ABSTRACT
Toxigenic Corynebacterium ulcerans may cause both respiratory and cutaneous diphtheria in humans. As a zoonotic emerging pathogen it has been isolated from a wide variety of animals living in captivity, such as livestock, pet, zoo and research animals and additionally in a large number of different wild animals. Here we report the isolation of tox-positive C. ulcerans in four hedgehogs with cutaneous diphtheria and pneumonia, respectively.
Introduction
Diphtheria and diphtheria-like illness are caused by Corynebacterium species harbouring the diphtheria toxin (DT) encoding tox gene. In recent years, diphtheria-like human infections with toxigenic Corynebacterium ulcerans have outnumbered those caused by toxigenic C. diphtheriae in many industrialized countries [Citation1–3]. While about 50 years ago human cases of C. ulcerans-caused disease were associated with consumption of raw milk and dairy products or contact to cattle [Citation3–5], nearly all C. ulcerans infections since then have been described after contact with domestic animals such as pet dogs and cats [Citation3,Citation6–11] or – less often – after occupational contact with livestock animals such as pigs [Citation12,Citation13]. Moreover, both non-toxigenic and toxigenic C. ulcerans as emerging zoonotic pathogens have been isolated from a wide variety of animal species, either from zoo, shelter, research or herd animals with human contact, e.g. water rats [Citation14], shelter dogs [Citation15,Citation16], macaques [Citation17,Citation18], killer whales [Citation19], a lion [Citation19], a dromedary [Citation20], ferrets [Citation21], a goat [Citation22], a cow [Citation23] and ground squirrels [Citation24] or from free-roaming animals such as otters [Citation25], roe deer [Citation26,Citation27], wild boars [Citation27,Citation28], red fox [Citation29], Ural owl [Citation30] and Japanese shrew-moles [Citation30] (). Interestingly, most of the toxigenic C. ulcerans strains were found either in carnivores or animals with (seasonal) group hierarchical fighting. Here we report on the unusual finding of toxigenic C. ulcerans in four hedgehogs (Erinaceus europaeus), three of them without known previous contact to humans.
Table 1. Characteristics of Corynebacterium ulcerans isolated from free-roaming wild animals.
Results
In December 2017, a young hedgehog (#1) was found with a weight of 1026 g in a garden with severe soft tissue damage after being cut by a mowing machine ((a)). The injured animal was brought to a local veterinarian and treated for 12 days with enrofloxacin and a proteinolytic ointment. Because of an extremely retarded wound healing and severe loss of weight the animal was transferred to a private hedgehog rescue station in March 2018, where the animal (750 g) was presented to another veterinarian and taken care of. A wound swab was taken for bacteriological diagnosis. The animal was treated with a third generation cephalosporin which was later switched to sulphonamides for 10 days. The wound continued to heal within three months ((b,c)) with diminishing necrotic wound margins.
Figure 1. (a,b) Wound infection due to toxigenic C. ulcerans in a hedgehog, healing progress under antibiotic treatment.

In April 2017, a male hedgehog (#2) was found in an allotment colony in Berlin, Germany, in a moribund condition. The animal died and was subjected to necropsy showing a weight of 570 g but considering the adipose tissue still had a good nutritional status. Gross pathological examination revealed otitis externa (left ear), anaemia and severe tick infestation. Thickening and redness of the lung suggestive of pneumonia were observed prompting further histological and bacteriological investigations.
In July 2018 two hedgehogs (#3 and #4) were found in Hanover, Germany and finally euthanized because of moribund conditions. Both were adult, male animals with a weight of 760 and 605 g, respectively. Pathological examination revealed myiasis and otitis externa in both cases. Further histological and bacteriological investigations were done because of gross pathological aspects of severe pneumonia and septicaemia in both cases. None of the four hedgehogs presented typical local or systemical findings of diphtheria toxin effects such as pseudomembranes or histopathological lesions indicating myocarditis or damage of the peripheral nervous system.
