Abstract
Inflammatory mediators released from activated mast cells and basophils during hypersensitivity reactions have direct pathological effects on the myocardium and coronary vasculature. It was traditionally thought that cardiovascular signs and symptoms in anaphylaxis are largely due to peripheral vasodilation and increased vascular permeability. However, there is extensive evidence of primary cardiac involvement during hypersensitivity reactions, most notably coronary vasoconstriction as well as atherosclerotic plaque erosion and rupture, leading to angina pectoris and acute coronary syndromes. Furthermore, mast cells are well established as effector cells in atherosclerosis, through their effects on atherosclerotic plaque progression and destabilisation. It was noted over 30 years ago that cardiac patients have a markedly higher concentration of biologic amines (especially histamine) in their coronary vasculature, and, additionally, are hyper-reactive to the effects thereof. This is borne out by the disproportionate mortality rate of those with cardiac disease that suffer a hypersensitivity reaction. Kounis syndrome refers to angina pectoris or an acute coronary syndrome secondary to a hypersensitivity reaction, with the subtypes dependent on the underlying state of the coronaries and presence of a drug-eluting stent or not. This review will focus mainly on the aetiology, pathophysiology, diagnoses and treatment of this important syndrome.
Introduction
There is extensive evidence of cardiac involvement in animal (in vivo and in vitro) anaphylaxisCitation1–3 as well as human anaphylaxis.Citation4–7 During anaphylaxis, the heart is both a source and a target of released chemical mediators, which have direct effects on the coronary vasculature and myocardium.
Figure 1: Timeline of Kounis syndrome.Citation21
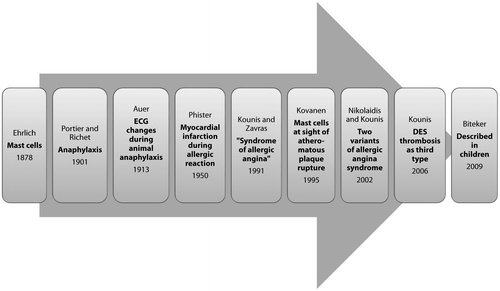
Kounis syndrome is the concurrent occurrence of acute coronary syndromes with hypersensitivity reactions (including anaphylaxis).Citation8 Two principal variants of the syndrome are described: type 1 is allergy-related angina due to coronary spasm with type II being allergy-related myocardial infarction due to plaque rupture and thrombus formation. Drug-eluting stent thrombosis (late stent thrombosis) secondary to hypersensitivity reactions has recently been proposed as a third type.Citation9
Historical background (see Figure )
Paul Ehrlich published his seminal description of mast cells in 1878.Citation10 Anaphylaxis was characterised by Richet and Portier in 1901, which won Richet the Nobel Prize in 1913.Citation11 In 1913 Auer reported ECG changes during animal anaphylaxis.Citation12
The first case of myocardial infarction during an allergic reaction in humans was reported in 1950.Citation13 In 1991, Kounis and Zavras described a ‘syndrome of allergic angina’ as the simultaneous occurrence of chest pain and allergic reactions, accompanied by clinical and laboratory findings of angina pectoris, caused by inflammatory mediators released during the allergic process.Citation8 Kovanen in 1995 found much greater coronary mast cell degranulation at the sites of erosion or plaque rupture than in adjacent areasCitation;14 later Braunwald noted that mediators such as histamine or leukotrienes released during allergic reactions could induce vasospastic angina by acting on coronary smooth muscle.Citation15
In 2002, two variants of the original ‘allergic angina syndrome’ were described,Citation16 with drug-eluting stent (DES) thrombosis later proposed as a third type.Citation9,17 It was first reported in children in 2009, including in a two-year-old.Citation18–20
Epidemiology
The lifetime prevalence of anaphylaxis is at least 1.6%, probably higher.Citation22 It is increasing in developing countries,Citation23–25 and leading to an increased number of UK critical care admissions.Citation26
Perioperative anaphylaxis occurs in 1:3 500–1:20 000 cases, with a mortality of 9%.Citation27 It accounts for 9–19% of anaesthetic-associated complications and 5–7% of deaths during anaesthesia.Citation27,28 The most common agents implicated in perioperative anaphylaxis are neuromuscular blocking agents (58%), latex (19.6%) and antibiotics (12.8%).Citation29
It is difficult to estimate the incidence of Kounis syndrome. It is probably under-reported, with anaesthesiologists and intensivists generally unaware of the condition. It is not unlikely to occur though. Two out of 21 healthy adults developed symptoms and ECG changes suggestive of myocardial ischaemia during a diagnostic sting challenge.Citation30 In a French survey of anaphylaxis under anaesthesia, 73.6% of cases had cardiovascular involvement.Citation31 A Swiss study showed an incidence of severe life-threatening anaphylaxis with circulatory signs of 7.9–9.6 per 100 000 people per year.Citation6 Many cases go unreported, although awareness and reporting is increasing.
