?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A research investigation was conducted on two variables that are indicative of plastic raw materials based on kaolin vs. interstratified illite-smectite clay and a non-plastic admixture including grog (quartz vs. alumina) during the sintering process of an anorthite porcelain body beginning at 950°C, up to the sintering temperature. A correlation was found between the mineralogical composition and various parameters of the fired bodies. A purely anorthic sintered body can be produced using interstratified illite-smectite clay with quartz as a grog, which has the lowest coefficient of linear thermal expansion in the temperature range of 30°C to 700°C and highest bending strength – modulus of rupture of sintered body. When alumina is present in the raw material mixture, the coefficient of linear thermal expansion is lower due to the absence of the SiO2 phases. Quartz in combination with interstratified illite-smectite clay significantly reduces the sintering temperature through the formation of an amorphous melt up to the sintering temperature.
HIGHLIGHTS
Summary of novel conclusions
Utilization of interstratified illite/smectite clay as a raw material
Utilization of quartz and alumina as grogs
Crystallization of anorthite bodies fired at 950°C
Alumina used as a grog lowered the coefficient of linear thermal expansion.
Quartz used as a grog lowered the required sintering temperature
1. Introduction
Generally, a sintered ceramic body can be produced with traditional non-calcareous raw material mixtures (usually a mixture of kaolinitic clay or kaolin, feldspars and quartz) or calcareous raw material mixtures (based on CaO clays, such as limestone, wollastonite, bone ash or calcium aluminate cement etc.). The main mineralogical phase of a sintered ceramic body based on a non-calcareous raw material mixture is mullite [Citation1,Citation2], as opposed to anorthite which is typical of calcareous porcelain raw material mixtures [Citation3,Citation4]. The advantages of anorthite ceramics are the relatively minor degree of contraction of the body during and after firing and the degree of body closure after hydration [Citation5]. A dense anorthite ceramic body (composed of kaolin and calcium hydroxide) with a bulk density of around 2300 kg·m−3 can be produced at low temperatures (from 950°C), while the sintering begins between 1000°C and 1150°C [Citation6]. It is possible to prepare (generally after the firing at about 1200°C) an anorthite porcelain body with a higher bending strength – modulus of rupture (MOR) using conventional materials that are used to produce stoneware materials based on mullite [Citation7–10] or with a sintered body with other beneficial properties, such as whiteness [Citation11] or a lower coefficient of linear thermal expansion (CLTE) [Citation10]. Technical porcelain (for example high-voltage electrical insulators) is typically made from clay, feldspar, and quartz. The addition of alumina, rather than the traditional quartz, led to an increase in the mechanical strength and electrical strength of traditional porcelain body [Citation12–14] – for instance, the addition of 5 wt.% of alumina increased the bending strength of the fired bodies by approximately 30% higher than in samples with 0.0% alumina addition due to the reduction of phase content of quartz and cristobalite [Citation14]. An optimal combination of both grogs (alumina and quartz) is another suitable option that increases the bending and compressive strength of the porcelain body [Citation15]. The utilization of waste casting sands (source of quartz), in place of clay and kaolin was successfully investigated in traditional porcelain tile production with a sintering temperature of 1200°C [Citation16]. Secondary waste materials (CaCO3, Ca(OH)2), marble dust, plaster cast, steelmaking slag) can also be successfully used in the production of anorthite porcelain bodies with a sintering temperature of about 1200°C [Citation4,Citation17,Citation18]. The use of wollastonite as a source of CaO can solve the issue of excessive shrinkage when firing sintered ceramic bodies based on anorthite [Citation8]. In these studies, an anorthite ceramic body was prepared using calcium aluminate cement (binder and source of CaO) instead of kaolin but the sintering temperature was relatively high at 1300°C [Citation19] or 1400°C, respectively, [Citation20].
This paper is a logical continuation of the work published in the previous paper [Citation21] that dealt with the sintering of anorthite ceramics with the exclusive use of a quartz grog in contrast to traditional mullite-based sintered bodies. The results of the previous research [Citation21] revealed that the illite/ smectite (I/S) clay is more sinterable compared to kaolin. A purely anorthic sintered body can be prepared using I/S clay with the highest strength (MOR) and lowest CLTE. The presence of calcite decreases the sintering temperature significantly. The resulting challenge of this research paper was in the utilization of quartz as a grog, cristobalite was formed and the CLTE was high due to the cristobalite inversion at lower temperatures. The advantages of sintered anorthic bodies are therefore obvious.
