 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA), also known as oxacillin-resistant S. aureus, is a leading cause of community and hospital associated infections globally. In this work, we found that deletion of the arlRS two-component system genes in the USA300 and USA500 strains resulted in increased susceptibilities to oxacillin (8–16-fold decrease in minimal inhibitory concentrations). In USA300ΔarlRS, transcriptional levels of mecA or blaZ showed no obvious change, while mRNA levels of spx showed a 4-fold decrease at 4 h and a 6.3-fold decrease at 10 h. Overexpression of spx in ΔarlRS restored oxacillin resistance to a similar level in USA300. In addition, gel shift assay showed that the recombinant ArlR bound to spx promoter region. Furthermore, silencing of spx led to a significant increase of oxacillin susceptibility in multiple MRSA isolates. Our results indicate that ArlRS plays a strong role in regulating oxacillin resistance in MRSA strains, which involves direct modulation of spx expression. Moreover, oritavancin showed inhibition to ATPase activity of the recombinant histidine kinase ArlS (IC50 = 5.47 μM). Oritavancin had synergy effect on oxacillin activity against the MRSA strains in both planktonic and biofilm state. Our data suggest that ArlRS is an attractive target for breaking antimicrobial resistance of MRSA.
Introduction
Staphylococcus aureus is a major pathogen causing both community-acquired and hospital-acquired infections globally [Citation1]. The emergence and prevalence of community-associated methicillin-resistant S. aureus (CA-MRSA) and healthcare-associated methicillin-resistant S. aureus (HA-MRSA), e.g. the predominant clones USA300 [Citation2] and USA500 [Citation3], have attracted attentions because of their virulence and antibiotic resistance, especially their high-level resistance to the β-lactam antibiotics [Citation4].
MRSA, also known as oxacillin-resistant S. aureus, is defined as an oxacillin minimum inhibitory concentration (MIC) of greater than or equal to 4 mg/L [Citation5]. Oxacillin is a β-lactam antibiotic targeting penicillin-binding protein 2 (PBP2). Oxacillin resistance involves multiple factors. Most MRSA strains possess a mecA gene that encodes an alternative form of PBP2 called PBP2a (or PBP2′). PBP2a can take over the transpeptidation function of PBP2, but it has a lower penicillin-binding affinity and is resistant to the action of oxacillin [Citation6]. PBP2a synthesis is modulated by the transcriptional regulator MecI and the signal transduction protein MecR1, which are encoded by mecI and mecR1 genes located adjacent to mecA on the staphylococcal chromosome [Citation7,Citation8]. MecI and MecR1 share high protein sequence similarity with BlaI and BlaR1 [Citation9], respectively, which may also have the function of regulating PBP2a expression. Thus, in many clinical isolates of MRSA, a plasmid carrying blaI and blaR1 genes can encode proteins modulating PBP2a expression [Citation10,Citation11]. Beside the main mechanism above, oxacillin resistance in S. aureus clinical isolates has been reported to involve other factors, including FemAB (peptidoglycan synthesis), Llm (autolytic activity related protein), Sar, Agr, SigB [Citation12–15] (global regulators), etc.
The two-component signal transduction system ArlRS is a global regulator of S. aureus virulence, modulating the extracellular proteolytic activity, bacterial autolysis, capsule formation and production of virulence factors [Citation16–21]. Recent studies has found that ArlRS regulates S. aureus cell aggregation [Citation22] and is vital for in vivo catheter associated biofilm formation by S. aureus [Citation23]. However, whether ArlRS is involved in the regulation of oxacillin resistance remains unclear.
Here, we demonstrate for the first time that ArlRS plays an important role in the regulation of oxacillin resistance in MRSA strains, mainly through the direct modulation of spx expression. Besides, oritavancin can inhibit ArlS kinase activity and it has synergetic effect on oxacillin activity against MRSA strains.
Materials and methods
Bacterial strains, plasmids, growth media and antibiotics
Bacterial strains and plasmids used in this study are listed in . The MRSA strains were collected from Zhongshan Hospital of Fudan University and identified by the VITEK 2 system (bioMerieux SA, Lyon, France).
Table 1. Bacterial strains and plasmids used in this study.
Tryptic soy broth (TSB, Oxoid, Cambridge, UK) were used for S. aureus cultivation. Mueller-Hinton Broth and Mueller-Hinton agar were used for antibiotic susceptibility tests. Media were supplemented with erythromycin (10 µg/ml), ampicillin (100 µg/ml) or chloramphenicol (10 µg/ml), when appropriate for purposes of selection. Oritavancin diphosphate (dissolved in DMSO at 10 mM) was purchased from MedChemExpress China. Oxacillin was purchased from Sangon BiotechCo., Ltd (Shanghai, China).