Aetiology
Sixty years ago, Pfister reported a case of antero-septal myocardial infarction and urticaria four days after treatment with penicillin.Citation13 Hundreds of cases of Kounis syndrome have been described since, including several case series.Citation32–35
Kounis syndrome has been described secondary to a large number of drugs, of various classes (e.g. antibiotics, muscle relaxants, anti-neoplastics, contrast media, NSAIDS, thrombolytics etc.).Citation21,36,37
Several environmental exposures are linked to the syndrome (with Hymenoptera stings accounting for the majority of serious reactions); in addition, there are a number of diseases and conditions related to Kounis syndrome (e.g. mastocytosis, bronchial asthma etc.).Citation36
One can argue that any drug or condition able to provoke an allergic reaction can lead to Kounis syndrome. It is well known that atopic individuals are at higher risk of acute coronary syndromes.Citation38–40
Pathophysiology
Anaphylaxis is a serious, potentially fatal, multi-organ syndrome caused by a triggered release of mast cell derived mediators into the systemic circulation.Citation41,42 The trigger is either (1) immunologic (IgE or IgG dependent) or (2) non-immunologic (direct stimulation of mast cells by certain drugs, cold air, exercise, etc.).Citation43–46
The immunologic pathway involves an allergen-induced crosslinking of IgE antibodies (formed during a clinically silent initial exposure) coupled to mast cells and basophils, which induces a transduction cascade activating mast cells. Note that a very small amount of allergen is needed to trigger the cascade.Citation47
Activated mast cells degranulate and release an array of vasoactive and pro-inflammatory mediators, namely (1) preformed granule associated mediators, (2) newly generated lipid-derived mediators plus (3) cytokines and chemokines (Table ).Citation48–51
Table 1: Mediators released by activated human mast cells (clinically important ones italicised)
Mast cells are widely distributed in tissues, particularly those with external environment contact (i.e. skin, gastrointestinal system, lung) but also in other organs such as the heart.Citation49 In the heart they are located mainly between myocardial fibres (in close proximity to myocytes), around both large coronary vessels and small intramural coronary arteries, and in the arterial intima and adventitia.Citation49,51,52
Uniquely, heart mast cells can be directly activated by non-allergenic stimuli (e.g. anaphylatoxins C3a and C5a and substance P),Citation49,53 as well as by drugs such as muscle relaxants, protamine and radio-contrast media.Citation54 Cardiac mast cells are some of the most important effector cells of anaphylaxis.Citation50,55
Effects of mast cell mediators on coronary circulation:
Histamine has important context-specific effects on cardiac tissues. It dilates coronary arteries (via H1-receptors on vascular smooth muscle cells) and causes arrhythmias and atrio-ventricular conduction blocks in healthy volunteers.Citation56 It further leads to a baroreceptor-mediated tachycardia by decreasing mean aortic pressure.Citation57
However, in patients with coronary artery disease and vasospastic angina, intravenous histamine causes a decrease in coronary blood flow, and in some cases severe coronary spasm.Citation56,58
Histamine also induces tissue factor expression in endothelial cells and vascular smooth muscle cells, thus mediating thrombus formation in acute coronary syndromes.Citation59
Heart mast cells release large quantities of chymase (compared with lung and skin), as well as significant quantities of renin,Citation2,60 activating the cardiac renin-angiotensin-aldosterone system. Chymase has potent angiotensin-converting enzyme (ACE) activity, even in the presence of ACE inhibitors.Citation60 Angiotensin is a coronary vasoconstrictor, causes arrhythmias and leads to fibrosis and apoptosis.
Platelet activating factor (PAF) causes a significant decrease in coronary blood flow and marked negative inotropy; it also has a direct arrhythmogenic effect.Citation61 It may also contribute to atherosclerotic plaque instability and rupture by inducing local platelet aggregation and the release of lytic enzymes by macrophages.Citation14 Systemic PAF causes peripheral vasodilatation.Citation62 One of the major causes of disseminated intravascular coagulation in fatal anaphylaxis is PAF-induced platelet activation.Citation63 Of note, PAF has shown significant correlation with the severity of anaphylaxis, better than either histamine or tryptase.Citation64,65
Intravenous or intra-coronary infusion of cysteinyl leukotrienes, such as leukotriene D4, leads to a rapid and sustained rise in coronary vascular resistance and subsequent decrease in coronary blood flow.Citation66,67
Thromboxane causes vasoconstriction and is an important mediator of platelet aggregation.Citation68 In experimental animal studies, prostaglandin D2 causes coronary vasoconstriction and arrhythmias.Citation69
Mast cells in cardiovascular disease
There is increasing evidence of the central role of mast cells and their mediators in cardiovascular disease, through their effects on atherosclerotic plaque progression and destabilisation.Citation52,70,71
Histamine and tryptase may contribute to fatty streak formation and the generation of unstable plaques susceptible to rupture.Citation52 Activation of matrix metalloproteinases by mast cell derived proteases is an important mechanism in atherosclerotic plaque destabilisation.Citation72
Higher levels of histamine are found in coronary arteries of patients who died from ischaemic heart disease.Citation73 They also have more degranulated mast cells at the site of atheromatous rupture, compared with adjacent normal intima.Citation14,52 Patients with peripheral vascular disease were found to have higher plasma levels of histamine compared with controls.Citation74 Mast cell derived mediators like histamine, serotonin, leukotrienes and prostaglandin D2 contribute to vascular responsiveness; increased numbers of mast cells are associated with coronary vasospasm.Citation75
Some authors have suggested using tryptase as a biomarker for atherosclerosisCitation76 and acute coronary syndromes.Citation77–79 Pre-existing cardiovascular disease, mastocytosis and elevated baseline serum tryptase are all risk factors for fatal anaphylactic reactions.Citation80,81
Hypersensitivity-induced coronary stent thrombosis (Kounis type III)
All the components of drug-eluting stents, namely (1) the metal strut (composed of nickel, molybdenum and chromium),Citation82 (2) the polymerCitation83 and (3) the impregnated paclitaxel/sirolimus,Citation84 can elicit hypersensitivity reactions with a release of pro-thrombotic mediators like PAF, cytokines and chemokines. Several hundred cases of hypersensitivity reactions related to drug-eluting stents (DES) have been reported, some of which were fatal.Citation85
Based on human autopsy series, DES late/very late stent thrombosis is caused by impaired arterial healing (with characteristic incomplete re-endothelialisation, persistent fibrin deposition and macrophage infiltration) when compared with bare metal stents.Citation86 Autopsies confirmed inflammatory cell infiltrates (plasma cells, macrophages, eosinophils and lymphocytes) that permeate all three vascular wall layers, a histopathological picture very similar to Kounis type I and type II.Citation86
Clinical variants
Systemic allergic signs and/or symptoms accompanied by clinical, electrocardiographic or laboratory findings of myocardial ischaemia constitutes Kounis syndrome.Citation8 Three variants have been described (Table ).Citation9,16,85
Table 2: Clinical variants of Kounis syndrome
Diagnosis
There are two aspects to the diagnosis: the hypersensitivity reaction plus the myocardial ischaemia. Dewachter suggested a triad of evidence to diagnose perioperative anaphylaxis (clinical, biologic and allergologic evidence).Citation47 It is important to actively look for and anticipate cardiac involvement if anaphylaxis is suspected or diagnosed.