The primary objective of this research paper was to find the optimal raw material composition for the preparation of an anorthite porcelain body using different plastic raw materials (based on double-layer clay minerals, such as kaolinite (kaolin) and triple-layer clay minerals, such as I/S clay and different non-plastic raw materials, that included grogs (quartz vs. alumina). The aim is to determine the lowest sintering temperature and coefficient of linear thermal expansion (CLTE) in combination with the highest mechanical strength. The sintering process used to produce the test samples is described with regard to the vacuum water absorption and CLTE for the various test samples with different mineralogical compositions after firing (especially the content of anorthite, mullite, quartz and cristobalite).
2. Methodology and the properties of the used raw materials
The sintering of the ceramic body is not only determined by the composition of the raw materials mixture (the aim of this article), but it also changes depending on the particle size [Citation22], mixing method, and molding method. However, these partial influences were eliminated by using the similar granulometry of the used grogs and the same method of preparation of the test samples. The selected plastic raw materials and grogs were chosen due to their utilization for the production of traditional whitewares based on mullite both at present (kaolin, quartz, alumina) or in the past (illite-smectite clay).
Four raw material mixtures were used for the preparation of the test samples, described in , based on different plastic materials – interstratified illite-smectite clay (ISC) and kaolin (KA), industrially milled quartz sand (99.8% of SiO2, D(50) = 6.0 m), alumina (Magyar Aluminium ALO-G4-4 G with greater than 99.5% Al2O3 content, D(50) = 5.0
m) and calcite (laboratory grade precipitated calcium carbonate with 99.0% of CaCO3 – Penta Chemicals unlimited) to produce an anorthite fired body. The composition of the raw material mixtures was influenced by the effort to maximize the anorthite content in the fired bodies (). Illite-smectite clay from Fuzzeradvanyi (Hungary), a triple-layered type of plastic clay with an interstratified illite-smectite structure (the detail of the technical properties of the material can be found in references [Citation21,Citation24,Citation25]) and washed kaolin from the Sedlecky kaolin company were used as the plastic raw materials. The chemical compositions of the plastic raw materials used can be found in . The mineralogical composition of the used kaolin was 91% kaolinite, 2% quartz and about 7% mica minerals. The mineralogical composition of IS clay is 80% interstratified illite/smectite, 12% quartz and 4% orthoclase as main phases.
Figure 1. Equilibrium phase diagram CaO – Al2O3 – SiO2 [Citation23]: the theoretical melting temperatures of the test samples; ISQC (1), KAQC (2), ISAC (3) and KAAC (4).
![Figure 1. Equilibrium phase diagram CaO – Al2O3 – SiO2 [Citation23]: the theoretical melting temperatures of the test samples; ISQC (1), KAQC (2), ISAC (3) and KAAC (4).](/cms/asset/bf04113a-61e3-4fee-a41c-d6dac9604b09/tace_a_2320448_f0001_b.gif)
Table 1. Composition and identification of test samples (wt.%).
Table 2. Chemical composition of the plastic raw materials in wt.% (LOI – loss on ignition).
The composition of the raw material mixtures () was based on a previously published research paper [Citation21]. The content of calcite (15 wt.%) in all raw material mixtures falls approximately in the area for the theoretical anorthite formation in the equilibrium phase diagram CaO – Al2O3 - SiO2 (). All the raw material mixtures () were milled in suspension (220% water content without deflocculant, which could affect the parameters achieved for the test samples) in a laboratory ball mill for 24 hours. Test samples of 10 × 10 × 50 mm each were prepared from this plastic dough and were found to have a deformation ratio of 0.4, according to a Pfefferkorn test (Czech standard no. 72 1074).