The wound swab obtained from the severe soft tissue wound of hedgehog #1 grew C. ulcerans (strain number KL 1151) and Streptococcus pyogenes. Toxigenicity was verified by real-time PCR and a modified Elek test both yielding positive results. Histopathological examination of lung tissues of hedgehogs #2, #3 and #4 showed pneumonia and a severe lungworm infection in hedgehog #2, respectively. Lung tissue material obtained from hedgehog #2 grew C. ulcerans (strain number KL 955) in pure culture. Toxigenicity testing by tox-PCR and Elek identified the isolate as non-toxigenic tox-bearing (NTTB). Lung and heart tissue material obtained from hedgehog #3 grew Enterococcus avium, Morganella morganii and C. ulcerans (KL 1203). C. ulcerans strain KL 1204 was isolated in pure culture from heart and lung tissue materials obtained from hedgehog #4. Both KL 1203 and KL 1204 were toxigenic as shown by positive tox-PCR and Elek testing, respectively. All C. ulcerans were identified by partial rpoB sequencing, FT-IR and MALDI-TOF analysis.
Commercially available biochemistry systems unequivocally identified all four isolates as C. ulcerans (VITEK, Omnilog) with the exception of isolate KL 955 which was falsely identified as C. pseudotuberculosis by VITEK CBC. All isolates were found to be resistant against penicillin (MICs 0.19–0.25 mg/l) and clindamycin (MICs 2–4 mg/l) according to EUCAST, but susceptible against erythromycin, cephalosporins and sulphonamides according to CLSI guidelines. NGS-derived MLST based on seven housekeeping loci was performed using NGS data and revealed three different sequence types (ST), 332 in hedgehog #1 (KL 1151) and hedgehog #4 (KL 1204), ST 330 in hedgehog #2 (KL 955), and ST 331 in hedgehog #3 (KL 1203), respectively.
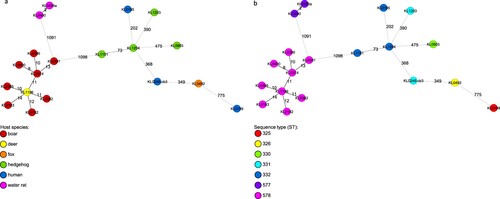
Phylogenetic minimum spanning trees, built from cgMLST results of NGS data showed that the genetic similarity of the four C. ulcerans isolates from hedgehogs was much lower to the NTTB wildlife cluster from wild boars and roe deer (>1000 alleles) than to human samples from different geographic regions (>200 alleles). However, genomic differences in cgMLST analysis were at least 73 alleles between isolates from hedgehog #1 and #4 which shared the same ST 332 based on the 7-gene scheme and more than 200 alleles compared to all other isolates. These differences show that the hedgehog-derived isolates are genetically not closely related to each other or to any other human or animal isolate ((a,b)).
Figure 2. (a,b) Phylogenetic minimum spanning trees of the cgMLST analysis of 19 C. ulcerans isolates originating from various host species with an in-house C. ulcerans-specific cgMLST scheme of 1211 target loci. Allele distances between samples are indicated. Samples are colour coded by the corresponding host organism (A) or by their ST based on the 7-gene scheme (B), as given in the legend.
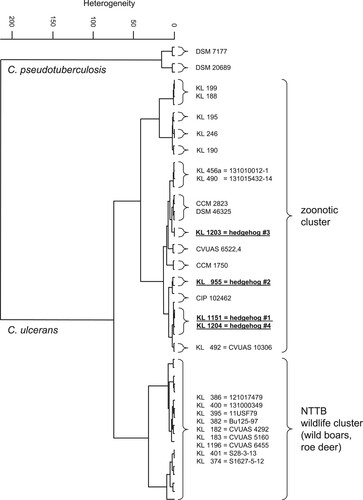
The comparison of FT-IR spectra () shows no similarity for the four hedgehog isolates with the NTTB wildlife cluster (wild boars, roe deer) observed in different parts of Germany [Citation26–28].
Figure 3. Dendrogram of FT-IR-spectra of C. ulcerans strains obtained from the four hedgehogs (underlined) in comparison with spectra from C. ulcerans DSM 46325 and several C. ulcerans isolates, including isolates from wild animals and humans in Germany. Spectra of two C. pseudotuberculosis strains are used as outgroup.