Clinical/ECG features
The modified Ring and Messmer clinical severity scale should be used to classify signs and symptoms of immediate hypersensitivity reactions (Table ).Citation11,47,87
Table 3: Clinical severity scale of immediate hypersensitivity reactionsCitation47
The diagnosis of anaphylaxis during anaesthesia can be challenging, because important features like hypotension or bronchospasm have many other, more common causes.Citation88 Note that cutaneous signs can be absent in rapidly progressing anaphylaxis, further clouding the diagnosis.
ECG changes are integral to the diagnosis of Kounis syndrome, in combination with the clinical picture and biochemical markers. The syndrome usually presents with changes in the anterior or inferior leads, the right coronary artery being commonly involved.Citation21
It may also show non-specific ST-segment/T-wave abnormalities, atrial fibrillation, nodal rhythms, ventricular ectopics, bigeminy, QRS-complex broadening and QT-segment prolongation; rarely do patients have a normal ECG.
Special investigations
Cardiac: Cardiac enzymes (troponins) should be sent as soon as cardiac involvement is suspected. Other blood tests such as d-dimer, full blood count, brain natriuretic peptide and serum cholesterol levels can be sent after the acute event.
Transthoracic or trans-oesophageal echocardiography can reveal regional wall motion abnormalities suggestive of acute coronary syndromes, and can help distinguish between other causes of chest pain and/or hypotension (such as aortic dissection, pericarditis, pericardial effusion and pulmonary embolism).
Coronary angiography will distinguish between types I and II Kounis syndrome, as well as being useful to guide therapy (e.g. intracoronary calcium channel blockers for vasospasm).
Hypersensitivity reaction: Tryptase, histamine levels, specific IgE antibody and eosinophil levels will help to identify an allergic reaction.
Mast cell tryptase is elevated in most patients with systemic anaphylaxis (though not all). Tryptase peaks at 1–1.5 hours after anaphylaxis onset and remains elevated for over 5 hours. The best time to measure is from 1–2 hours, but no longer than 6 hours after onset. Histamine has a half-life of less than 30 minutesCitation89 and is thus not commonly measured.
Although PAF correlates better with the severity of anaphylaxis than histamine or tryptase,Citation64,65 the extremely short half-life precludes its clinical use.Citation48
Anaphylatoxins like C5a can cause mast cell degranulation,Citation90 thus complement levels may be useful.
Allergologic testing
Allergy testing, such as patch testing, skin prick, intradermal testing and serum-specific IgE testing for IgE antibodies should be done 4–6 weeks after the reaction (to avoid false negative test results because of mast cell depletion). However, mast cells can degranulate from direct stimulation (e.g. morphine, codeine), leading to the absence of IgE antibodies.Citation91
It should be performed based on clinical history, with the aim to prove the pathophysiological mechanism and identify a culprit to avoid next time.
Treatment
At present there are no clear treatment guidelines for Kounis syndrome; most of the data are derived from case reports. Two aspects need treatment, (1) the allergic reaction and (2) the acute coronary syndrome (ACS). Treating the hypersensitivity reaction may abolish the type I variant, but the type II variant often needs ACS treatment in addition.
Allergic reaction/anaphylaxis
The World Allergy Organization recently published a consensus document summarising the evidence-based anaphylaxis guidelines developed and published independently from 2010 to 2014 by four allergy/immunology organisations.Citation92 More specifically, there are numerous protocols for perioperative anaphylaxis.Citation93,94 It is recommended to follow one of these.
The first-line pharmacologic treatment is prompt injection of adrenaline,Citation92,95 although this is not supported by any randomised controlled trial.Citation95,96 In addition to the cardiovascular and respiratory beneficial effects, it suppresses further mediator release from mast cells and basophils during anaphylaxis (via β2-adrenergic receptors).Citation97 The therapeutic plasma concentration of adrenaline for successful treatment of anaphylaxis is unknown. However, one should titrate small intravenous boluses to clinical effect, the dose depending on clinical presentation (using the Ring and Messmer grading scale).Citation88,98,99 Some authors suggest never using adrenaline in grade I reactions.Citation47
IV fluids (crystalloids) should be given to compensate for the intravascular loss, as up to 35% of the intravascular volume can leak into the extravascular space within 10 minutes.Citation100
Although most guidelines advocate the use of anti-histamines and glucocorticoids, no robust studies confirming the effectiveness of these exist.Citation95,101 However, in those with type I variant and milder grades of anaphylaxis, hydrocortisone (1–2 mg/kg/day) and H1 plus H2-antihistamines (diphenhydramine 1–2 mg/kg and ranitidine 1 mg/kg) may be adequate treatment.Citation102
Refractory anaphylaxis should be managed with a continuous infusion of vasopressors rather than IV boluses, but only by those experienced with their use.Citation103 In addition, vasopressin,Citation104–106 glucagon,Citation107,108 anti-cholinergic drugs (atropine), methylene blue,Citation109 and α1-agonistsCitation110–112 are all proposed treatments for refractory cases.
Acute coronary syndromes
The treatment of acute coronary syndromes is based on the latest ACC/AHA guidelines, and will depend on the presentation.Citation113,114 In those with an ST-elevation myocardial infarction (STEMI) the focus is on obtaining an urgent angiogram, with subsequent management dependent on findings (medical therapy, percutaneous coronary intervention or coronary artery bypass grafting). Note that fibrinolytics like streptokinase carry a high risk of anaphylaxis.Citation115
Anaphylaxis guidelines suggest using 100% O2, but routine oxygen administration in myocardial infarction may be associated with increased mortality.Citation116 Oxygen should thus be titrated to normoxia.
