Abstract
Objective: This study was performed to compare the effectiveness of intravenous dexmedetomidine with that of pethidine and tramadol in the treatment of post-neuraxial anaesthesia shivering.
Design: This was a prospective, randomised, double-blinded study.
Setting and subjects: One hundred and two patients of both genders, aged 18–70 years with American Society of Anesthesiologists physical status I and II undergoing spinal or combined spinal and epidural anaesthesia for elective surgery were enrolled in this study. Sixty of them developed shivering after an intrathecal injection of 0.5% hyperbaric bupivacaine 15 mg. They were then randomly allocated to receive either intravenous dexmedetomidine 0.5 μg/kg, pethidine 0.5 mg/kg or tramadol 0.5 mg/kg.
Outcome measures: The response rate to treatment, the degree of sedation and the side-effects were recorded.
Results: The response rate to treatment was highest in the dexmedetomidine group, and it was only significant when compared to tramadol group (p = 0.0012). It was noted that the response rate was higher in the pethidine than in the tramadol group. This difference was not statistically significant (p = 0.082). The sedation score post treatment was similar in all three groups, but more patients in the dexmedetomidine group developed hypotension and bradycardia (p < 0.05).
Conclusion: Dexmedetomidine 0.5 μg/ml was more effective than tramadol 0.5 mg/ml and pethidine 0.5 mg/ml, and both tramadol and pethidine were found to have similar efficacy, in the treatment of post-neuraxial anaesthesia shivering. However, dexmedetomidine caused a higher incidence of hypotension and bradycardia.
Introduction
Shivering may be defined as an involuntary, repetitive activity in the skeletal muscle. Shivering usually occurs as a thermoregulatory response to cold, although non-thermoregulatory shivering may also occur. The core temperature in humans varies with the circadian rhythm (and with the menstrual cycle in females), but is normally maintained within the narrow range of 36.5–37.0 °C.Citation1
Shivering is a relative common problem encountered after neuraxial (spinal and epidural) anaesthesia. An incidence of shivering of up to 55% has been reported.Citation1 Neuraxial anaesthesia produces vasodilatation, which facilitates rapid heat loss and the core to peripheral redistribution of body heat, causing the core temperature to decrease. Therefore, the shivering threshold is reached sooner, and more shivering is required to prevent further hypothermia.Citation2 According to the study conducted by Kurz et al., neuraxial anaesthesia, either spinal or epidural anaesthesia, impaired the centrally mediated thermoregulatory responses. The mechanism remains unknown, but is most likely to result from altered afferent thermal input from the blocked region.Citation3 Although shivering is not a life-threatening process, it can be a source of patient discomfort, and may interfere with the monitoring of the electrocardiogram, blood pressure and pulse oxygen saturation.Citation4 It can also have deleterious metabolic and cardiovascular effects, which include increased expenditure of cardiac and systemic energy, increased oxygen consumption by approximately 100%, increased carbon dioxide production and increased cardiac work.Citation5,6 Therefore, shivering should ideally be prevented or treated by pharmacological or other means.
Tramadol hydrochloride, a centrally acting analgesic drug, is effective in the treatment of post-anaesthetic shivering after general and neuraxial anaesthesia.Citation2 It inhibits the neuronal reuptake of noradrenaline and 5-hydroxytryptamine (5-HT), facilitates 5-HT release and activates the μ-opioid receptors. Each of these actions is likely to influence thermoregulatory control.Citation4 The study by De Witte et al. showed that tramadol reduced the sweating, vasoconstriction and shivering threshold.Citation7 In the study by Chan et al., intravenous tramadol effectively controlled shivering during Caesarean delivery under neuraxial anaesthesia with minimal side-effects.Citation8
Pethidine, an opioid derivative, is frequently recommended for the treatment of post-neuraxial anaesthesia shivering.Citation4 Pethidine is a combined μ- and κ-receptor agonist. Although its mechanism of anti-shivering effect has yet to be fully elucidated, it was indicated in a study in which naloxone was used that pethidine may act via the κ-, rather than μ-opioid, receptors. The anti-shivering action of pethidine was inhibited by high-dose naloxone, which blocked the μ- and κ-receptors, but not by low-dose naloxone which only blocked the μ-receptors.Citation9 Activation of the κ-opioid receptors decreased the shivering threshold twice as much as the vasoconstriction threshold.Citation10 However, pethidine probably acts directly on the thermoregulatory centre, and not only through receptor activation.Citation4
Dexmedetomidine, a potent alpha 2-adrenergic receptor agonist, has been used as a sedative agent and is known to reduce the shivering threshold. It acts by decreasing the vasoconstriction and shivering thresholds. Bicer et al. showed that one dose of prophylactic administration of intraoperative dexmedetomidine (1.0 μg/kg) before the end of the surgery reduced vasoconstriction, as well as shivering threshold.Citation11 A study performed by Easley et al. showed that dexmedetomidine (0.5 μg/kg) was effective in treating post-anaesthetic shivering in children.Citation12
Objective
The purpose of this study was to compare the efficacy of intravenous dexmedetomidine 0.5 μg/kg with that of pethidine 0.5 mg/kg and tramadol 0.5 mg/kg in the treatment of shivering in patients undergoing elective surgery under neuraxial anaesthesia in Universiti Kebangsaan Malaysia Medical Centre (UKMMC), Kuala Lumpur.