The dried test samples were fired in an electric muffle kiln at temperatures from 950°C to 1400°C (increased in 50°C steps). The heating rate was 5°C·min−1 and the soaking time was 1 hour at the maximum temperature. Vacuum-saturated water absorption Ev (EquationEquation (1)(1)
(1) ) was determined according to EN ISO 10,545–3:
Where:
mwet … weight of water-saturated sample (g),
mdry … weight of dried sample (g).
The sintering process was observed in accordance with the following criteria:
sintering temperature, denoted as Ts, which is defined as the temperature at which the fired body reaches a water absorption level of exactly 2.0%, according to the Czech standard no. 72 1072 (Determination of sinterability of ceramic materials and masses) and
thermodilatometric analysis (CLASIC instrument) – heating rate 5°C·min−1 without soaking time, determination of CLTE was carried out in temperature range 30–700°C. The coefficient of linear thermal expansion (CLTE) was calculated from the thermodilatometric curve according to EquationEquation (2)
(2)
(2) :
(2)
(2)
Where:
ΔL … change in length of the test sample due to heating in the temperature range of ΔT (m).
L0 … the original length of the test sample at room temperature (here 30°C) (m).
ΔT … temperature change during the test (here 30–700°C) (°C).
The MOR (modulus of rupture) of the fired bodies was carried out according to ASTM C1505 as a three-point bending test (Testometric M350-20CT). The mineralogical composition of the fired bodies was determined by X-ray diffraction analysis (XRD) using a PANalytical Empyrean from PANalytical B.V. (CuKα was used as the radiation source, acceleration voltage of 45 kV, beam current of 40 mA, a diffraction angle of 2θ in a range of 10° to 50° and scan step of 0.01°). Calcium fluoride CaF2 (15,0 wt.%) was incorporated as an internal standard for the quantitative analysis of the samples. Weighted samples and calcium fluoride were milled in a laboratory mill with corundum cylinders together with isopropyl alcohol and then dried in a laboratory drier. The quantitative representation of minerals and amorphous phase was then calculated after Rietveld refinement.
Scanning electron microscopy (SEM) was used to evaluate the microstructure of sintered bodies. This test was performed with a Tescan Mira3 instrument (Tescan Orsay Holding a.s., Brno, Czech Republic) with an energy-dispersive X-ray spectroscopy (EDS) probe for the elemental analysis of the microstructure. The surface of test samples was previously etched with 5% hydrofluoric acid (HF) for 30 min, which dilutes the amorphous glass phase.
3. Results and discussion
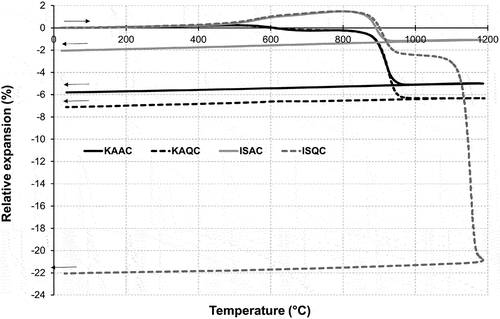
In the first stage of the presented research, changes in the test sample length (thermodilatometric analysis) during firing were studied to establish the sintering properties of the various raw material mixtures. The results of the thermodilatometric analysis of the test samples KAAC, KAQC, ISAC, and ISQC can be seen in . It is apparent that the thermodilatometric curves are very similar in the temperature range of 20–900°C and are dependent on the used plastic material (kaolin vs. illite/smectite clay) or the predominant clay mineral used (kaolinite vs. interstratified illite/smectite structure). The test samples based on ISC have a higher degree of expansion between 500°C and 850°C due to their higher quartz content and the presence of clay minerals (the difference between the thermodilatometric curves of kaolin and illite and smectite are presented in detail in [Citation21,Citation26]). Surprisingly, there is no visible change in the appearance of the curves due to the different grogs used (quartz vs. alumina), the quartz modification change at 573°C had no visible influence. As the temperature increased, samples shrunk over a narrow temperature interval from 850°C to 950°C, which corresponds to the decomposition of limestone. With the further increase in temperature, a distinct area of formation (crystallization) of anorthite occurred, which typically causes minimal change in the volume of the sample or, if the raw material mixture has a higher CaO content, even an increase in volume. The ISQC mixture begins to behave completely differently from 1100°C, as the sample begins to sinter, the firing shrinkage sharply increases. This can be explained by the formation of an eutectic melt that promotes the sintering process. If kaolin-based mixtures are used, the effect of the grog (quartz vs. alumina) on firing shrinkage is not as pronounced as with ISC mixtures, where the alumina significantly reduces (equalizes) firing shrinkage in the temperature range of 900–1200°C. On the contrary, with a firing temperature of 1100°C, a quartz admixture supports the formation of an amorphous glass phase that has an extreme effect on the changes in volume (shrinkage) during firing. This is also reflected in the sintering temperature Ts when the difference in Ts is exactly 200°C for mixtures based on IS clay (ISQC vs. ISAC) but only 90°C for mixtures based on kaolin. The presence of ISC in the raw material mixture leads to entirely distinct effects resulting from the use of the two different types of grogs. Alumina significantly reduces changes in length (shrinkage), due to the firing of the body, while quartz supports significant shrinkage in the body at the temperature of 1100°C. This is then reflected in the significant difference in the sintering temperatures Ts of the ISQC and ISAC samples as shown in .