Construction of gene knockout mutants and complementation strains
The arlRS genes in methicillin-resistant S. aureus USA300 TCH1516 strain (locus tag: USA300HOU_1349 and USA300HOU_1350) and USA500 2395 strain (locus tag: CH51_07425 and CH51_07430) were deleted using the temperature-sensitive vector pKOR-1 [Citation27]. Then the regions flanking arlRS gene were amplified by PCR and inserted into pKOR-1. Primers for PCR were designed according to the genomic sequence of S. aureus TCH1516 strain, and the sequences are listed in Supplementary Table 1. The recombinant plasmid, designated pKOR-arlRS, was transformed to Escherichia coli DC10B strain then into S. aureus USA300 and USA500 strains by electroporation respectively. A procedure for allelic displacement of the arlRS genes was performed as previous described [Citation30,Citation31]. The mutants, designated USA300ΔarlRS and USA500ΔarlRS, were verified by PCR, RT–PCR and direct sequencing. The vector pCN51 [Citation25] and pRB475 were used for arlRS complementation. The DNA fragment of the arlRS genes with their promoter region were amplified by PCR and inserted into the vectors. The resulting plasmids were transformed by electroporation into USA300ΔarlRS and USA500ΔarlRS, forming complementary strains PCNarlRS and PRBarlRS, respectively.
Construction of the gene silencing strains and the gene overexpressing strains
To silence the arlS, arlR or spx (locus tag: USA300HOU_0955) gene in S. aureus strains, the shuttle plasmid pMX6 [Citation26] with the paired termini 7 segment (which can form a hairpin structure) was used for constructing asRNA expression vectors. The expression plasmid of asRNAarlR or asRNAspx (named pMX6-arlR or pMX6-spx) was constructed by first amplifying the predicted Shine-Dalgarno (SD) sequence plus ∼100 nt downstream of the start codon of each gene and then inserting the fragment in the reverse direction between EagI and BglII sites downstream of the anhydrotetracycline (ATc) inducible promoter in pMX6. As for generating the expression plasmid of arlS asRNA (named pMX6-arlS), a ∼120 nt sequence downstream from the start codon of the arlS gene was amplified.
To overexpress spx in S. aureus strains, the coding sequence of S. aureus spx gene were amplified with the primers SA-spx-RBS-F and SA-spx-RBS-R (Supplementary Table 1), and was inserted between the BamHI and EcoRI sites downstream of the cadmium chloride (CdCl2) inducible promoter of the shuttle plasmid pCN51, generating pCN51-spx.
The sequences of all the asRNA expression plasmids and gene overexpression plasmids were verified by DNA sequencing. Then each plasmid was transformed into S. aureus strains by electroporation.
Detection of bacterial growth curves
The growth curves of the S. aureus strains were determined by measuring the optical density at a wavelength of 600 nm using an automated growth curve detector (Bioscreen C, Finland). Briefly, overnight cultures were diluted (1:200) and incubated at 37°C with shaking at 220 rpm. The OD600 of the bacterial culture was measured at 1 h intervals for 24 h.
Antimicrobial susceptibility testing
Susceptibility of S. aureus strains to various antibiotics was detected by the broth microdilution method and the disk-diffusion method according to the guidelines of American Clinical and Laboratory Standards Institute (CLSI). To determine the minimal inhibitory concentrations (MICs) of the antibiotics for S. aureus, two-fold dilutions of antibiotics in 96 wells microplates containing Mueller-Hinton broth were made to concentrations from 256 to 0.125 mg/L. Overnight cultures of the bacteria were adjusted to the 0.5 McFarland standards and inoculated 1:200 into the MH broth (for oxacillin, MHB + 2% NaCl) and then incubated at 35°C for 24 h. The lowest concentration inhibiting visible growth of the bacteria was recorded as MIC.
