?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We successfully prepared Hydroxyapatite/Zinc oxide (HAp/ZnO) composite particles with hexagonal plate-like shapes. The surface of the ZnO particles was treated with (3-aminopropyl)triethoxysilane (APTES) as a scaffold for the crystallization of HAp from the precursor solution. From the results of XRD measurements and SEM images of composite particles, formation of HAp on the ZnO particles was revealed. In addition, diffraction peaks associated with the (0 0 2) and (0 0 4) planes of HAp were clearly observed. They also revealed that the HAp/ZnO composite particles had a c-face orientation. Furthermore, element mapping analysis by EPMA showed that the elemental distributions of Ca, P and Zn of the composites were almost coincident. These results suggested that the formation of HAp occurred on the ZnO particles. In contrast, the ZnO particles without APTES treatment readily dissolved in the precursor solution and the diffused Zn2+ ions reacted with PO43- ions and Ca2+ ions to form CaZn2(PO4)2. APTES treatment of the ZnO surfaces appeared to prevent the dissolution of ZnO in the solution and induce the adsorption of anions such as phosphate on ZnO.
1. Introduction
Hydroxyapatite (HAp, Ca5(PO4)3OH) is a well-known biocompatible ceramic material that has high biocompatibility and bioactivity [Citation1–4]. HAp has been applied as a biomaterial in the fields of medicine and tissue engineering as a bone replacement agent [Citation5,Citation6], coating agent on implants [Citation7,Citation8] and adsorbent for the separation of proteins and viruses [Citation9,Citation10]. These functions change depending on the shape of the HAp particles. Therefore, controlling the particle shape is important. Much effort has gone into particle shape control, and plate [Citation11,Citation12], rod [Citation13,Citation14], sphere [Citation15,Citation16] and other particle shapes have been reported. HAp belongs to a hexagonal crystal system and exhibits polarity along the c axis. Also, the ab plane of HAp is rich in Ca2+ ions and is positively charged, whereas the c plane is rich in PO43- ions and OH− ions and is negatively charged [Citation17]. Since the surface properties change depending on the orientation of the crystal plane, the selective synthesis of particles with specific orientations of crystal planes is important for developing functional materials.
In contrast, photocatalytic materials such as titanium dioxide (TiO2) and zinc oxide (ZnO) are expected to be applied as functional inorganic materials in areas such as environmental purification [Citation18,Citation19], cancer therapy [Citation20,Citation21], and the decomposition of organic compounds [Citation22,Citation23]. A combination of HAp and such photocatalysts permits the simultaneous adsorption and decomposition of viruses, bacteria and other toxic agents [Citation24–26]. As mentioned above, since the adsorption properties of HAp depend on the crystal plane, it is necessary to control the shape of composite particles according to the target substance. However, there are few reports on shape control of composite particles focusing on the orientation of crystal planes. We considered that it important to limit the reaction field as well as to control the direction and reaction rate of crystal growth. There are several reports on the synthesis of HAp using chelating agents such as EDTA to control the reaction rate [Citation27–29]. In addition, one method of limiting the reaction field is the epitaxial growth of particles on substrates used for thin films [Citation30–32]. If the reaction field can be limited to particles rather than planar substrates, composite particles can be synthesized that are oriented along specific crystal planes.
In our recent work, we have succeeded in preparing hexagonal plate-like ZnO particles with crystal growth control [Citation33–35]. We focused on surface treatment by (3-aminopropyl)triethoxysilane (APTES) as a method to enable the use of these hexagonal plate-like ZnO particles as substrates. APTES is known to induce the adsorption of anionic species such as phosphate [Citation36,Citation37]. Therefore, treating the surface of the ZnO particles with APTES promotes the adsorption of phosphate ions and the formation of HAp on the ZnO. We hypothesized that crystal growth of HAp could be controlled on the ZnO particles. In this report, we propose a novel synthetic method that incorporates a combination of these techniques.
2. Experimental section
2.1. Materials
Hexagonal plate-like ZnO particles prepared by our previously reported method [Citation33–35] were used. APTES (Sigma Aldrich) was used for the surface treatment of ZnO particles. Ca(NO3)2 · 4H2O (Wako Pure Chemical Co., Ltd., Japan) and (NH4)2HPO4 (Wako Pure Chemical Co., Ltd., Japan) were used as the calcium and phosphorus sources for HAp, respectively. Ethylenediamine-N,N,N’,N’-tetraacetic acid (EDTA; Dojindo Laboratories, Japan) was used as a chelating agent for Ca ions for the preparation of HAp/ZnO composite particles.
2.2. Surface treatment of hexagonal plate-like ZnO using APTES
First, the specific surface area of the hexagonal plate-like ZnO particles was measured using nitrogen adsorption/desorption measurements by the BET method. From the specific surface area values, the APTES concentration (n) for surface treatment of ZnO particles was calculated as follows.