Figure 3. Sintering curves – the effect of firing temperature on water absorption and the determination of sintering temperature Ts.
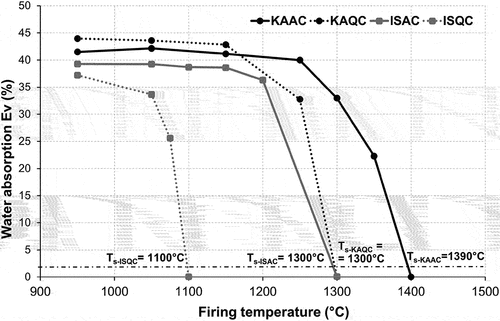
The shape of the sintering curve () is similar for all the raw materials used – up to a certain firing temperature between 1150°C and 1200°C. The porosity of the fired body, as defined by its water absorption is more or less constant. Subsequently, the sintering of the body is indicated by a steep decrease in water absorption up to the sintering temperature Ts. The exception to this is the ISQC mixture, which, as predicted by the thermodilatometry curve, sinters much earlier and the sharp decrease in water absorption is already evident at the lowest test firing temperature of 950°C. The corresponding sintering temperature (Ts) is also significantly lower (1100°C) in comparison with the other test samples.
An admixture of alumina increases the sintering temperature of an anorthite ceramic body when compared to quartz, by about 200°C for ISC and 90°C for kaolin-based mixtures as can be seen in . The lower sintering temperatures of quartz-based mixtures (ISQC and KAQC) in comparison to alumina-based mixtures can be explained by the inert behavior of the alumina grog (with a high theoretical melting temperature of about 2050°C according to the grain size [Citation27]) during firing, all of the fired bodies contained a crystalline corundum phase at all firing temperatures.
The creation of new phases after firing at temperatures of 950°C, 1050°C, 1150°C and the sintering temperature was established from the results of the XRD analyses, show the XRD patterns of the fired bodies (all patterns were adjusted to start from 10° 2theta angle, since there were no reflections observed at lower angles). Anorthite was the main crystalline phase found in all fired bodies from the lowest firing temperature (950°C), with wollastonite as the preliminary phase in the test samples based on a quartz grog. Wollastonite was found up to a firing temperature of 1050°C (the actual minimum firing temperature required for a low-temperature synthesis of wollastonite is about 800°C [Citation28,Citation29]). In the case of the IS clay and quartz combination (ISQC mixture), anorthite is the only crystalline phase of the sintered body. In regards to the IS clay-alumina mixture, anorthite and corundum are typically the crystalline phases found. Samples that contained kaolin (KAQC, KAAC) were supplemented by mullite, and the KAQC mixture was also supplemented with cristobalite. Due to the lower sintering temperatures, the ISC-based sintered bodies contained quartz instead of cristobalite. The results of the quantitative analysis of the sintered samples are shown in .
Figure 4. XRD patterns of the test samples fired at 950°C (A – anorthite, Q – quartz, W-wollastonite, Al – corundum, mu – muscovite).