Though nitroglycerin may be used for ischaemic pain, it can worsen the hypotension of anaphylaxis and should be used cautiously.
Opioids such as morphine, codeine and pethidine can induce mast cell degranulation and aggravate allergic reactions. Fentanyl and its derivatives (with minimal mast cell activation) may be better-suited narcotic analgesics.
β blockers offset the beneficial effects of adrenaline in anaphylaxis and should be used with extreme caution, if at all. Glucagon can be used for patients with anaphylaxis and hypotension who are on β blockers.Citation107
Long-acting calcium channel blockers (CCB) and nitrates are generally recommended for patients with hypersensitivity-induced coronary vasospasm.Citation102 Based on the pathophysiology of Kounis syndrome, CCB may be considered as the initial anti-ischaemic drugs of choice.
Uncoated aspirin should be given to all patients promptly after presentation. However, aspirin and NSAIDS are two of the most common causes of drug-induced anaphylaxis. Aspirin influences the pathogenesis of anaphylaxis through cyclooxygenase inhibition, by shunting arachidonic acid into the leukotriene pathway, producing more anaphylaxis mediators.Citation117
In the type III variant, in addition to ACS treatment, aspiration of the intra-stent thrombus should be considered, followed by histological examination of the clot (staining for mast cells and eosinophils).Citation102
Based on pathophysiological findings, mast cell blockers have been proposed as potential therapeutic agents.Citation70,71 Disodium cromoglycate seems ineffective; however, the flavonoid quercetin showed some promise.Citation118 Mast cell stabilisation may be an important future therapeutic option for Kounis syndrome.
Controversies regarding adrenaline
The use of adrenaline in anaphylaxis is based on a century of clinical experience, epidemiological studies,Citation119 fatality studiesCitation81,120,121 and prospective studies,Citation4,30,122 as well as in-vitroCitation123 and animal studies.Citation103,104,124
Delayed administration of adrenaline in anaphylaxis is associated with increased mortality.Citation81,120,121,124 Furthermore, animal models suggest that giving adrenaline in established anaphylactic shock does not hasten recovery, and may cause LV impairment.Citation124,125 In practice it is a difficult balance between timeous administration to those needing it and avoiding it for low-grade anaphylactic reactions.
Apical ballooning syndrome (ABS), or Tako-Tsubo cardiomyopathy (a reversible left ventricular dysfunction) is thought to be a result of excess catecholamine stimulation of the heart, with several pathophysiological postulates (epicardial coronary artery spasm, microvascular spasm, or direct myocyte injury).Citation126 There are several reports in the literature of ABS following adrenaline therapy during anaphylaxis.Citation127–134
While it is the first-line therapy in anaphylaxis, its use in acute coronary syndromes is a slightly more challenging decision. It can cause serious side effects like arrhythmias (e.g. tachycardia leading to aggravated myocardial ischaemia), hypertension (causing intra-cerebral bleeds, pulmonary oedema etc.) and coronary vasospasm. Elderly patients and those with a history of hypertension, peripheral vascular disease, ischaemic heart disease and untreated hyperthyroidism are especially prone to these side effects.Citation135 A study looking at fatal anaphylaxis attributed 3 out of 20 (15%) deaths directly to adrenaline overdose.Citation120
Adrenaline is not an innocuous drug in anaphylaxis. It has a narrow therapeutic indexCitation97, and should be carefully titrated to effect.
Prognosis
This will depend on the magnitude of the initial reaction, which in turn depends on the patient’s sensitivity (i.e. previous exposure), other comorbidities (e.g. pre-existing coronary atherosclerosisCitation80,81,136 and mastocytosisCitation39,137,138), the site of antigen–antibody reaction, the allergen concentration and the route of allergen entrance (e.g. intravenous versus topical).
The prognosis is generally good beyond the acute phase, with left ventricular function recovering over three days to several weeks. In the acute phase patients may develop life-threatening arrhythmias, pulmonary oedema and plaque rupture leading to a coronary thrombus, but fortunately death is rare.Citation139
There are some reports that the type I variant of Kounis has a better prognosis than type II.Citation33
Conclusions
Mediators released from cardiac mast cells during a hypersensitivity reaction are key to the subsequent adverse CVS effects. It would be prudent to actively look for cardiac involvement whenever hypersensitivity is suspected, keeping in mind that patients with cardiovascular disease have a significantly higher risk of developing severe or fatal anaphylaxis.Citation80,81 Additionally, commonly used CVD drugs such as β blockers and ACE inhibitors could exacerbate anaphylaxis and make it resistant to treatment.Citation140
Adrenaline remains the first-line treatment for higher-grade anaphylaxis. However, in those with CVD (and specifically ACS) it needs to be carefully considered, weighing the side effects of adrenaline against the risks of untreated anaphylaxis. There is scope to develop multispecialty guidelines to manage this complex condition.
Acknowledgement
The author would like to thank Andrew Levin for proofreading an early draft of the manuscript.
References
- Capurro N, Levi R. The heart as a target organ in systemic allergic reactions: comparison of cardiac analphylaxis in vivo and in vitro. Circ Res. 1975;36(4):520–528.10.1161/01.RES.36.4.520
- Mackins CJ, Kano S, Seyedi N, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116(4):1063–1070.10.1172/JCI25713
- del Balzo UH, Levi R, Polley MJ. Cardiac dysfunction caused by purified human C3a anaphylatoxin. Proc Natl Acad Sci USA. 1985;82(3):886–890.10.1073/pnas.82.3.886
- Smith PL, Kagey-Sobotka A, Bleecker ER, et al. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66(5):1072–1080.10.1172/JCI109936
- Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327(6):380–384.10.1056/NEJM199208063270603
- Helbling A, Hurni T, Mueller UR, et al. Incidence of anaphylaxis with circulatory symptoms: a study over a 3-year period comprising 940,000 inhabitants of the Swiss Canton Bern. Clin Exp Allergy. 2004;34(2):285–290.10.1111/cea.2004.34.issue-2
- Brown SGA. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol. 2005;5:359–364.10.1097/01.all.0000174158.78626.35
- Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45(2):121–128.