Here, M is the molecular weight of APTES, S is the amount of ZnO added, and AZnO and AAPTES are the specific surface area of hexagonal plate-like ZnO particles and the area occupied by APTES molecules, respectively. AAPTES was calculated as the area of a sphere having a radius the length of the Si-O bond. APTES coverage corresponding to a monolayer film on the total surface area of hexagonal plate-like ZnO particles was set to n(coverage) = 1.0, and surface treatments were performed at n = 0.5, 1.0, 3.0, and 5.0. For comparison, ZnO particles without surface treatment were also prepared, with n = 0.
Second, the surface treatment was performed as follows. A mixture of ethanol and APTES with a volume ratio of 1:1 was prepared for each concentration. A total of 0.2 g of ZnO particles was added to the solution and then left to stand for 12 hours at room temperature. In addition, samples were held at 70°C for 12 h to obtain surface-treated ZnO(ZnO/APTES) particles with various coverage levels (n = 0.5–5.0). The ZnO/APTES particles were collected by centrifugation and washed three times with ethanol and deionized water. These samples were characterized by Fourier transform infrared spectroscopy (FT-IR; FT/IR4200, JASCO, Japan). The morphology of the particles was observed by scanning electron microscopy (SEM; VE-7800, Keyence, Japan).
2.3. Preparation of HAp/ZnO composite particles with hexagonal plate-like shapes
A 10 mM calcium nitrate solution and a 10 mM EDTA solution were mixed. Subsequently, a 6 mM diammonium hydrogen phosphate solution was added to the mixed solution to prepare a precursor solution of HAp. The Ca/P molar ratio in the precursor solution was 1.67. Then, 0.2 g of various ZnO/APTES particles were added to the solution and allowed to stand at room temperature for 6–48 hours to synthesize HAp/ZnO composite particles. All obtained composites were collected by centrifugation and washed three times with deionized water, then dried at 70°C for 24 hours. The sample was characterized by X-ray diffraction (XRD; MiniFlex600, Rigaku, Japan Cu-Kα radiation) and FT-IR (FT/IR4200, JASCO, Japan). The morphology of the particles was observed by SEM (VE-7800, Keyence, Japan). An elemental mapping analysis of composites was performed by an Electron Probe Micro Analyzer (EPMA; EPMA-1720, Shimazu, Japan).
3. Result and Discussion
3.1. Surface treatment of ZnO particles by APTES
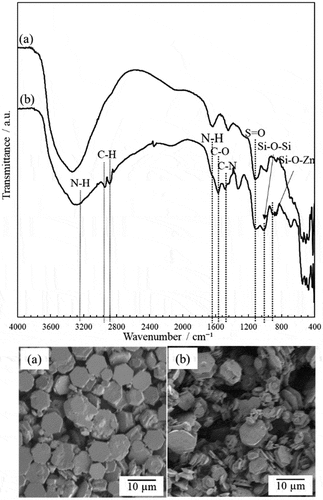
shows the FT-IR spectra and SEM images of ZnO/APTES(n = 1.0), respectively. For comparison, the FT-IR spectrum and SEM image of hexagonal plate-like ZnO without APTES treatment (ZnO/APTES(n = 0)) is also shown. In the spectrum of ZnO/APTES(n = 1.0) in ), the Si-O-Si stretching vibration at 1020 cm−1 [Citation38–40] and C-N stretching vibration at 1477 cm−1 [Citation38–40] attributed to APTES were observed. Furthermore, C-H stretching vibrations at 2875 cm−1 and 2931 cm−1 [Citation38–40] attributed to the alkyl chain in APTES were observed. The peaks of the asymmetric stretching mode of NH2 at 3300 cm−1 [Citation38–40] and bending vibration of NH2 at 1620 cm−1 [Citation38–40] were also observed. The peak appearing at 900 cm−1 could be assigned to Zn-O-Si bonds [Citation39,Citation40]. These absorption peaks were not observed for the ZnO particles without APTES ()). In addition, SEM observation was performed to investigate the effect of surface treatment on particle shape and morphology. There was no difference observed between the SEM images of particles before and after APTES treatment. APTES modifies the surface of ZnO particles by dehydration-condensation but does not seem to cause associated particle aggregation. These results suggest that the surface of the ZnO particles was successfully treated by APTES.
3.2. Preparation of HAp/ZnO composite particles
In the XRD patterns of the composites prepared with a reaction time of 6 hours, diffraction peaks attributed to ZnO were observed in the samples with ZnO/APTES(n = 0.5–5.0) (Supporting information ). There were no differences compared with the patterns of single crystal ZnO particles. In contrast, not only the diffraction peaks of ZnO, but also a diffraction peak attributed to CaZn2(PO4)2 [Citation41,Citation42] was observed in the sample with ZnO/APTES(n = 0). This peak was not observed in the other patterns. Under this reaction condition, we found that only ZnO/APTES(n = 0) formed CaZn2(PO4)2, while the others did not.