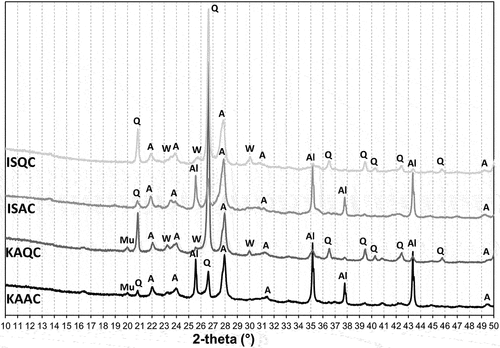
Figure 5. XRD patterns of the test samples fired at 1050°C (A – anorthite, Q – quartz, Al – corundum).
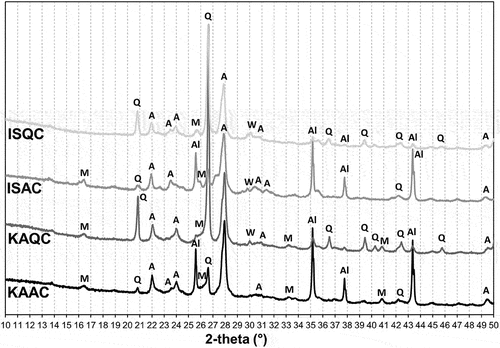
Figure 6. XRD patterns of the test samples fired at 1150°C (A – anorthite, Q – quartz, M – mullite, C – cristobalite, Al – corundum), ISQC is already sintered ().
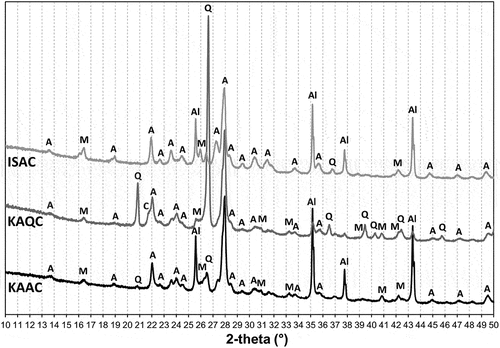
Figure 7. XRD analysis of sintered bodies at the sintering temperature Ts, according to (A – anorthite, M – mullite, C – cristobalite, Al – corundum).
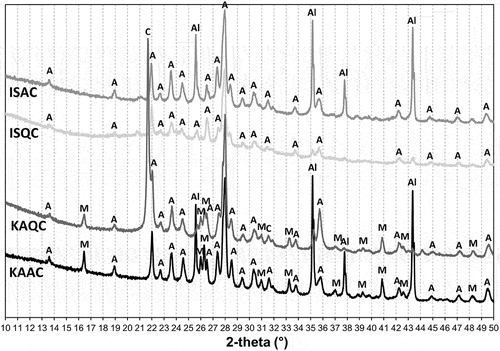
Table 3. Summary of mineral composition of sintered bodies – quantitative XRD analysis.
Alumina functions as a non-reactive admixture (grog) that remains in the sintered body in the crystalline form of corundum, in contrast to quartz. For example, the ISQC sintered body does not contain any crystalline SiO2 phases (quartz or cristobalite), because it is integrated into the amorphous glass phase that is formed. This is evident from the XRD patterns () [Citation30,Citation31]. Therefore, quartz simultaneously with flux oxides from raw materials promotes the formation of a eutectic melt that promotes the sintering process and all crystalline quartz is incorporated into the amorphous glass matrix – this is especially evident in the ISQC sample with the lowest sintering temperature Ts and was also confirmed by the XRD analysis patterns () and quantitative analysis calculations – a greater extent of the liquid glass phase is evident in the fired bodies (using the methodology in [Citation30]).