- Kounis NG, Kounis GN, Kouni SN, et al. Allergic reactions following implantation of drug-eluting stents: a manifestation of Kounis syndrome? J Am Coll Cardiol. 2006;48(3):592–593; author reply 3–4.10.1016/j.jacc.2006.05.007
- Crivellato E, Beltrami C, Mallardi F, et al. Paul Ehrlich’s doctoral thesis: a milestone in the study of mast cells. Br J Haematol. 2003;123(1):19–21.
- Ring J, Behrendt H, de Weck A. History and classification of anaphylaxis. Chem Immunol Allergy. 2010;95:1–11.10.1159/000315934
- Auer J, Robinson GC. An electrocardiographic study of the anaphylactic rabbit. J Exp Med. 1913;18:450–460.10.1084/jem.18.4.450
- Pfister CW, Plice SG. Acute myocardial infarction during a prolonged allergic reaction to penicillin. Am Heart J. 1950;40(6):945–947.10.1016/0002-8703(50)90191-8
- Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92(5):1084–1088.10.1161/01.CIR.92.5.1084
- Braunwald E. Unstable angina: an etiologic approach to management. Circulation. 1998;98(21):2219–2222.10.1161/01.CIR.98.21.2219
- Nikolaidis LA, Kounis NG, Gradman AH. Allergic angina and allergic myocardial infarction: a new twist on an old syndrome. Can J Cardiol. 2002;18(5):508–511.
- Chen JP, Hou D, Pendyala L, et al. Drug-eluting stent thrombosis. JACC Cardiovasc Interv. 2009;2(7):583–593.10.1016/j.jcin.2009.04.017
- Biteker M, Duran NE, Biteker FS, et al. Kounis syndrome secondary to amoxicillin/clavulanic acid use in a child. Int J Cardiol. 2009;136(1):e3–e5.10.1016/j.ijcard.2008.04.064
- Biteker M, Ekşi Duran NE, Sungur Biteker FS, et al. Allergic myocardial infarction in childhood: Kounis syndrome. Eur J Pediatr. 2010;169(1):27–29.10.1007/s00431-009-0965-5
- Parent B, Wearden P, Kounis NG, et al. Kounis syndrome or allergic coronary vasospasm in a two-year-old. Congenit Heart Dis. 2011;6(5):499–503.10.1111/chd.2011.6.issue-5
- Biteker M. Current understanding of Kounis syndrome. Expert Rev Clin Immunol. 2010;6(5):777–788.10.1586/eci.10.47
- Wood RA, Camargo CA Jr, Lieberman P, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133(2):461–467.10.1016/j.jaci.2013.08.016
- Lawson JA, Senthilselvan A. Asthma epidemiology: has the crisis passed? Curr Opin Pulm Med. 2005;11(1):79–84.10.1097/01.mcp.0000147861.60768.24
- Patel DA, Holdford DA, Edwards E, et al. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128(1):110–115.e5.10.1016/j.jaci.2011.03.013
- Gupta R, Sheikh A, Strachan DP, et al. Time trends in allergic disorders in the UK. Thorax. 2007;62(1):91–96.10.1136/thx.2004.038844
- Gibbison B, Sheikh A, McShane P, et al. Anaphylaxis admissions to UK critical care units between 2005 and 2009. Anaesthesia. 2012;67(8):833–839.10.1111/anae.2012.67.issue-8
- Galvão VR, Giavina-Bianchi P, Castells M. Perioperative anaphylaxis. Curr Allergy Asthma Rep. 2014;14(8):452.10.1007/s11882-014-0452-6
- Mertes PM, Laxenaire MC, Lienhart A, et al. Reducing the risk of anaphylaxis during anaesthesia: guidelines for clinical practice. J Investig Allergol Clin Immunol. 2005;15(2):91–101.
- Mertes PM, Alla F, Tréchot P, et al. Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128(2):366–373.10.1016/j.jaci.2011.03.003
- Brown SG, Blackman KE, Stenlake V, et al. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004;21(2):149–154.10.1136/emj.2003.009449
- Laxenaire MC, Mertes PM. Anaphylaxis during anaesthesia. Results of a two-year survey in France. Br J Anaesth. 2001;87(4):549–558.10.1093/bja/87.4.549
- Ridella M, Bagdure S, Nugent K, et al. Kounis syndrome following beta-lactam antbiotic use: review of literature. Inflamm Allergy Drug Targets. 2009;8(1):11–16.
- Mori E, Ikeda H, Ueno T, et al. Vasospastic angina induced by nonsteroidal anti-inflammatory drugs. Clin Cardiol. 1997;20(7):656–658.10.1002/clc.v20:7
- Gázquez V, Dalmau G, Gaig P, et al. Kounis syndrome: report of 5 cases. J Investig Allergol Clin Immunol. 2010;20(2):162–165.
- Almpanis G, Siahos S, Karogiannis NC, et al. Kounis syndrome: two extraordinary cases. Int J Cardiol. 2011;147(2):e35–e38.10.1016/j.ijcard.2009.01.031
- Kounis NG, Hahalis G, Manola A, et al. Kounis syndrome (allergic angina and allergic myocardial infarction). In: Gallo AP, Jones ML, editors. Angina pectoris: etiology, pathogenesis and treatment. Nova Science Publishers; New York; 2008. p. 77–150.