Discussion
In contrast to the classical diphtheria agent C. diphtheriae which is basically a human pathogen and has only extremely rarely been reported to be isolated from animals [Citation32], the emerging pathogen C. ulcerans is a zoonotic pathogen with an increasing spectrum of affected animals. While originally only reported from livestock (cattle, pigs) and pet (dog, cat) animals, C. ulcerans has been meanwhile detected in a wide variety of species living in captivity as zoo (killer whales, lion, water rats), shelter (dogs), herd (dromedary, goat, cow) or research (macaques, ground squirrels) animals with contact to humans. In recent years, isolation of C. ulcerans has also been reported in wildlife (). Interestingly, the broad majority of wild animals affected by C. ulcerans showed pathologic lesions of internal organs such as lymph nodes [Citation26–28], lung [Citation25] – also in hedgehogs #2, #3 and #4 of the current study – or spleen [Citation29] suggesting systemic infection. These findings are in contrast to human diphtheria cases due to C. diphtheriae or C. ulcerans exhibiting respiratory or cutaneous manifestations or to C. ulcerans infections in animals living in captivity which were reported to be either asymptomatic carriers or to present with skin or mucosal ulceration. One could assume that only wild animals with a deteriorating disease, possibly aggravated by C. ulcerans infection are found and diagnosed, while the asymptomatic carriership of C. ulcerans in wildlife is likely as usual as in other animals. Asymptomatic pharyngeal carriage is known for C. diphtheriae and – rarely – C. ulcerans in humans, but also for C. ulcerans primarily in pets and less frequently in livestock animals. In wildlife, however, it has so far only been reported in a recent surveillance study among wild birds and their prey animals [Citation30]. Most reported C. ulcerans strains in animals – both with and without human contact – harbour the tox gene [Citation10]. Further studies are needed to determine if this is only a reporting bias or reflects the real distribution of toxigenic and non-toxigenic C. ulcerans strains among animals and also humans. Since DT producing strains among wild animals were until very recently detected only in carnivores (otters, red fox, Ural owl) with non-toxigenic strains isolated from omnivorous (e.g. wild boars) and herbivorous (e.g. roe deer) animals, it seemed possible that C. ulcerans toxigenicity might be associated with a carnivorous lifestyle involving predatory hunting behaviour with the potential of acquiring an infection while fighting. However, the recent detection of asymptomatic carriage of toxigenic C. ulcerans in two Japanese shrew-moles [Citation30], as well as the current cases of hedgehogs (this study), broadens the spectrum of affected animals also to primarily insectivorous species.
In contrast to the majority of reported C. ulcerans infections in humans causing cutaneous diphtheria [Citation33] as well as in pet or livestock animals [Citation3] with mucosal or skin involvement, superficial soft tissue infection in wild animals has so far only been reported in the current hedgehog #1. Similar to most cases of human cutaneous C. diphtheriae-caused diphtheria the infection in hedgehog #1 was associated with a previous trauma, but the source of C. ulcerans remains unclear. The strain might be acquired from the environment, an anatomical site of the hedgehog, during its stay in the hedgehog rescue station or from another carnivorous animal trying to feed on the heavily injured hedgehog. However, no signs of animal-afflicted bite wounds were noticed. In hedgehog #3 and #4 otitis externa induced by myiasis could possibly be the portal of entry for the C. ulcerans strains.
Notably, according to the recently revised German recommendations [Citation31], public health measures including personal protection, antibiotic prophylaxis and screening for C. ulcerans carriage for close contact persons were advised, since zoonotic transmission from pet animals to humans has been clearly demonstrated using molecular typing techniques [Citation6,Citation8–12]. The analysed dataset also indicates closer genetic similarity of the hedgehog-derived isolates to human isolates than to those from wild animals, although no close relationship of the hedgehog isolates to any other isolate was detected. However, C. ulcerans carrier status of persons with direct contact to hedgehogs was not a subject of the investigation. As a bacteriological examination with detection of C. ulcerans was performed after intensive care treatment of hedgehog #1, all close contact persons refused recommended measures and only engaged in hygienic behaviour and self-observation for clinical signs of diphtheria, raising the general awareness of zoonotic agents in wildlife care. Toxigenic C. ulcerans harbour either prophages or, an alternative pathogenicity island (PAI) described previously and can therefore act as a beta corynephage reservoir [Citation10].
In conclusion, the finding of toxigenic C. ulcerans in hedgehogs, an increasingly synanthropic species known to reside in urban and suburban environments in close proximity to humans, highlights potential transmission risks and should raise the public health awareness towards zoonotic infections.