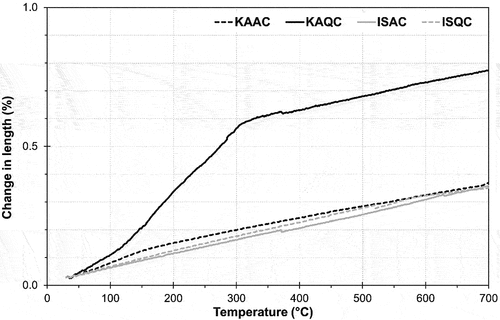
The highest coefficient of linear thermal expansion, CLTE, is exhibited by the KAQC sintered body – due to the use of quartz as a grog and the high sintering temperature (1300°C), cristobalite was formed during firing. At a temperature of about 200°C (), the transformation modification of cristobalite [Citation32] leads to an extremely high CLTE value in comparison to the other test samples. The CLTE of all the other sintered bodies is almost identical. The theoretical coefficient of linear thermal expansion of alumina films is 10.3 × 10−6 K−1 in the temperature range of 20–900°C [Citation33]. Undeniably, the inclusion of cristobalite significantly increases the CLTE (for the KAQC mixture) – only the sintered body from the KAQC sample contains cristobalite which is also seen in the incomparably high value of CLTE. Sintered bodies based on IS clay generally have a lower value of CLTE.
Figure 8. Change in the length of sintered bodies for the determination of the coefficient of linear thermal expansion, CLTE.
In the case of an anorthite ceramic body, using alumina as a grog does not increase the strength of the fired body, contrary to what is typically found with traditional mullite based porcelain bodies [Citation12–14]. This can be explained by the minimal formation of mullite (alumina supports its formation [Citation34]) when using calcareous raw material mixtures and therefore anorthite ceramics [Citation17]. If mullite is present in the sintered body (KAAC, KAQC), then alumina has a positive effect on the modulus of rupture compared to a quartz grog ().
Table 4. Coefficient of linear thermal expansion (CLTE) of sintered bodies in the temperature range of 30–700°C. Modulus of rupture (MOR) at sintering temperature.
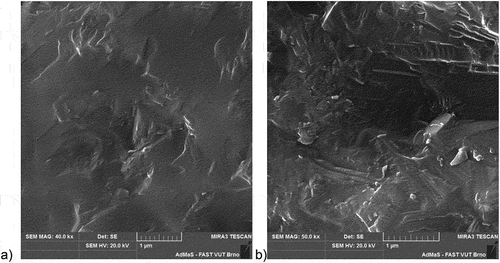
The microstructure of sintered bodies, thus fired at their sintering temperature TS, was studied by scanning electron microscope (SEM) and showed a high content of amorphous glass phase without any porosity (). The presented sintered structure is very similar for all tested samples and no distinctive grains can be detected. After etching the amorphous glass phase with a 5% HF solution, small (lower than 1 µm) grains of triclinic anorthite are typical for all sintered bodies.
Figure 9. SEM – examples of typical fractured surface of ISQC (a) and KAQC (b) sintered bodies without HF application.
Larger alumina grains (around 2 µm in size) are evident in the KAAC () sample and a very similar microstructure with the same identified mineral was also observed in ISAC sample. These grains are unchanged by firing. In the sintered KAQC body, typical rod-shaped grains of well-crystallized mullite are clearly visible (). Only anorthite grains are visible in the microstructure of the ISQC sample (). The elemental composition of the grains in the SEM microphotographs was identified by the EDS probe and their elemental composition can be found in .
Figure 10. SEM – microstructure of fractured and HF etched sintered body KAAC (al-corundum, A-anorthite).

Figure 11. SEM – microstructure of fractured and HF etched sintered body KAQC (M-mullite, A-anorthite).

Table 5. Results of the EDS analysis at the points highlighted in the SEM microphotographs.
4. Conclusion
The effect of two different non-plastic materials – grogs (quartz vs. alumina) and plastic materials (kaolin vs. interstratified illite/smectite clay) was examined during the sintering of an anorthite ceramic body to investigate the possible differences in mineralogical composition and linear thermal expansion. It was found that:
If quartz is utilized as a grog, the ceramic body may be sintered at a lower temperature, as quartz can form a melt, in contrast, alumina functions as an inert grog for an anorthite-based ceramic body.
Anorthite is the main crystalline phase in all fired bodies from the lowest firing temperature used (950°C). Mullite is present in all sintered bodies based on kaolin. A purely anorthic sintered body can be prepared using IS clay with quartz as a grog.
The addition of alumina into the raw material mixtures reduced the coefficient of linear thermal expansion (CLTE) due to the absence of SiO2 phases. Quartz in combination with IS clay creates an amorphous glass phase in the sintered body and does not influence CLTE.