Method
This was a prospective, double-blinded and block randomisation study conducted in the UKMMC, Kuala Lumpur, following approval from the Dissertation Committee of the Department of Anaesthesiology and Intensive Care, UKMMC, and the Research and Ethics Committee of the UKMMC (Research No. FF-231-2011).
Following patient informed consent, 102 patients of both genders, aged 18–70 years, with American Society of Anesthesiologists (ASA) physical status I or II undergoing neuraxial anaesthesia (spinal, or combined spinal and epidural anaesthesia) for elective orthopaedic, gynaecology or general surgery were enrolled in this study. The following groups of patients were excluded from the study: patients with a history of convulsions, hypo- or hyperthyroidism, cardiopulmonary disease, psychiatric disorders, neuromuscular pathology, an allergy to the study drugs, those with an initial heart rate < 50 beats/minute, systolic blood pressure < 100 mmHg and body temperature > 38.0 °C or < 36.0 °C prior to anaesthesia. Shivering was graded with a scale similar to that validated by Crossley and MahajanCitation13 (Appendix A). Only patients who developed grade 3 or 4 shivering were included in this study. Body temperature (tympanic membrane temperature) was measured with a Microlife Ear Digital Thermometer IR 1DE1-1® at the start of shivering and 5, 10 and 15 minutes after commencement of treatment of shivering. The ambient temperature of the operating room was set at 20 ± 1 °C, with a relative humidity of 60%. None of the patients were given premedication drugs. Standard monitoring was used throughout the operation.
Subarachnoid anaesthesia was instituted at the lumbar vertebra 3–4 or 4–5 interspaces, with 15 mg hyperbaric bupivacaine. The volume of intravenous fluid and the use of ephedrine for hypotension, atropine for bradycardia and metoclopramide for nausea or vomiting were determined by the attending anaesthesiologist. The administration of pre- or intraoperative opioids was not permitted. Patients were supplemented with oxygen 5.0 l/minute by face mask, and covered with one layer of surgical drape and one layer of cotton blanket (over the non-surgical field area), but not actively warmed during anaesthesia. The preloading fluids were preheated to 37 °C.
Of the 102 patients, the 60 who shivered (grade 3 or 4) during the operation under neuraxial anaesthesia were randomly allocated to receive either dexmedetomidine 0.5 μg/kg (n = 20), pethidine 0.5 mg/kg (n = 20) or tramadol 0.5 mg/kg (n = 20), using computer-generated randomised numbers. The study drugs were diluted to the same volume of 5.0 ml, and given intravenously over 3–5 minutes.
Once the patients began shivering, the anaesthesiologist would administer the treatment drugs and measure the time that elapsed from commencement of the treatment to the cessation of shivering. If the shivering did not cease after 15 minutes, the treatment was regarded as ineffective. Treatment efficacy was evaluated subjectively by the patient as “no improvement”, “partial improvement” or “marked improvement”. Vital signs were measured before, and 5, 10 and 15 minutes after treatment. Side-effects, such as pruritus, hypotension (a fall in systolic blood pressure > 20% from baseline), bradycardia (< 45 beats/minute), and nausea, vomiting and dizziness were recorded. The degree of sedation was recorded according to the Ramsay Sedation ScoreCitation6 (Appendix A).
Statistical analysis
A study population of 20 patients for each group was determined to have statistical power of 80% at an α-value of 0.05 to enable detection of a difference of 47% in the cessation of shivering within 15 minutes post treatment compared with the dexmedetomidine group, in response to pethidine and tramadol groups.