In the XRD patterns of composites prepared with reaction times of 12 and 24 hours, not only diffraction peaks attributed to ZnO, but also diffraction peaks attributed to other products were observed (Supporting information ). Composites prepared using ZnO/APTES(n = 0) showed formation of CaZn2(PO4)2 at both 12 hours and 24 hours of reaction. In contrast, composites prepared using ZnO/APTES(n = 0.5–5.0) showed a new peak at 2θ = 25.94 °. This peak was not consistent with either product. This 2θ value is almost consistent with the diffraction peak attributed to the (0 0 2) plane of HAp; thus, these results suggested the possibility of HAp formation.
Figure 2. XRD patterns of ZnO single crystal particles (a) and composite particles prepared using (b) ZnO/APTES(n = 0), (c) ZnO/APTES(n = 0.5), (d) ZnO/APTES(n = 1.0), (e) ZnO/APTES(n = 3.0), (f) ZnO/APTES(n = 5.0) at reaction time 48 h. (B) shows the same patterns as (A) with the vertical axis expanded.
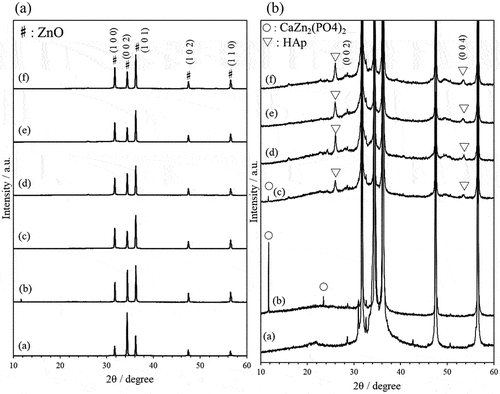
indicates the XRD patterns of composites prepared using ZnO/APTES(n = 0–5.0) with a reaction time of 48 hours. In the case of the composite prepared using ZnO/APTES(n = 0), CaZn2(PO4)2 was formed as well as in some other systems. However, composites prepared using ZnO/APTES(n = 0.5–5.0) showed new peaks at 2θ = 25.94 ° and 2θ = 53.5 °. These peaks are nearly identical to the peak positions of the (0 0 2) and (0 0 4) planes of HAp, respectively [Citation43–45]. On comparing these 2θ values with the standard values for HAp reported by Rodriguez-Lorenzo et al. [Citation45], each peak was found to shift to a higher angle. Zinc-substituted HAp is known to show an increase in the “a” lattice parameter and decrease in the “c” lattice parameter [Citation46,Citation47]. This is due to the replacement of Ca ions with Zn ions, which have a smaller ionic radius. Therefore, the composites prepared using ZnO/APTES(n = 0.5–5.0) are likely to contain zinc-doped HAp. Focusing on the diffraction peak of the (0 0 2) plane, this began to appear at a reaction time of 12 hours. The diffraction peak intensity increased with increasing reaction time. It appeared that the formation of HAp starts at around 12 hours of reaction time, and that crystal growth proceeds with the passage of time. These results suggested that the HAp/ZnO/APTES composite was formed under these reaction conditions.
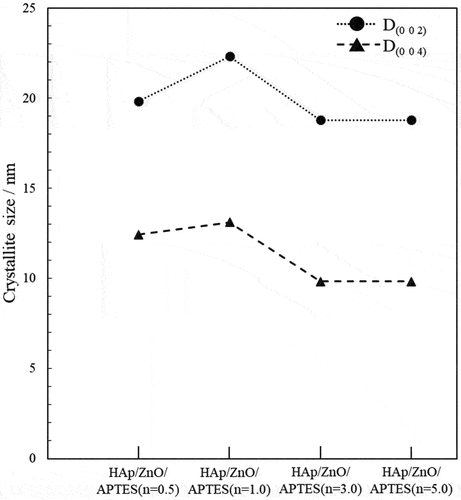
The crystallite size was calculated using Scherrer’s equation from the diffraction peaks attributed to the (0 0 2) and (0 0 4) crystal planes of HAp (). The crystallite size tended to increase at surface treatment concentrations up to n = 1.0. In contrast, crystallite size decreased at the surface treatment concentrations with n > 1.0. When the composite particles were prepared using ZnO/APTES (n = 1.0), produced HAp particles would have most crystalline.