From an economic point of view, the optimal solution is the use of IS clay as a plastic component in combination with quartz as the grog, which results in the lowest sintering temperature (1100°C) and the lowest CLTE value for the temperature range 30–700°C, which guarantees that the sintered body will have good resistance to thermal shock. A sintered body based on an IS clay and quartz (ISQC) is a purely anorthic body, which ensures it will have the highest modulus of rupture.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data presented in this paper are available upon request from the corresponding author.
Additional information
Funding
References
- Martín-Márquez J, Rincón JM, Romero M. Mullite development on firing in porcelain stoneware bodies. J Eur Ceram Soc. 2010;30(7):1599–1607. doi: 10.1016/j.jeurceramsoc.2010.01.002
- Montoya N, Serrano FJ, Reventós MM, et al. Effect of TiO2 on the mullite formation and mechanical properties of alumina porcelain. J Eur Ceram Soc. 2010;30(4):839–846. doi: 10.1016/j.jeurceramsoc.2009.10.009
- Sokolar R. Non-traditional whiteware based on calcium aluminate cement. Mater Tehnol. 2016;50(6):885–889. doi: 10.17222/mit.2015.182
- Kurama S, Ozel E. The influence of different CaO source in the production of anorthite ceramics. Ceram Int. 2009;35(2):827–830. doi: 10.1016/j.ceramint.2008.02.024
- Sokolar R. Effect of calcite on the brick body closing. InterCeram Int Ceramic Rev. 2010;59(2):123–127.
- Ouali A, Sanhoune F, Belhouchet H, et al. Effect of CaO addition on the sintering behaviour of anorthite formed from kaolin and CaO. Acta Phys Pol A. 2017;131(1):159–161. doi: 10.12693/APhysPolA.131.159
- Taskiran MU, Demirkol N, Capoglu A. A new porcelainised stoneware material based on anorthite. J Eur Ceram Soc. 2005;25(4):293–300. doi: 10.1016/j.jeurceramsoc.2004.03.017
- Choi JH, Kim US, Cho WS. Development and characteristics of anorthite-based traditional ceramic materials to suppress sintering deformation. J Korean Ceram Soc. 2017;54(1):55–60. doi: 10.4191/kcers.2017.54.1.09
- Harabi A, Zaiou S, Guechi A, et al. Mechanical properties of anorthite based ceramics prepared from kaolin DD2 and calcite. Cerâmica. 2017;63(367):311–317. doi: 10.1590/0366-69132017633672020
- Cheng X, Ke S, Wang Q, et al. Fabrication and characterization of anorthite-based ceramic using mineral raw materials. Ceram Int. 2012;38(4):3227–3235. doi: 10.1016/j.ceramint.2011.12.028
- Selli NT. Development of anorthite based white porcelain stoneware tile compositions. Ceram Int. 2015;41(6):7790–7795. doi: 10.1016/j.ceramint.2015.02.112
- Noori NR, Mamoory RS, Mehraeen S. Effect of materials design on properties of porcelain insulators. Am Ceram Soc Bull. 2007;86(3):9201–9203.