Statistical analysis was performed using Statistical Package for Social Sciences® software. Parametric data were analysed using one-way analysis of variance. Non-parametric data were analysed by using the chi-square test. A p-value of < 0.05 was considered to be statistically significant.
Results
In our study, 60 of the 102 patients (59%) experienced grade 3 or 4 shivering. Those who shivered were randomly allocated to receive exmedetomidine, pethidine or tramadol. Table shows that the three groups were comparable with respect to age, weight, height, gender, race, ASA status and type of surgery.
Table 1: Demographic, anaesthetic and surgical dataTable Footnote*
The responses of post-neuraxial anaesthesia shivering after treatment are shown in Table . The response rate was found to be highest in the dexmedetomidine group, and it was only significant when compared to the tramadol group (p = 0.0012). It was noted that although the response rate was higher in the pethidine group than in the tramadol group, the difference was not statistically significant (p = 0.082). There was no significant difference in terms of the time that elapsed from the start of treatment to the cessation of shivering and patient-assessed treatment efficiency between the three groups.
Table 2: Responses of post-neuraxial anaesthesia shivering after treatmentTable Footnote*
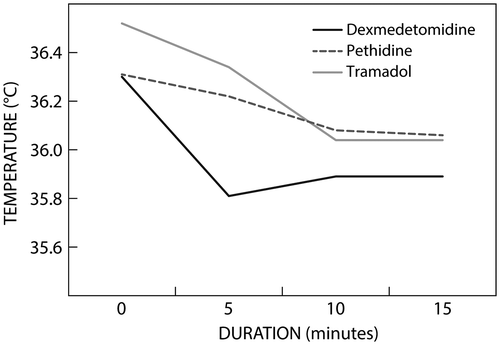
It was noted in all three groups that core body temperature at 15 minutes post treatment was lower than the core body temperature at the baseline, but the difference was not significant. Patients in the dexmedetomidine group had the lowest core body temperature at 15 minutes post treatment, but the difference between the three groups was not significant (Figure ).
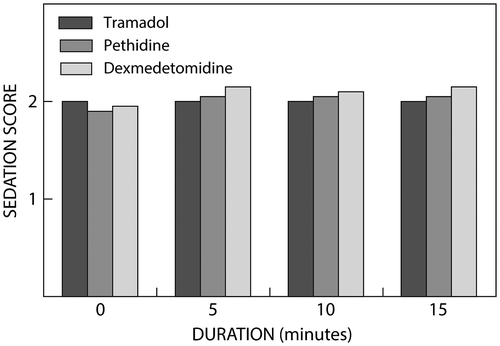
Figure shows the Ramsay Sedation Score for all three groups post treatment. The difference in the sedation score in the three groups was statistically insignificant (p > 0.05).
In this analysis, hypotension and bradycardia were more common in the dexmedetomidine group during the 15-minutes study period, and the difference was significant when compared with the pethidine and tramadol groups. Only one patient in the tramadol group experienced nausea and vomiting (Table ).
Table 3: Observed side-effectTable Footnote*
Discussion
The incidence of shivering during neuraxial anaesthesia was 59% in this study. This is comparable to that in studies conducted by Sagir et al. (55%)Citation14 and Bilotta et al. (57%).Citation15 Larry and Donal mentioned in their article that in a broad sample of 21 studies, the median incidence of shivering in the control group was 55%.Citation1
The efficacy of dexmedetomidine in treating and preventing shivering in various clinical scenarios has been demonstrated in previous studies.Citation12 In our study, all patients treated with dexmedetomidine stopped shivering within 15 minutes post treatment. This result corresponds with that in the study conducted by Easley et al. which showed that 24 children ranging in age from 7–16 years old experienced the cessation of shivering behaviour within five minutes following the completion of dexmedetomidine administration.Citation12 The difference between our study and their study was that ours involved adult patients who experienced shivering post neuraxial anaesthesia, while theirs involved children who shivered post general anaesthesia. The shivering that occurs during neuraxial anaesthesia and general anaesthesia share a common pathogenesis. Thus, agents that have proven successful in the treatment of shivering following neuraxial anaesthesia might also be useful in the management of shivering during general anaesthesia.Citation1
Dexmedetomidine has been shown to reduce the core temperature and our results correlate with these findings.Citation16 Studies in healthy volunteers have demonstrated that dexmedetomidine controls shivering by reducing the shivering threshold.Citation17−19 Doufas et al. carried out a study in which shivering was induced by infusing cold Ringer’s lactate solution (≈ 4 °C) into healthy adult volunteers. The results showed that the shivering threshold was 35.5–36.5 °C in the group treated with dexmedetomidine.Citation17 However, in our study, patients did not shiver, even though their temperature was in the range of 35.8–35.9 °C. This might be owing to a difference in the mechanism of shivering under neuraxial anaesthesia compared to that induced by cold Ringer’s lactate solution.