(and Supporting information ) shows the FT-IR spectra of various HAp/ZnO composite particles. The peaks corresponding to PO43-(ν2) and PO43-(ν4) appeared at about 472 cm−1, 564 cm−1 and 599 cm−1, respectively [Citation48–50]. Bands observed at about 1046 cm−1 and 1096 cm−1 were assigned to the asymmetric stretching vibration mode of PO43-(ν3) [Citation48–50]. In addition, bands attributed to CO3 were observed at around 1440–1470 cm−1 [Citation51,Citation52]. This suggests that the composite contains some B-type carbonated HAp [Citation51,Citation52]. These peaks were observed for all composite particles prepared with ZnO/APTES(n = 0.5–5.0) regardless of reaction time; whereas, in the case of reaction times from 6 to 24 hours, C-H stretching vibrations attributed to the alkyl chain of APTES were observed. It is possible that for the composite particles obtained under these reaction conditions, the products do not completely coat the surface of the ZnO particles, and the APTES-coated surface is exposed. However, these C-H peaks were not observed for the reaction time of 48 hours. These results suggested increasing coverage of the HAp occurs on the ZnO particle surface due to crystal growth of HAp with increasing reaction time.
Figure 4. FT-IR spectra of ZnO single crystal particles (a) and composite particles prepared using (b) ZnO/APTES(n = 0), (c) ZnO/APTES(n = 0.5), (d) ZnO/APTES(n = 1.0), (e) ZnO/APTES(n = 3.0), (f) ZnO/APTES(n = 5.0) at reaction time 48 h.
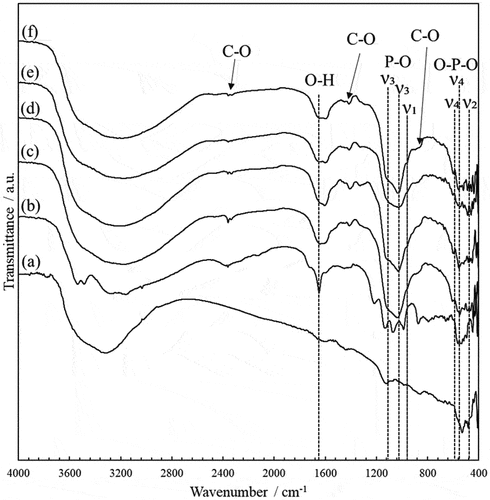
Figure 6. Elemental mapping analysis of Zn, Ca and P of HAp/ZnO/APTES(n = 0–5.0) composite particles prepared at reaction time 48 h, performed by EPMA.
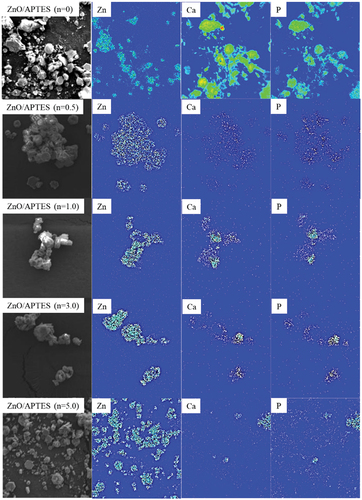
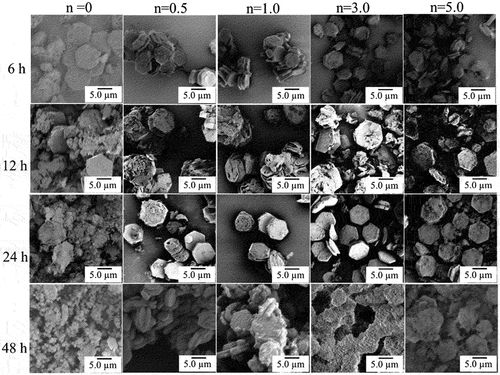
shows SEM images of HAp/ZnO composite particles prepared under various conditions. As shown in the SEM image in ), the hexagonal plate-like ZnO single-crystal particles have very smooth c faces, although the composite particles prepared using ZnO/APTES(n = 0) have hexagonal plates with rounded corners and microscopic pores on the c faces. Furthermore, as the reaction time was increased to 12 and 24 hours, the ZnO particles were observed to gradually dissolve, and when the reaction time reached 48 hours, the hexagonal plate-like shape of the ZnO particles could not be maintained. This indicates the dissolution of ZnO particles in the precursor solution. In contrast, no micropores were observed on the c face of the ZnO particles, and their surfaces were observed to be coated by different products for the composite particles prepared using ZnO/APTES(n = 0.5–5.0). As the reaction time was increased, the product formation on the surface became more pronounced.
Elemental mapping analysis was performed on the composite particles prepared with a reaction time of 48 hours (). In the case of ZnO/APTES(n = 0), the elemental distribution positions of Ca and P do not match those of Zn. It is clear that CaZn2(PO4)2, which is formed when ZnO without APTES treatment is used, is not formed on the ZnO surface but in the bulk solution. In contrast, the elemental distribution positions of Ca, P and Zn are nearly coincident when using ZnO/APTES(n = 0.5–1.0). However, distribution of Ca and P was observed in the vicinity of ZnO but mostly on the side (ab plane) of ZnO particles and almost no distribution on the c plane was observed for ZnO/APTES(n = 0.5–3.0). In particular, the composite particles prepared using ZnO/APTES(n = 5.0) resulted in significantly lower Ca and P elemental distributions.