- Sedghi A, Riahi-Noori N, Hamidnezhad N, et al. Effect of chemical composition and alumina content on structure and properties of ceramic insulators. Bull Mater Sci. 2014;37(2):321–325. doi: 10.1007/s12034-014-0641-x
- Yahya H, Ahmad A, Ibrahim I. Effect of alumina (Al2O3) to the properties of whiteware porcelain. Mater Sci Forum. 2020;1010:194–199. doi: 10.4028/www.scientific.net/MSF.1010.194
- Mehta NS, Sahu PK, Tripathi P, et al. Influence of alumina and silica addition on the physico-mechanical and dielectric behavior of ceramic porcelain insulator at high sintering temperature. Bol Soc Esp Ceram V. 2018;57(4):151–159. doi: 10.1016/j.bsecv.2017.11.002
- Bahtli T, Erdem Y. The use of foundry waste sand from investment casting in the production of porcelain tiles. Ceram Int. 2022;48(19A):27967–27972. doi: 10.1016/j.ceramint.2022.06.100
- Sokolář R, Vodová L, Grygarová S, et al. Mechanical properties of ceramic bodies based on calcite waste. Ceram Int. 2012;38(8):6607–6612. doi: 10.1016/j.ceramint.2012.05.046
- Li B, He M, Hwang JY, et al. Synthesis and characteristics of anorthite ceramics from steelmaking slag. Char Minerals Metals Mater. 2016;263–269. doi: 10.1007/978-3-319-48210-1_32
- Tai WP, Kimura K, Jinnai K. A new approach to anorthite porcelain bodies using non plastic raw materials. J Eur Ceram Soc. 2002;22(4):463–470. doi: 10.1016/S0955-2219(01)00317-X
- Pal M, Das S, Das SK. Anorthite porcelain: synthesis, phase and microstructural evolution. Bull Mater Sci. 2015;38(2):551–555. doi: 10.1007/s12034-015-0855-6
- Sokolar R, Nguyen M. Sintering of anorthite ceramic body based on interstratified illite-smectite clay. Ceram Int. 2022;48(21):31783–31789. doi: 10.1016/j.ceramint.2022.07.105
- Dvořák K, Macháčková A, Ravaszová S, et al. Effect of imposed shear strain on steel ring surfaces during milling in high-speed disintegrator. Materials. 2020;13(10):2234. doi: 10.3390/ma13102234
- Levin EM, Robbins CR, McMurdie HF. Phase diagrams for ceramists: fig. 630. 2nd ed. Columbus (OH): American Ceramic Society; 1975. ISBN: 0916094049.
- Pécskay Z, Molnar F, Itaya T, et al. Geology and K-Ar geochronology of illite from the clay deposit at Füzérradvány, Tokaj Mts., Hungary. Acta Mineralogica Petrographica, Szeged. 2005;46:1–7.
- Húlan T, Štubňa I, Ondruška J, et al. Young’s modulus of different illitic clays during heating and cooling stage of firing. Materials. 2020;13(21):4968. doi: 10.3390/ma13214968
- Foldvári M. Handbook of thermogravimetric system of minerals and its use in geological practice. Budapest: Geological Institute of Hungary; 2011. ISBN: 978-963-671-288-4.
- Joshi N, Mathur N, Mane T, et al. Size effect on melting temperatures of alumina monocrystals: molecular dynamics simulations and thermodynamic modelling. Comp Mater Sci. 2018;145:140–153. doi: 10.1016/j.commatsci.2017.12.064
- Zulumyan N, Migorodski A, Isahakyan A, et al. A low-temperature method of the beta-wollastonite synthesis. J Therm Anal Calorim. 2015;122:97–104. doi: 10.1007/s10973-015-4752-4
- Ribas RG, Bastos Campos TM, Modelski Schatkoski V, et al. α-wollastonite crystallization at low temperature. Ceram Int. 2020;46(5):6575–6580. doi: 10.1016/j.ceramint.2019.11.143
- Kemethmüller S, Roosen A, Goetz-Neunhoeffer F, et al. Quantitative Analysis of Crystalline and amorphous phases in glass–ceramic composites like LTCC by the Rietveld Method. J Am Ceram Soc. 2006;89(8):2632–2637. doi: 10.1111/j.1551-2916.2006.01113.x
- Klika Z, Valasková M, Bartonova L, et al. Quantitative evaluation of crystalline and amorphous phases in clay-based cordierite ceramic. Minerals. 2020;10(12):1122. doi: 10.3390/min10121122
- Walker RF, Zerfoss S, Holley SF, et al. Temperature of the inversion in cristobalite. J Res Nat Bur Stand. 1958;61(4):251–261. doi: 10.6028/jres.061.026
- Huntz AM, Marechal L, Lesage B, et al. Thermal expansion coefficient of alumina films developed by oxidation of a FeCrAl alloy determined by a deflection technique. Appl Surf Sci. 2006;252(22):7781–7787. doi: 10.1016/j.apsusc.2005.08.116
- Behera PS, Bhattacharyya S. Effect of different sources on phase formation and densification of single-phase mullite ceramic – reference clay alumina system. Mater Today Comm. 2021;26:101818. doi: 10.1016/j.mtcomm.2020.101818