Horn et al. showed that 0.5 mg/kg of pethidine was an effective dose for the treatment of shivering, which is why we administered pethidine 0.5 mg/kg to our patients with grade 3 shivering or higher.Citation20 In 1999, Wang et al. showed that the response rate with pethidine (0.4 mg/kg) was 83% at five minutes and 93% at 30 minutes.Citation21 On the other hand, in the study by Bhatnagar et al., the response rate to pethidine (0.5 mg/kg) was only 80%.Citation22 In our study, the response rate with pethidine was 85%, which is comparable with these two studies, although they were performed in post-general anaesthesia patients and not in post-neuraxial anaesthesia patients. The disadvantages of pethidine treatment are the side-effects of nausea, vomiting, sedation and respiratory depression, but these did not occur in our patients with the dosage we used.
In the study by Chan et al., intravenous tramadol 0.25 mg/kg effectively controlled shivering (92%) during Caesarean delivery under neuraxial anaesthesia with minimal side-effects.Citation8 Tsai and Chu conducted a study on parturients who shivered post epidural anaesthesia, and found that 87% who received tramadol (0.5 mg/kg) stopped shivered within 15 minutes of treatment.Citation4 In our study, 55% patients stopped shivering within 15 minutes of receiving tramadol (0.5 mg/kg). The better response of patients to tramadol in the Tsai and Chu study may relate to the fact that their patients were pregnant women. The mechanism of shivering during epidural anaesthesia in parturients may be different from that in non-parturients. The disadvantages of tramadol are the side-effects of nausea, vomiting and sedation. In our study, one patient developed nausea and vomiting, two developed hypotension and none were sedated post treatment with tramadol.
We have demonstrated that dexmedetomidine, pethidine and tramadol effectively treat post-neuraxial anaesthesia shivering. Dexmedetomidine appears to be more effective than pethidine and tramadol (100% vs. 85% vs. 55%, respectively). However, the only significant difference statistically was demonstrated between dexmedetomidine and tramadol (p = 0.0012), not between dexmedetomidine and pethidine, or pethidine and tramadol, in reducing post-neuraxial anaesthesia shivering. Tsai and Chu showed that both tramadol (0.5 mg/kg) and pethidine (0.5 mg/kg) effectively treated post-epidural anaesthesia shivering.Citation4 Seifi et al. noted that both tramadol (1.0 mg/kg) and pethidine (0.5 mg/kg) effectively reduced postoperative shivering, but there was no significant difference between them.Citation23
Hypotension and bradycardia are known haemodynamic effects of dexmedetomidine.Citation24−26 In our study, five patients developed hypotension and four developed bradycardia after receiving this drug. Nausea was also one of the adverse effects experienced, as shown in previous studies.Citation26,27 But, in our study, none of the patients developed nausea or vomiting. All of the patients who received dexmedetomidine were cooperative, orientated, tranquil and could respond to commands, and these findings were similar to the results obtained in the study conducted by Elvan et al.Citation6
Conclusion
In conclusion, dexmedetomidine 0.5 μg/kg was more effective than tramadol 0.5 mg/kg and pethidine 0.5 mg/kg, and both tramadol and pethidine were found to have similar efficacy, in the treatment of post-neuraxial anaesthesia shivering. However, dexmedetomidine caused a higher incidence of hypotension and bradycardia.
References
- Larry JC, Donal JB. Shivering and neuraxial anaesthesia. Reg Anesth Pain Med. 2008;33:241–52.
- Federico B, Paolo P, Raffaele S, et al. Nefopam and tramadol for the prevention of shivering during neuraxial anaesthesia. Reg Anesth Pain Med. 2002;27:380–4.
- Kurz A, Sessler DI, Schroeder M, et al. Thermoregulatory response thresholds during spinal anaesthesia. Anesth Analg. 1993;1993(77):721–6.
- Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–92.10.1097/00000539-200111000-00052
- Imrie MM, Hall GM. Body temperature and anaesthesia. Br J Anaesth. 1990;64(3):346–54.10.1093/bja/64.3.346
- Elvan EG, Öç B, Uzun Ş, et al. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesth. 2008;25:357–64.10.1017/S0265021507003110
- De Witte JL, Kim JS, Sessler DI, et al. Tramadol reduces the sweating, vasoconstriction and shivering thresholds. Anesth Analg. 1998;87:173–9.