First, the effect of the presence or absence of APTES treatment on the products was considered. ZnO without APTES treatment is expected to easily dissolve in a pH 4 precursor solution containing EDTA. Therefore, we considered that high dissolution of Zn2+ ions from inside the crystal occurs with diffusion into the solution, and that CaZn2(PO4)2 is formed in regions other than the surface of ZnO particles. In contrast, APTES treatment of ZnO is expected to prevent the dissolution of Zn2+ ions from the ZnO crystal. In addition, APTES has an amino group, so the surface of the ZnO particles coated by APTES will have a positive charge. Anionic species such as PO43- ions adsorb on the ZnO/APTES surface by electrostatic interaction; thus, ZnO/APTES limited the reaction field of HAp formation.
Second, the effect of the APTES treatment concentration on the product was considered. In the treatment concentrations with n ≤ 1.0, the ZnO/APTES particle surfaces were smooth due to APTES forming a monolayer. In contrast, the ZnO/APTES surface is expected to become irregular at APTES concentrations with n > 1.0 because APTES may oligomerize locally [Citation53]. As a result, the smoother surfaces are more likely to form HAp than the irregular ones.
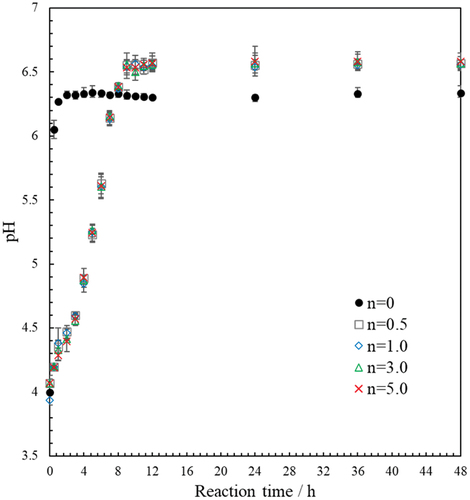
Finally, to elucidate the formation mechanism of HAp/ZnO composite particles and CaZn2(PO4)2 particles in our system, the change in the solution pH from 0 hours (immediately after the addition of various ZnO/APTES particles to the precursor solution) to 48 hours of reaction was investigated (). The increase in pH with reaction time was found to be significantly different between ZnO/APTES(n = 0) and ZnO/APTES(n = 0.5–5.0). When ZnO/APTES(n = 0) was used, the pH increased rapidly from 0.5–1.0 hours, reaching a pH of about 6.3 at 2 hours, and then remained constant. In contrast, when ZnO/APTES(n = 0.5–5.0) was used, the pH gradually increased with reaction time independent of the APTES concentration. The pH reached 6.5 at 10 hours and then did not change further up to 48 hours. This difference is thought to be due to the dissolution rate of Zn2+ ions and affects the composition of the product.
In this experiment, we added EDTA to inhibit the reaction between Ca2+ ions and PO43-ions. The Ca-EDTA chelate is formed in the precursor solution, but this chelate structure changes when Zn2+ ions dissolve from the ZnO particles. The chelate formation reaction between completely dissociated EDTA (E−4) and metal ions (Mn+) is given as follows:
and its equilibrium constant k is given as follows:
The value of k changes depending on conditions such as pH, but it is known that under the same conditions, a chelate composed of a metal ion having a low k value will be replaced with a metal ion having a higher k value. Zn-EDTA has a larger k value than Ca-EDTA [Citation54,Citation55]. Therefore, the Zn2+ ions undergo a substitution reaction with the Ca-EDTA chelate, and Ca2+ ions are released into the solution with Zn-EDTA chelate formation. It is considered that the released Ca2+ ions react with PO43- ions and OH− ions adsorb on the ZnO/APTES(n = 0.5–5.0) particles to obtain plate-like HAp/ZnO composite particles ()). Wang et al. reported that -NH3+ groups of amino acid forms hydrogen bonds with -PO43- on the HAp faces [Citation56]. Therefore, the crystal plane of HAp in contact with the ZnO/APTES particles is expected to be the c plane. Since the c plane of HAp is fixed on the ZnO/APTES particles, HAp grows epitaxially along the c axis. HAp/ZnO composite particles are considered to have a large proportion of the c plane of HAp.
Figure 8. Schematic illustration of plate-like HAp/ZnO/APTES composite particles (a) and CaZn2(PO4)2 formation.