- Mazarakis A, Koutsojannis CM, Kounis NG, et al. Cefuroxime-induced coronary artery spasm manifesting as Kounis syndrome. Acta Cardiol. 2005;60(3):341–345.10.2143/AC.60.3.2005015
- Criqui MH, Lee ER, Hamburger RN, et al. Ige and cardiovascular disease. Am J Med. 1987;82(5):964–968.10.1016/0002-9343(87)90159-8
- González-de-Olano D, Matito A, Sánchez-López P, et al. Mast cell-related disorders presenting with Kounis syndrome. Int J Cardiol. 2012;161(1):56–58.10.1016/j.ijcard.2012.06.041
- Ridolo E, Triggiani M, Montagni M, et al. Mastocytosis presenting as cardiac emergency. Intern Emerg Med. 2013;8(8):749–752.10.1007/s11739-013-1012-0
- Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002;110(3):341–348.10.1067/mai.2002.126811
- Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. Ann Emerg Med. 2006;47(4):373–380.10.1016/j.annemergmed.2006.01.018
- Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11(4):319–325.10.1097/ACI.0b013e3283481ab6
- Simons FE. Anaphylaxis pathogenesis and treatment. Allergy. 2011;66(Suppl 95):31–34.10.1111/all.2011.66.issue-s95
- Simons FE. 9. Anaphylaxis. J Allergy Clin Immunol. 2008;121(2):S402–S407; quiz S20.10.1016/j.jaci.2007.08.061
- Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2):S161–S181.10.1016/j.jaci.2009.12.981
- Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia. Anesthesiology. 2009;111(5):1141–1150
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079.
- Patella V, Marinò I, Lampärter B, et al. Human heart mast cells. J Immun. 1995;154:2855–2865.
- Genovese A, Rossi FW, Spadaro G, et al. Human cardiac mast cells in anaphylaxis. In J Ring (Ed.), Anaphylaxis Chem Immunol Allergy. 2010;95:98–109.10.1159/000315945
- Marone G, Genovese A, Varricchi G, et al. Human heart as a shock organ in anaphylaxis. Allergo J Int. 2014;23(2):60–66.10.1007/s40629-014-0007-3
- Kovanen PT. Mast cells in atherogenesis: actions and reactions. Curr Atheroscler Rep. 2009;11(3):214–219.10.1007/s11883-009-0033-7
- Marone G, de Crescenzo G, Adt M, et al. Immunological characterization and functional importance of human heart mast cells. Immunopharmacology. 1995;31:1–18.10.1016/0162-3109(95)00037-3
- Stellato C, Casolaro V, Ciccarelli A, et al. General anaesthetics induce only histamine release selectively from human mast cells. BJA: Br J Anaesth. 1991;67(6):751–758.10.1093/bja/67.6.751
- Triggiani M, Montagni M, Parente R, et al. Anaphylaxis and cardiovascular diseases. Curr Opin Allergy Clin Immunol. 2014;14(4):309–315.10.1097/ACI.0000000000000071
- Vigorito C, Giordano A, De Caprio L, et al. Effects of histamine on coronary hemodynamics in humans: role of H1 and H2 receptors. J Am Coll Cardiol. 1987;10(6):1207–1213.10.1016/S0735-1097(87)80120-1
- Vigorito C, Russo P, Picotti GB, et al. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983;5(4):531–537.10.1097/00005344-198307000-00004
- Vigorito C, Poto S, Picotti GB, et al. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986;73(6):1175–1182.10.1161/01.CIR.73.6.1175
- Steffel J, Akhmedov A, Greutert H, et al. Histamine induces tissue factor expression: implications for acute coronary syndromes. Circulation. 2005;112(3):341–349.10.1161/CIRCULATIONAHA.105.553735
- Reid AC, Silver RB, Levi R. Renin: at the heart of the mast cell. Immunol Rev. 2007;217:123–140.10.1111/imr.2007.217.issue-1
- Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80(4):1669–1699.
- Braquet P, Rola-Pleszczynski M. The role of PAF in immunological responses: a review. Prostaglandins. 1987;34(2):143–147.10.1016/0090-6980(87)90190-0
- Choi IH, Ha TY, Lee DG, et al. Occurrence of disseminated intravascular coagulation (DIC) in active systemic anaphylaxis: role of platelet-activating factor. Clin Exp Immunol. 1995;100(3):390–394.
- Vadas P, Gold M, Perelman B, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Eng J Med. 2008;358(1):28–35.10.1056/NEJMoa070030
- Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131(1):144–149.10.1016/j.jaci.2012.08.016
- Marone G, Giordano A, Cirillo R, et al. Cardiovascular and metabolic effects of peptide leukotrienes in man. Ann N Y Acad Sci. 1988;524:321–333.10.1111/nyas.1988.524.issue-1
- Vigorito C, Giordano A, Cirillo R, et al. Metabolic and hemodynamic effects of peptide leukotriene C4 and D4 in man. Int J Clin Lab Res. 1997;27(2-4):178–184.10.1007/BF02912454
- Arshad M, Vijay V, Floyd BC, et al. Thromboxane receptor stimulation suppresses guanylate cyclase-mediated relaxation of radial arteries. Ann Thorac Surg. 2006;81(6):2147–2154.10.1016/j.athoracsur.2006.01.024
- Hattori Y, Levi R. Effect of PGD2 on cardiac contractility: a negative inotropism secondary to coronary vasoconstriction conceals a primary positive inotropic action. J Pharmacol Exp Ther. 1986;237(3):719–724.
- Bot I, Shi GP, Kovanen PT. Mast cells as effectors in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(2):265–271.10.1161/ATVBAHA.114.303570
- Bot I, de Jager SC, Zernecke A, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein e-deficient mice. Circulation. 2007;115(19):2516–2525.10.1161/CIRCULATIONAHA.106.660472
- Johnson JL, Jackson CL, Angelini GD, et al. Activation of matrix-degrading metalloproteinases by mast cell proteases in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1998;18(11):1707–1715.10.1161/01.ATV.18.11.1707
- Kalsner S, Richards R. Coronary arteries of cardiac patients are hyperreactive and contain stores of amines: a mechanism for coronary spasm. Science. 1984;223(4643):1435–1437.10.1126/science.6701530
- Gill DS, Barradas MA, Fonseca VA, et al. Increased histamine content in leukocytes and platelets of patients with peripheral vascular disease. Am J Clin Pathol. 1988;89(5):622–626.