- Chan AM, Ng KF, Nung Tong EW, et al. Control of shivering under regional anesthesia in obstetric patients with tramadol. Can J Anaesth. 1999;46:253–8.10.1007/BF03012605
- Kurz M, Belani KG, Sessler DI, et al. Naloxone, meperidine, and shivering. Anesthesiology. 1993;79:1193–201.10.1097/00000542-199312000-00009
- Kurz A, Ikeda T, Sessler DI, et al. Meperidine decreases the shivering threshold twice as much as the vasoconstriction threshold. Anesthesiology. 1997;86:1046–54.10.1097/00000542-199705000-00007
- Bicer C, Esmaoglu A, Akin A, et al. Dexmedetomidine and meperidine prevent postanaesthetic shivering. Eur J Anaesth. 2006;23:149–53.10.1017/S0265021505002061
- Blaine Easley R, Brady KM, Tobias JD. Dexmedetomidine for the treatment of postanesthesia shivering in children. Pediatric Anaesth. 2007;17(4):341–6.10.1111/pan.2007.17.issue-4
- Crossley AWA, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994;49:205–7.
- Sagir O, Gulhas N, Toprak H, et al. Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron. Acta Anaesthesiol Scan. 2007;51:44–9.10.1111/aas.2007.51.issue-1
- Bilotta F, Pietropaoli P, Sanita R, et al. Nefopam and tramadol for the prevention of shivering during neuraxial anesthesia. Reg Anesth Pain Med. 2002;27:380–4.
- El-Tahir K. Dexmedetomidine a sedative-analgesic drug for the 21st century. M.E.J. Anesth. 2002;16(6):577–85.
- Doufas AG, Lin CM, Suleman MI, et al. Dexmedetomidine and meperidine additively reduce the shivering threshold in humans. Stroke. 2003;34:1218–23.10.1161/01.STR.0000068787.76670.A4
- Talke P, Tayefeh F, Sessler DI, et al. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–41.10.1097/00000542-199710000-00017
- Talke P, Richardson CA, Scheinin M, et al. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42.
- Horn EP, Standl T, Sessler DI, et al. Physostigmine prevents postanesthetic shivering as does meperidine or clonidine. Anesthesiology. 1998;88:108–13.10.1097/00000542-199801000-00018
- Wang JJ, Ho ST, Lee SC, et al. A comparison among naluphine, meperidine and placebo for treating post anaesthetic shivering. Anesth Analg. 1999;88:686–9.
- Bhatnagar S, Saxena A, Kannan TR, et al. Tramadol for post-operative shivering. A double blind comparison with pethidine. Anaesth Intens Care. 2001;29:149–54.
- Seifi A, Avestimehr S, Mowla A, et al. Comparative study of the effect of tramadol and pethidine on postoperative shivering. J Anesth. [ Internet]. 2008 [ cited 2012 Apr 25];16(2). Available from: http://www.ispub.com/journal/the-internet-journal-of-anesthesiology/volume-16-number-2/a-comparative-study-of-the-effect-of-tramadol-and-pethidine-on-postoperative-shivering.html
- Jaionen J, Hynynen M, Kuitunen A, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997;86(2):331–45.10.1097/00000542-199702000-00009
- Ebert TJ, Hall JE, Barney JA. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94.10.1097/00000542-200008000-00016
- Burhanettin U, Muhammet G, Adnan Y. Dexmedetomidine for the prevention of shivering during spinal anaesthesia. Clinics (Sao Paulo). 2011;66(7):1187–91.
- Bhana N, Goa KL. Mc Clellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8.
Appendix A
Shivering score
0 – No shivering
1 – Piloerection or peripheral vasoconstriction, but no visible shivering
2 – Muscular activity in only one muscle group
3 – Muscular activity in more than one muscle group, but not generalised
4 – Shivering involving the whole body.
Ramsay Sedation Score
1 – The patient is anxious and agitated, or restless, or both
2 – The patient is cooperative, oriented and tranquil
3 – The patient responds to commands only
4 – The patient exhibits a brisk response to a light glabellar tap or to a loud auditory stimulus
5 – The patient exhibits a sluggish response to a light glabellar tap or a loud auditory stimulus
6 – The patient exhibits no response.