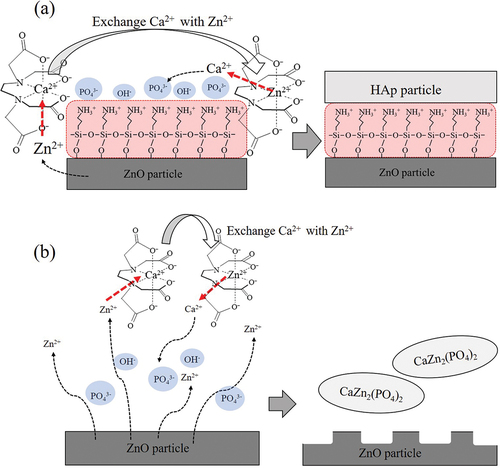
When ZnO/APTES(n = 0) is used, PO43- ions and OH− ions cannot be adsorbed on the surface, so a product cannot be formed on the surface ()). In addition, CaZn2(PO4)2 was produced when the dissolution rate of Zn2+ ions exceeded the amount of Ca2+ ions released by the substitution reaction. On the other hand, when ZnO/APTES(n = 0.5–5.0) was used, the dissolution rate of Zn2+ ions was slowed, so the release rate of Ca2+ ions due to the substitution reaction also slowed down, and HAp was generated on the surface of the ZnO particles.
For comparison, experiments were performed under the same conditions using spherical silica and plate-like mica as examples of metal oxide particles different from ZnO particles. There was no change in the pH of the precursor solution, and there was no change in the composition of the product before versus after the treatment (Supporting information –9). This revealed that Si2+, Mg2+, and Al3+ ions did not replace Ca2+ in the Ca-EDTA chelate, and HAp and other calcium phosphate compounds were not produced. The above results show that the ZnO particles treated with APTES not only induce the adsorption of PO43- ions and OH− ions on the surface, but also change the release rate of Zn2+ ions from ZnO and Ca2+ ions from the Ca-EDTA chelate. By slowing down the release of ions in solution, it became possible to limit the HAp formation reaction to the particle surface.
4. Conclusion
In this study, we succeeded in preparing plate-like HAp/ZnO composite particles by controlling the crystal growth of HAp on ZnO particles using APTES as a surface treatment agent. When ZnO/APTES(n = 0) that had not been surface-treated with APTES was used, CaZn2(PO4)2 was newly generated in the solution instead of on the ZnO particle surface. In contrast, when ZnO/APTES(n = 0.5–5.0) with APTES treatment was used, HAp was generated on the surface of ZnO particles for reaction times of 12 hours or more regardless of the APTES concentration. It was also found that the thickness of HAp generated on the ZnO particles increased as the reaction time increased. The formation of HAp and CaZn2(PO4)2 occurred when Zn2+ ions dissolved from ZnO particles replaced Ca2+ ions in the Ca-EDTA chelate to release Ca2+ ions into the solution. The surface treatment of APTES has two effects on the formation of HAp. One is that the amino group of APTES imparts a positive charge to the surface of the ZnO particles, so PO43- ions and OH− ions are adsorbed on the surface to limit the production field of HAp. The other is to reduce the release rate of Zn2+ ions in the precursor solution and slow down their substitution reaction with Ca2+ ions in the Ca-EDTA chelate. When ZnO/APTES(n = 0) was used, the dissolution rate of Zn2+ ions and the substitution reaction rate of the Ca-EDTA chelate were relatively high, and CaZn2(PO4)2 was produced, which did not lead to shape control for the particles. In contrast, when ZnO/APTES(n = 0.5–5.0) was used, the dissolution rate of Zn2+ ions and the substitution reaction rate of Ca-EDTA chelate with Ca2+ ions were relatively slow, and the formation reaction rate of HAp was slow, so the shape of the particles was controlled.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Disclosure statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Cornell CN, Lane JM. Current understanding of osteoconduction in bone regeneration. Clin Orthop Relat Res. 1998;355:267–273.
- Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20(23–24):2287–2303.
- Hench LL. Bioceramics: from Concept to Clinic. J. Am. Cream. Soc. 1991;74(7):1487–1510
- Wahl DA, Czernuszka JT. Collagen-hydroxyapatite composites for hard tissue repair. Eur Cells Mater. 2006;11:43–56.
- Galgut PN. Oxidized cellulose mesh II. Using hydroxy-apatite bone grafting material in the treatment of infrabony defects. Biomater. 1990;11(8):565–567.
- Heise U, Osborn JF, Duwe F. Hydroxyapatite ceramic as a bone substitute. Int Orthop. 1990;14(3):329–338.
- Zablotsky M. Hydroxyapatite coatings in implant dentistry. Implant Dent. 1992;1:253–257.
- Habibovic P, Marrère F, Blitterswijk CAV, et al. Biomimetic Hydroxyapatite Coating on Metal Implants. J Am Chem Soc. 2004;85(3):517–522.
- Shen JW, Wu T, Wang Q, et al. Molecular simulation of protein adsorption and desorption on hydroxyapatite surfaces. Biomaterials. 2008;29(5):513–532.
- Akazawa A, Kobayashi M, Kodaira K. A Newly Designed Adsorbent Prepared from Hydroxyapatite Originating from Cattle-Bones for Chromatographic Separation of Albumin and Lysozyme. Bull Chem Soc Jpn. 1997;70(9):2323–2329.
- Zhang H, Zhou K, Li Z, et al. Plate-like hydroxyapatite nanoparticles synthesized by the hydrothermal method. J Phys Chem Solids. 2009;70(1):243–248.