Material and methods
For bacteriological examination, clinical material obtained from all four animals (i.e. wound swabs and lung or heart tissue, respectively) were plated on Columbia agar with 5% sheep blood, chocolate agar supplemented with Vitox (5% CO2 atmosphere) and Gassner agar (Oxoid, Wesel, Germany) and incubated for up to 48 hours at 37 °C. Bacteriological species identification was performed as recently described [Citation34] using MALDI-TOF MS analysis (Microflex LT Mass Spectrometer, MALDI Biotyper™; Bruker Daltonics, Bremen, Germany) and the MBT 7311 commercial library. Supplementary species identification by commercial biochemistry assays (VITEK2-compact with card systems for anaerobes and corynebacteria [ANC] and coryneform bacteria [CBC; all bioMérieux, Nürtingen, Germany] and Omnilog [Biolog, Hayward, USA]) was done according to the manufactureŕs prescriptions. Fourier-transform infrared (FT-IR) spectroscopy with cluster analysis, and partial sequencing of the rpoB gene were carried out as described previously [Citation27,Citation28,Citation35]. Toxigenicity was investigated by real-time PCR [Citation36] and a modified Elek test [Citation37]. Next-generation sequencing (NGS) of the isolates was performed on an Illumina MiSeq (Illumina, San Diego, CA, USA) as reported previously [Citation38]. Multilocus sequence typing (MLST) based on seven housekeeping loci [Citation39] was done using the NGS data. The sequence type (ST) was determined with the respective MLST database (http://pubmlst.org/cdiphtheriae/). For cg (core genome) MLST typing an ad-hoc C. ulcerans-specific cgMLST scheme was generated by using the SeqSphere+ target definer tool (Ridom, Munster, Germany) with default settings [Citation40]. As a reference, the genome of strain 809 with accession number NC_018101 was used. 11 complete C. ulcerans genomes from NCBI were used as query sequences for core genome scheme definition (accession nos. NC_018101.1, NZ_CP009716.1, NZ_CP010818.1, NZ_CP011095.1, NZ_CP009583.1, NZ_CP009500.1, NC_015683.1, NZ_CP009622.1, NZ_CP011913.1, NZ_LT906443.1, NZ_CP021417.1). The resulting cgMLST scheme consisted of 1,211 target loci. cgMLST with the described ad-hoc scheme was performed using NGS data as described [Citation38]. NGS raw datasets are available in the NCBI sequence read archive (SRA) at https://www.ncbi.nlm.nih.gov/sra (accession numbers in Supplementary Table 1). Antibiotic susceptibility testing was performed according to both CLSI (CLSI: Performance standards for antimicrobial susceptibility testing. M100, 28th. Ed., Jan 2018;
CLSI: Methods for Antimicrobial Dilution and Disk Susceptibility Testing of infrequently isolated or fastidious bacteria. M45 3rd Ed, 2015) and EUCAST guidelines (http://www.eucast.org/clinical_breakpoints, version 8.1).
For histopathological examination, small slices of lung tissue were fixed in 4% buffered formalin, processed using standard methods and embedded in liquid paraffin. Sections were stained with hematoxylin-eosin (HE).
Summary of the conclusions
The first isolation of tox-positive Corynebacterium ulcerans from four hedgehogs underlines both the veterinary and the human public health importance of a variety of wild animals which might serve as zoonotic C. ulcerans reservoirs for pet or livestock animals and humans.
Acknowledgements
We thank Wolfgang Schmidt, Sabine Wolf, Marion Lindermayer, Katja Meindl, Jasmin Fräßdorf, Vanessa Nowak, Martin Dyk, Nadine Jahn, Zoltan Mezö, Sabine Schiller and Anna Eckert for excellent technical assistance. AB, MP, KM, SB, TE, CAS, RK, SH, NA and AS were involved in primary bacteriological and histopathological diagnostics, RK performed PCRs, AB and AS performed special diagnostics for diphtheria, AD performed the NGS analysis, JR performed the FT-IR analysis. All authors participated in conducting the study and in writing the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bonmarin I, Guiso N, Le Flèche-Matéos A, et al. Diphtheria: a zoonotic disease in France? Vaccine. 2009;27:4196–4200. doi: 10.1016/j.vaccine.2009.04.048
- Wagner KS, White JM, Crowcroft NS, et al. Diphtheria in the United Kingdom, 1986-2008: the increasing role of Corynebacterium ulcerans. Epidemiol Infect. 2010;138:1519–1530. doi: 10.1017/S0950268810001895
- Hacker E, Antunes CA, Mattos-Guaraldi AL, et al. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016;11:1191–1208. doi: 10.2217/fmb-2016-0085
- Bostock AD, Gilbert FR, Lewis D, et al. Corynebacterium ulcerans infection associated with untreated milk. J. Infect. 1984;9:286–288. doi: 10.1016/S0163-4453(84)90662-5
- Hart RJ. Corynebacterium ulcerans in humans and cattle in North Devon. J Hyg. 1984;92:161–164. doi: 10.1017/S0022172400064172
- Lartigue MF, Monnet X, Le Fleche A, et al. Corynebacterium ulcerans in an immunocompromised patient with diphtheria and her dog. J Clin Microbiol. 2005;43:999–1001. doi: 10.1128/JCM.43.2.999-1001.2005
- Hogg RA, Wessels J, Hart J, et al. Possible zoonotic transmission of toxigenic Corynebacterium ulcerans from companion animals in a human case of fatal diphtheria. Vet Rec. 2009;165:691–692.
