2020;40(5):1820–1828.
- Lin J, Zhao L, Li G, et al. Effect of Nb on oxidation behavior of high Nb containing TiAlalloys. Intermetallics. 2011;19(2):131–136.
- Yoshihara M, Imamura N, Kobayashi E, et al. Oxidation resistance and effects of the heat treatment under law partial pressure oxygen atmosphere in intermetallic compound TiAl containing Nb. J Japan Inst Metals. 1993;57(5):574–581.