- Bian T, Zhao K, Meng Q, et al. Synthesis of plate-like single-crystal hydroxyapatite rods with c-axis orientation by biotemplate small intestinal submucosa. Ceram Int. 2017;43(15):11807–11814.
- D’Elía NL, Gravina AN, Ruso JM, et al. Manipulating the bioactivity of hydroxyapatite nano-rods structured networks: effects on mineral coating morphology and growth kinetic. Biochim. Biophys. Acta. Gen. 2013;1830(11):5014–5026.
- Zhao XY, Zhu YJ, Chen F, et al. Hydrothermal synthesis of hydroxyapatite nanorods and nanowires using riboflavin-5′-phosphate monosodium salt as a new phosphorus source and their application in proteinadsorption. Cryst Eng Comm. 2013;15(39):7926–7935.
- Zhang Y, Lu J. A simple method to tailor spherical nanocrystal hydroxyapatite at low temperature. J. Nanoparticle. Res. 2007;9(4):589–594
- Oral ÇM, Çalışkan A, Kapusuz D, et al. Facile control of hydroxyapatite particle morphology by utilization of calcium carbonate templates at room temperature. Ceram Int. 2020;46(13):21319–21327.
- Kawasaki T. Hydroxyapatite as a liquid chromatographic packing. J Chromatogr A. 1991;544:147–184.
- Fernández A, Lassaletta G, Jiménez VM, et al. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel). Comparative studies of photocatalytic activity in water purification. Appl. Catl. B. 1995;7(1–2):49–63.
- Yu QL, Browers HJH. Indoor air purification using heterogeneous photocatalytic oxidation. Part I: experimental study. Appl Catal B. 2009;92(3–4):454–461.
- Zhao B, Wang Y, Yao X, et al. Photocatalysis-mediated drug-free sustainable cancer therapy using nanocatalyst. Nat Commun. 2021;12(1):1345.
- Han X, Huang J, Jing X, et al. Oxygen-Deficient Black Titania for Synergistic/Enhanced Sonodynamic and Photoinduced Cancer Therapy at Near Infrared-II Biowindow. ACS Nano. 2018;12(5):4545–4555.
- Tang J, Zou Z, Ye J. Efficient Photocatalytic Decomposition of Organic Contaminants over CaBi2O4 under Visible-Light Irradiation. Ang. Chem. Int. Ed. 2004;43(34):4563–4566
- Minabe T, Tryk DA, Sawunyama P, et al. TiO2-mediated photodegradation of liquid and solid organic compounds. J Photochem Photobiol A Chem. 2000;137(1):53–62.
- Hirakura S, Kobayashi T, Ono S, et al. Fibrous nanocrystals of hydroxyapatite loaded with TiO2 nanoparticles for the capture and photocatalytic decomposition of specific proteins. Colloids Surf B. 2010;79(1):131–135.
- Nakajima A, Rakakuwa K, Kameshima Y, et al. Preparation and properties of titania–apatite hybrid films. J Photochem Photobiol A Chem. 2006;177(1):94–99.
- Hung J, Gong Y, Liu Y, et al. Developing titania-hydroxyapatite-reduced graphene oxide nanocomposite coatings by liquid flame spray deposition for photocatalytic applications. J. Eur. Cream. Soc. 2017;37(12):3705–3711.
- Yusufoglu Y, Akinc M. Effect of pH on the Carbonate Incorporation into the Hydroxyapatite Prepared by an Oxidative Decomposition of Calcium–EDTA Chelate. J. Am. Cream. Soc. 2007;91(1):77–82
- Xie R, Feng Z, Li S, et al. EDTA-Assisted Self-Assembly of Fluoride-Substituted Hydroxyapatite Coating on Enamel Substrate. Cryst Growth Des. 2011;11(12):5206–5214.
- Kumar GS, Girjia EK, Venkatesh M, et al. One step method to synthesize flower-like hydroxyapatite architecture using mussel shell bio-waste as a calcium source. Ceram Int. 2017;43(3):3457–3461.
- Soten I, Ozin GA. Porous hydroxyapatite-dodecylphosphate composite film on titania-titanium substrate. J. Mater. Chem. 1999;9(3):703–710
- Forsgren J, Svahn F, Jarmar T, et al. Formation and adhesion of biomimetic hydroxyapatite deposited on titanium substrates. Acta Biomater. 2007;3(6):980–984.
- Jin B, Shao C, Wang Y, et al. Anisotropic Epitaxial Behavior in the Amorphous Phase-Mediated Hydroxyapatite Crystallization Process: a New Understanding of Orientation Control. J Phys Chem Lett. 2019;10(24):7611–7616.
- Shibata H, Iizuka Y, Kawai T, et al. Preparation of Hexagonal Plate-like ZnO Single-crystal Particles in the Presence of Anionic Amphiphiles. J Oleo Sci. 2020;69(7):783–787.