- Forman MB, Oates JA, Robertson D, et al. Increased adventitial mast cells in a patient with coronary spasm. N Engl J Med. 1985;313(18):1138–1141.10.1056/NEJM198510313131807
- Moreno M, Puig J, Serrano M, et al. Circulating tryptase as a marker for subclinical atherosclerosis in obese subjects. PLoS ONE. 2014;9(5):e97014.10.1371/journal.pone.0097014
- Pastorello EA, Morici N, Farioli L, et al. Serum tryptase: a new biomarker in patients with acute coronary syndrome? Int Arch Allergy Immunol. 2014;164(2):97–105.10.1159/000360164
- Xiang M, Sun J, Lin Y, et al. Usefulness of serum tryptase level as an independent biomarker for coronary plaque instability in a Chinese population. Atherosclerosis. 2011;215(2):494–499.10.1016/j.atherosclerosis.2011.01.006
- Kaartinen M, van der Wal AC, van der Loos CM, et al. Mast cell infiltration in acute coronary syndromes: implications for plaque rupture. J Am Coll Cardiol. 1998;32(3):606–612.10.1016/S0735-1097(98)00283-6
- Mueller UR. Cardiovascular disease and anaphylaxis. Curr Opin Allergy Clin Immunol. 2007;7:337–341.10.1097/ACI.0b013e328259c328
- Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Ann Allergy Asthma Immunol. 2007;98(3):252–257.10.1016/S1081-1206(10)60714-4
- Oppel T, Schnuch A. Häufigste Auslöser allergischer Kontaktekzeme. Dtsch Med Wochenschr. 2006;131(28/29):1584–1589.10.1055/s-2006-947800
- Revell PA, Braden M, Freeman MA. Review of the biological response to a novel bone cement containing poly(ethyl methacrylate) and n-butyl methacrylate. Biomaterials. 1998;19(17):1579–1586.10.1016/S0142-9612(97)00118-X
- Zanotti KM, Markman M. Prevention and management of antineoplastic-induced hypersensitivity reactions. Drug Saf. 2001;24(10):767–779.10.2165/00002018-200124100-00005
- Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents. J Am Coll Cardiol. 2006;47(1):175–181.10.1016/j.jacc.2005.07.071
- Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans. J Am Coll Cardiol. 2006;48(1):193–202.10.1016/j.jacc.2006.03.042
- Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;309(8009):466–469.10.1016/S0140-6736(77)91953-5
- Harper NJ, Dixon T, Dugue P, et al. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64(2):199–211.
- Brown MJ, Ind PW, Jenner DA. Platelet histamine. N Engl J Med. 1980;303(13):756.
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2):S73–S80.10.1016/j.jaci.2009.11.017
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142.10.1038/ni1158
- Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7(1):9.10.1186/1939-4551-7-9
- The Association of Anaesthetists of Great Britain & Ireland. Management of a Patient with Suspected Anaphylaxis During Anaesthesia 2009 [cited 2015 Jun 16]. Available from: http://www.aagbi.org/sites/default/files/ana_laminate_2009.pdf.
- Australian & New Zealand Anaesthetic Allergy Group. ANZAAG Anaphylaxis Management Guidelines 2013 [cited 2015 Jun 16]. Available from: http://www.anzaag.com/MgmtResources.aspx.
- Dhami S, Panesar SS, Roberts G, et al. Management of anaphylaxis: a systematic review. Allergy. 2014;69(2):168–175.10.1111/all.12318
- Sheikh A, Shehata YA, Brown SG, et al. Adrenaline for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2009;64(2):204–212.10.1111/all.2009.64.issue-2
- Kemp SF, Lockey RF, Simons FE. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63(8):1061–1070.10.1111/all.2008.63.issue-8
- Kroigaard M, Garvey LH, Gillberg L, et al. Scandinavian clinical practice guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51(6):655–670.10.1111/aas.2007.51.issue-6
- Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: Cardiac arrest in special situations: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18_suppl_3): S829–S861.10.1161/CIRCULATIONAHA.110.971069
- Fisher MM. Clinical observations on the pathophysiology and treatment of anaphylactic cardiovascular collapse. Anaesth Intensive Care. 1986;14(1):17–21.
- Nurmatov UB, Rhatigan E, Simons FE, et al. H2-antihistamines for the treatment of anaphylaxis with and without shock: a systematic review. Ann Allergy Asthma Immunol. 2014;112(2):126–131.10.1016/j.anai.2013.11.010
- Kounis NG. Coronary hypersensitivity disorder: the Kounis syndrome. Clin Ther. 2013;35(5):563–571.10.1016/j.clinthera.2013.02.022
- Mink SN, Simons FE, Simons KJ, et al. Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin Exp Allergy. 2004;34(11):1776–1783.10.1111/cea.2004.34.issue-11
- Dewachter P, Jouan-Hureaux V, Lartaud I, et al. Comparison of arginine vasopressin, terlipressin, or epinephrine to correct hypotension in a model of anaphylactic shock in anesthetized brown Norway rats. Anesthesiology. 2006;104(4):734–741.10.1097/00000542-200604000-00018
- Schummer W, Schummer C, Wippermann J, et al. Anaphylactic shock: is vasopressin the drug of choice? Anesthesiology. 2004;101:1025–1027.10.1097/00000542-200410000-00032
- Hussain AM, Yousuf B, Khan MA, et al. Vasopressin for the management of catecholamine-resistant anaphylactic shock. Singapore Med J. 2008;49(9):e225–8.