- Berger A. Toxigenic Corynebacterium ulcerans in woman and cat. Emerg Infect Dis. 2011;17:1767–1769. doi: 10.3201/eid1709.110391
- Meinel DM, Konrad R, Berger A, et al. Zoonotic transmission of toxigenic Corynebacterium ulcerans strain, Germany, 2012. Emerg Infect Dis. 2015;21:356–358. doi: 10.3201/eid2102.141160
- Meinel DM, Margos G, Konrad R, et al. Next generation sequencing analysis of nine Corynebacterium ulcerans isolates reveals zoonotic transmission and a novel putative diphtheria toxin-encoding pathogenicity island. Genome Med. 2014;6:113. doi: 10.1186/s13073-014-0113-3
- Vandentorren S, Guiso N, Badell E, et al. Toxigenic Corynebacterium ulcerans in a fatal human case and her feline contacts, France, March 2014. Euro Surveill. 2014;19:20910. doi: 10.2807/1560-7917.ES2014.19.38.20910
- Schuhegger R, Schoerner C, Dlugaiczyk J, et al. Pigs as source for toxigenic Corynebacterium ulcerans. Emerg Infect Dis. 2009;15:1314–1315. doi: 10.3201/eid1508.081568
- Berger A, Boschert V, Konrad R, et al. Two cases of cutaneous diphtheria associated with occupational pig contact in Germany. Zoonoses Public Health. 2013;60:539–542. doi: 10.1111/zph.12031
- Eisenberg T, Mauder N, Contzen M, et al. Outbreak with clonally related isolates of Corynebacterium ulcerans in a group of water rats. BMC Microbiol. 2015;15:42. doi: 10.1186/s12866-015-0384-x
- Katsukawa C, Komiya T, Yamagishi H, et al. Prevalence of Corynebacterium ulcerans in dogs in Osaka, Japan. J Med Microbiol. 2012;61:266–273. doi: 10.1099/jmm.0.034868-0
- Dias AA, Silva FC, Pereira GA, et al. Corynebacterium ulcerans isolated from an asymptomatic dog kept in an animal shelter in the metropolitan area of Rio de Janeiro, Brazil. Vector Borne Zoonotic Dis. 2010;10:743–748. doi: 10.1089/vbz.2009.0132
- Fox JG, Frost WW. Corynebacterium ulcerans mastitis in a bonnet macaque (Macaca radiata). Lab Anim Sci. 1974;24:820–822.
- Venezia J, Cassiday PK, Marini RP, et al. Characterization of Corynebacterium species in macaques. J Med Microbiol. 2012;61:1401–1408. doi: 10.1099/jmm.0.045377-0
- Seto Y, Komiya T, Iwaki M, et al. Properties of corynephage attachment site and molecular epidemiology of Corynebacterium ulcerans isolated from humans and animals in Japan. Jpn J Infect Dis. 2008;61:116–122.
- Tejedor MT, Martin JL, Lupiola P, et al. Caseous lymphadenitis caused by Corynebacterium ulcerans in the dromedary camel. Can Vet J. 2000;41:126–127.