- Shibata H, Iizuka Y, Amano M, et al. Effect of Anionic Amphiphiles on the Morphology of Hexagonal Plate-like ZnO Particles. J Oleo Sci. 2021;70(7):919–925.
- Amano M, Hashimoto K, Shibata H. Preparation and Photocatalytic Activity of Hexagonal Plate-like ZnO Particles Using Anionic Surfactants. J Oleo Sci. 2022;71(6):927–932.
- Saad R, Hamoudi S, Belkacemi K. Adsorption of phosphate and nitrate anions on ammonium-functionnalized mesoporous silicas. J Porous Mater. 2008;15:315–323.
- Saad R, Belkacemi K, Hamoudi S. Adsorption of phosphate and nitrate anions on ammonium-functionalized MCM-48: effects of experimental conditions. J Colloid Interface Sci. 2007;311(2):375–381.
- Rahman IA, Jafarzadeh M, Sipaut CS. Synthesis of organo-functionalized nanosilica via a co-condensation modification using γ-aminopropyltriethoxysilane (APTES). Ceram Int. 2009;35(5):1883–1888.
- Jaramillo AF, Curz RB, Montoya LF, et al. Estimation of the surface interaction mechanism of ZnO nanoparticles modified with organosilane groups by Raman Spectroscopy. Cream. Int. 2017;43(15): 11838–11847
- Murugan C, Murugan N, Sundramoorthy AK, et al. Gradient Triple-Layered ZnS/ZnO/Ta2O5–SiO2 Core–Shell Nanoparticles for Enzyme-Based Electrochemical Detection of Cancer Biomarkers. ACS Appl Nano Mater. 2020;3(8):8461–8471.
- Bhanvase BA, Kutbuddin Y, Borse RN, et al. Ultrasound assisted synthesis of calcium zinc phosphate pigment and its application in nanocontainer for active anticorrosion coatings. Chem Eng J. 2013;231:345–354.
- Krishnapriya T, Jose A, Jose TA, et al. An insight into the luminescent properties and Judd–Ofelt analysis of Eu3+ doped CaZn2(PO4)2 phosphors. J Mater Sci Mater Electron. 2020;31:22452–22466.
- Abbona F, Baronnet A. A XRD and TEM study on the transformation of amorphous calcium phosphate in the presence of magnesium. J Cryst Growth. 1996;165(1–2):98–105.
- Rahavi S, Monshi A, Emadi R, et al. Determination of Crystallite Size in Synthetic and Natural Hydroxyapatite: a Comparison between XRD and TEM Results. Adv Mat Res. 2012;620:28–34.
- Rodriguez-Lorenzo LM, Hart JN, Gross KA. Structural and Chemical Analysis of Well-Crystallized Hydroxyfluorapatites. J Phys Chem B. 2003;107(33):8316–8320.
- Ergun C, Webster TJ, Bizios R, et al. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. I. Structure and microstructure. J Biomed Mater Res. 2001;59(2):305–311.
- Miyaji F, Kuno Y, Suyama Y. Formation and structure of zinc-substituted calcium hydroxyapatite. Mater Res Bull. 2005;40(2):209–220.
- Liao CJ, Lin FH, Chen KS, et al. Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomater. 1999;20(19):1807–1813.
- Rhee SH, Lee JD, Tanaka J. Nucleation of Hydroxyapatite Crystal through Chemical Interaction with Collagen. J Am Ceram Soc. 2004;83(11):2890–2892.
- Gasga JR, Piñeiro ELM, Álvarez GR, et al. XRD and FTIR crystallinity indices in sound human tooth enamel and synthetic hydroxyapatite. Mater Sci Eng C. 2013;33(8):4568–4574.
- Legeros RZ. Effect of carbonated on the lattice parameters of apatite, crystallographic studies of the carbonated subsitution in the apatite structure. Nature. 1965;206(982):403–404.
- Okazaki M, Takahashi J, Kimura H, et al. Crystallinity, solubility, and dissolution rate behavior of fluoridated CO3 apatites. J Biomed Mater Res. 1982;16(6):851–860.
- Yuan P, Southon PD, Liu Z, et al. Functionalization of Halloysite Clay Nanotubes by Grafting with γ-Aminopropyltriethoxysilane. J Phys Chem C. 2008;112(40):15742–15751.
- Xue H, Sigg L, Kari FG. Speciation of EDTA in Natural Waters: exchange Kinetics of Fe-EDTA in River Water. Environ Sci Technol. 1995;29(1):59–68.
- Sun B, Zhao FJ, Lombi E, et al. Leaching of heavy metals from contaminated soils using EDTA. Environ Pollut. 2001;113(2):111–120.
- Wang Z, Xu Z, Zhao W, et al. A potential mechanism for amino acid-controlled crystal growth of hydroxyapatite. J Mater Chem B. 2015;3:9157–9167.