Visualization of biofilm formation by confocal laser scanning microscopy
Overnight cultures of S. aureus strains were diluted with TSB supplemented with 1% glucose at a ratio of 1:200, then inoculated in cell culture dishes (23 mm diameter) with glass bottoms (FluoroDish, WPI, Florida, USA) and incubated at 37°C for 24 h. After removal of non-adhered cells, the biofilms were washed with phosphate-buffered saline, stained with a Live/Dead BacLight Viability Kit (Molecular Probes, Eugene, Oregon, USA), and subsequently analyzed with a Leica confocal laser scanning microscope (TCS SP8; Leica, Heidelberg, Germany) detecting the fluorescence intensities of SYTO9 and propidium iodide (PI). A series of images were acquired at 1 μm intervals in the Z section to measure the biofilm thickness. IMARIS 9.0 software (Bitplane, Zurich, Switzerland) was used to generate three-dimensional view of the biofilms.
To evaluate the effects of the antibacterial agents on 24-h biofilms, three biofilm-forming MRSA strains (strain USA300, strain 234 and strain 15098) were selected and cultured as described above for 24 h. After removal of the suspension cultures, four-fold MIC concentrations of the agents in fresh media were added and incubated at 37°C for an additional 24 h. The biofilms were then stained with the Live/Dead BacLight Viability Kit (Viable cells in the biofilms exhibited green fluorescence, and dead cells exhibited red fluorescence). The effects of the agents on 24-h biofilms were analyzed by visualizing three-dimensional biofilm structures with CLSM and calculating the ratio of dead/live bacterial cells with ImageJ software (Wayne Rasband, NIH, Bethesda, MD, USA).
Expression and purification of recombinant ArlR and ArlS’
To construct the recombinant S. aureus ArlR and ArlS’ (histidine kinase domain of ArlS) expression plasmids (pET-SAarlR and pET-SAarlS), the DNA fragments were amplified from the genomic DNA of USA300 TCH1516 strain by PCR with the primers REarlR-f/REarlR-r and RarlSHK-f/RarlSHK-r (Supplementary Table 1), digested with XbaI/XhoI and NcoI/XhoI respectively, and inserted into a pET-28a(+) plasmid [Citation29] at the corresponding sites. After transformation into BL21 (DE3), the bacteria were cultured in LB medium at 37°C for 4 h and incubated for another 12 h at 25°C with 0.4 mM isopropyl-1-thio-b-D-galactopyranoside. The cells were harvested, disrupted using sonication in lysis buffer (50 mM Tris-Cl and 300 mM NaCl, pH 8.0), and then centrifuged at 15,000 g for 15 min at 4°C. The recombinant His-tagged ArlR protein (rArlR) and histidine kinase domain of ArlS (ArlS’) in the supernatants were purified using affinity chromatography with a Ni-nitrilotriacetic acid column (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s protocol.
Inhibition assay for ArlS kinase activity
The inhibitory activity of oritavancin on the ATPase activity of the ArlS’ protein was measured using the Kinase-GloTM Luminescent Kinase Assay (Promega, Madison, WI, USA). The reactions were carried out in solid black, flat-bottomed 96-well plates. Briefly, 3 μg purified ArlS’ protein was pre-incubated with a series of dilutions of oritavancin in reaction buffer [40 mM Tris (pH 7.5), 20 mM MgCl2 and 0.1 mg/ml BSA] at 4°C for 30 min, then 4 μM ATP was added and incubated for 30 min at room temperature. Afterwards, an equal volume of the Kinase-GloTM Reagent was added to each well, mixed and kept at room temperature for 10 min before the final recording of the luminescence (RLU) with a Victor X5 Multilabel Plate Reader (PerkinElmer, Boston, Massachusetts, USA). The rate of inhibiting ATPase activity by oritavancin was calculated by the following formula:
Checkerboard dilution assay
To detect if oritavancin and oxacillin shows synergism against MRSA strains, a synergism assay was performed using the checkerboard method [Citation32]. Briefly, in order to prepare a range of drug concentrations which allows detection of antagonism, indifference/additive, and synergism, a series of twofold dilutions of oritavancin and oxacillin in 96-well microplates starting from the concentration of double the MIC were made to obtain combination of varying concentrations of the two antibiotics. Bacteria were prepared according to broth microdilution assay. The microplates were incubated overnight at 35°C and MIC was read as the least dilution without any turbidity. The combinational effect of the two antibiotics was defined according to the fractional inhibitory concentration (FIC) index, whereby FIC = MIC of oritavancin in combination/MIC of oritavancin alone + MIC of oxacillin in combination/MIC of oxacillin alone. The combination is considered synergistic when the FIC index is ≤0.5. Indifference was indicated by a FIC index >0.5 to ≤4, while antagonism when the FIC index is >4.