- Thomas M, Crawford I. Glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J. 2005;22(4):272–273.10.1136/emj.2005.023507
- Rosic M, Parodi O, Jakovljevic V. Glucagon effects on 3H-histamine uptake by the isolated guinea-pig heart during anaphylaxis. 2014;2014:782709.
- Jang DH, Nelson LS, Hoffman RS. Methylene blue for distributive shock: a potential new use of an old antidote. J Med Toxicol. 2013;9(3):242–249.10.1007/s13181-013-0298-7
- McBrien ME, Breslin DS, Atkinson S, et al. Use of methoxamine in the resuscitation of epinephrine-resistant electromechanical dissociation. Anaesthesia. 2001;56(11):1085–1089.
- Higgins DJ, Gayatri P. Methoxamine in the management of severe anaphylaxis. Anaesthesia. 1999;54(11):1126.10.1046/j.1365-2044.1999.01197.x
- Heytman M, Rainbird A. Use of alpha-agonists for management of anaphylaxis occurring under anaesthesia: case studies and review. Anaesthesia. 2004;59(12):1210–1215.10.1111/ana.2004.59.issue-12
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130(25):e344–e426.10.1161/CIR.0000000000000134
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61(4):e78–e140.10.1016/j.jacc.2012.11.019
- Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiol Drug Saf. 2003;12(3):195–202.
- Cabello JB, Burls A, Emparanza JI, et al. Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev. 2013;8:Cd007160.
- Berkes EA. Anaphylactic and anaphylactoid reactions to aspirin and other NSAIDs. Clin Rev Allergy Immunol. 2003;24(2):137–148.10.1385/CRIAI:24:2
- Weng Z, Zhang B, Asadi S, et al. Quercetin Is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE. 2012;7(3):e33805.10.1371/journal.pone.0033805
- Simons FE, Peterson S, Black CD. Epinephrine dispensing patterns for an out-of-hospital population: a novel approach to studying the epidemiology of anaphylaxis. J Allergy Clin Immunol. 2002;110(4):647–651.10.1067/mai.2002.127860
- Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30(8):1144–1150.10.1046/j.1365-2222.2000.00864.x
- Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018.10.1016/j.jaci.2006.12.622
- Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108(5):871–873.10.1067/mai.2001.119409
- Rawas-Qalaji M, Simons FE, Collins D, et al. Long-term stability of epinephrine dispensed in unsealed syringes for the first-aid treatment of anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(6):500–503.10.1016/S1081-1206(10)60124-X
- Mink SN, Bands C, Becker A, et al. Effect of bolus epinephrine on systemic hemodynamics in canine anaphylactic shock. Cardiovasc Res. 1998;40:546–556.10.1016/S0008-6363(98)00199-0
- Bautista E, Simons FE, Simons KJ, et al. Epinephrine fails to hasten hemodynamic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Immunol. 2002;128(2):151–164.10.1159/000059406
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539–548.10.1056/NEJMoa043046
- Manivannan V, Li JT, Prasad A, et al. Apical ballooning syndrome after administration of intravenous epinephrine during an anaphylactic reaction. Mayo Clin Proc. 2009;84(9):845–846.10.4065/84.9.845
- Litvinov IV, Kotowycz MA, Wassmann S. Iatrogenic epinephrine-induced reverse Takotsubo cardiomyopathy: direct evidence supporting the role of catecholamines in the pathophysiology of the ‘broken heart syndrome’. Clin Res Cardiol. 2009;98(7):457–462.10.1007/s00392-009-0028-y
- Vultaggio A, Matucci A, Del Pace S, et al. Tako-Tsubo-like syndrome during anaphylactic reaction. Eur J Heart Fail. 2007;9(2):209–211.10.1016/j.ejheart.2006.05.011
- Winogradow J, Geppert G, Reinhard W, et al. Tako-tsubo cardiomyopathy after administration of intravenous epinephrine during an anaphylactic reaction. Int J Cardiol. 2011;147(2):309–311.10.1016/j.ijcard.2010.12.063
- Scheiba N, Viedt-Suelmann C, Schäkel K. Tako-tsubo cardiomyopathy after a hymenoptera sting and treatment with catecholamines. Acta Derm Venereol. 2011;91(5):593–594.10.2340/00015555-1119
- Laínez B, Ureña M, Álvarez V, et al. Miocardiopatía de tako-tsubo iatrogénica secundaria a catecolaminas. Rev Esp Cardiol. 2009;62(12):1498–1499.10.1016/S0300-8932(09)73140-9
- Dewachter P, Tanase C, Levesque E, et al. Apical ballooning syndrome following perioperative anaphylaxis is likely related to high doses of epinephrine. J Anesth. 2011;25(2):282–285.10.1007/s00540-010-1085-0
- Dewachter P, Mouton-Faivre C. Possible link between apical ballooning syndrome during anaphylaxis and inappropriate administration of epinephrine. Mayo Clin Proc. 2010;85(4):396–397; author reply 8.10.4065/mcp.2009.0699
- Cevik C, Nugent K, Shome GP, et al. Treatment of Kounis syndrome. Int J Cardiol. 2010;143(3):223–226.10.1016/j.ijcard.2010.02.040
- Yilmaz R, Yuksekbas O, Erkol Z, et al. Postmortem findings after anaphylactic reactions to drugs in Turkey. Am J Forensic Med Pathol. 2009;30(4):346–349.10.1097/PAF.0b013e3181c0e7bb
- Brockow K, Jofer C, Behrendt H, et al. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63(2):226–232.10.1111/j.1398-9995.2007.01569.x
- Akin C. Anaphylaxis and mast cell disease: what is the risk? Curr Allergy Asthma Rep. 2010;10(1):34–38.10.1007/s11882-009-0080-8
- Kogias JS, Sideris SK, Anifadis SK. Kounis syndrome associated with hypersensitivity to hymenoptera stings. Int J Cardiol. 2007;114(2):252–255.10.1016/j.ijcard.2005.11.059
- Lieberman P, Simons FE. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy. 2015;45(8):1288-95.