- Marini RP, Cassiday PK, Venezia J, et al. Corynebacterium ulceransin Ferrets. Emerg Infect Dis. 2014;20:159–161. doi: 10.3201/eid2001.130675
- Morris WE, Uzal FA, Cipolla AL. Pyogranulomatous meningoencephalitis in a goat due to Corynebacterium ulcerans. Vet Rec. 2005;156:317–318. doi: 10.1136/vr.156.10.317
- Murakami K, Hata E, Hatama S, et al. Eosinophilic granuloma with Splendore-Hoeppli material caused by toxigenic Corynebacterium ulcerans in a heifer. J Vet Med Sci. 2014;76:931–935. doi: 10.1292/jvms.13-0582
- Olson ME, Goemans I, Bolingbroke D, et al. Gangrenous dermatitis caused by Corynebacterium ulcerans in Richardson ground squirrels. J Am Vet Med Assoc. 1988;193:367–368.
- Foster G, et al. Corynebacterium ulcerans in free-ranging otters. Vet Rec. 2002;150:524.
- Rau J, Blazey B, Contzen M, et al. Corynebacterium ulcerans infection roe deer (Capreolus capreolus). Berl Münch Tierärztl Wschr. 2012;125:159–162.
- Eisenberg T, Kutzer P, Peters M, et al. Nontoxigenic tox-bearing Corynebacterium ulcerans infection among game animals, Germany. Emerg Infect Dis. 2014;20:448–452. doi: 10.3201/eid2003.130423
- Contzen M, Sting R, Blazey B, et al. Corynebacterium ulcerans from diseased wild boars. Zoonoses Public Health. 2011;58:479–488. doi: 10.1111/j.1863-2378.2011.01396.x
- Sting R, Ketterer-Pintur S, Contzen M, et al. Toxigenic Corynebacterium ulcerans isolated from a free-roaming red fox (Vulpes vulpes). Berl Munch Tierärztl Wochenschr. 2015;128:204–208.
- Katsukawa C, Umeda K, Inamori I, et al. Toxigenic Corynebacterium ulcerans isolated from a wild bird (Ural owl) and its feed (shrew-moles): comparison of molecular types with human isolates. BMC Res Notes. 2016;9:181. doi: 10.1186/s13104-016-1979-5
- RKI: Ratgeber für Ärzte – Diphtherie https://www.rki.de/DE/Content/Infekt/EpidBull/Merkblaetter/Ratgeber_Diphtherie.html
- Sing A, Konrad R, Meinel DM, et al. Corynebacterium diphtheriae in a free-roaming red fox: case report and historical review on diphtheria in animals. Infection. 2016;44:441–445. doi: 10.1007/s15010-015-0846-y
- Moore LSP, Leslie A, Meltzer M, et al. Corynebacterium ulcerans cutaneous diphtheria. Lancet Infect Dis. 2015;15:1100–1107. doi: 10.1016/S1473-3099(15)00225-X
- Konrad R, Berger A, Huber I, et al. Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry as a tool for rapid diagnosis of potentially toxigenic Corynebacterium species in the laboratory management of diphtheria-associated bacteria. Euro Surveill. 2010;15:19699. doi: 10.2807/ese.15.43.19699-en
- Khamis A, Raoult D, La Scola B. Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J Clin Microbiol. 2005;43:1934–1936. doi: 10.1128/JCM.43.4.1934-1936.2005
- Schuhegger R, Lindermayer M, Kugler R, et al. Detection of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by a novel real-time PCR. J Clin Microbiol. 2008;46:2822–2823. doi: 10.1128/JCM.01010-08
- Engler KH, Glushkevich T, Mazurova IK, et al. A modified Elek test for detection of toxigenic corynebacteria in the diagnostic laboratory. J Clin Microbiol. 1997;35:495–498.
- Dangel A, Berger A, Konrad R, et al. Geographically diverse clusters of nontoxigenic Corynebacterium diphtheriae infection, Germany, 2016-2017. Emerg Infect Dis. 2018;24:1239–1245. doi: 10.3201/eid2407.172026
- König C, Meinel DM, Margos G, et al. Multilocus sequence typing of Corynebacterium ulcerans provides evidence for zoonotic transmission and for increased prevalence of certain sequence types among toxigenic strains. J Clin Microbiol. 2014;52:4318–4324. doi: 10.1128/JCM.02291-14
- Ruppitsch W, Pietzka A, Prior K, et al. Defining and evaluating a core genome multilocus sequence typing scheme for whole-genome sequence-based typing of Listeria monocytogenes. J Clin Microbiol. 2015;53:2869–2876. doi: 10.1128/JCM.01193-15