RNA extraction and quantitative real-time (qRT)-PCR
For RNA extraction, the S. aureus strains were cultured at 37 °C with shaking for 4 or 10 h. The cell pellets were collected and washed with ice-cold normal saline and then homogenized using 0.1-mm Zirconia-silica beads in a Mini-BeadBeater-16 (Biospec, Bartlesville, USA) at a speed of 3,600 rpm for 1 min following cooling on ice for 1 min. This homogenization and cooling cycle were repeated five times, then the samples were centrifuged at 13,000 rpm and the bacterial RNA in the supernatant was purified using a RNeasy Mini kit (Qiagen) and quantified using an ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, USA). RNA samples that had a 260/280 ratio between 2.0 and 2.2 were reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad) following the manufacturer’s protocol. The mRNA levels were quantified by using qRT-PCR with SYBR green PCR reagents (Takara, Japan) and the primers listed in Supplementary Table 3, using the housekeeping gene gyrB as an endogenous control. The amplification efficiency of all primer pairs was determined according to the standard curve with four magnitudes of templates. The specificity of primer pairs was determined with melting curve. All the qRT-PCR experiments were carried out in triplicate and the relative gene expression data were analyzed using the 2−△△CT method [Citation33].
Promotor-reporter assay
To detect mgrA (locus tag: USA300HOU_0709) and spx expression in S. aureus strains, a green fluorescent protein -reporter assay was performed by using the shuttle vector pCM29 [Citation28]. A fragment containing the putative mgrA promoter or spx promoter region was amplified from the genomic DNA of USA300 TCH1516 strain using corresponding primers listed Supplementary Table 1. The PCR products were digested with NheI and BamHI, and subsequently ligated to upstream of the GFP gene in pCM29 to generate the plasmids pCM29-mgrA and pCM29-spx respectively. Each plasmid was transformed into E. coli DC10B, then into S. aureus USA300 TCH1516 and ΔarlRS strains by electroporation. To monitor the mgrA and spx expression, S. aureus strains containing pCM29-mgrA or pCM29-spx were cultivated in TSB at 37°C with shaking respectively, and bacterial cultures were collected at different time points. After centrifugation, the pellets were washed three times with normal saline, resuspended and adjusted to OD600 = 1.0. The bacterial suspension was transferred to a black 96-well microplate and the fluorescence intensity was measured using a Victor X5 multilabel plate reader (PerkinElmer, Inc., USA) with excitation at 480 nm and emission at 515 nm. Values from quadruplicate wells were averaged, and the experiment was repeated at least once.
Electrophoretic mobility shift assay (EMSA)
To analyze binding of the recombinant rArlR and the promoter regions of spx, EMSA were performed using the DIG Gel Shift Kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, rArlR was phosphorylated prior to gel shift reaction by incubating ArlR with 50 mM acetylphosphate for 1 h. Meanwhile, the DNA fragment upstream spx (Pspx) were amplified with corresponding primers listed in Supplementary Table 1 and linked with digoxin-labelled dd-UTP respectively. The resulting DNA fragment, DIG-Pspx was used as a probe which was loaded with increasing amounts of rArlR (0, 0.5, 1, 1.5 and 2 μg). The digoxin-labelled DNA fragment upstream arlR (ParlR) was used as a control. All samples were incubated at 25°C for 30 min. After electrophoresis on 6% non-denaturing polyacrylamide gel, the DNA fragments were transferred to positively charged nylon membranes (GE Healthcare Life Sciences, Pittsburgh, PA, USA) by electro-blotting and detected by an enzyme immunoassay following the manufacturer’s instructions.
Results
Effect of arlRS mutation in MRSA USA300 and USA500 strains on their susceptibility to oxacillin
The methicillin-resistant S. aureus USA300 FPR3757 strain (GenBank Accession Number: NC_007793) showed high resistance to oxacillin (MIC = 64 mg/L), while its isogenic mutant with transposon insertion in either arlS gene or arlR gene showed 4-fold to 8-fold decrease in MIC to oxacillin (MIC = 8 mg/L or 16 mg/L, Supplementary Table 2).
To investigate the effect of ArlRS two-component system on oxacillin resistance in MRSA, an arlRS genes knockout mutant strain was constructed by homologous recombination using USA300 TCH1516 (GenBank Accession Number: NC_010079) as a parent strain, designated USA300ΔarlRS. The arlRS complementation strain PCN-arlRS was constructed by introducing a plasmid-expressing arlRS into the mutant. The wild type, mutant and complementation strains showed no obvious difference in bacterial growth in MHB + 2% NaCl at 35°C ((a)). However, compared to the wild type strain, USA300ΔarlRS showed much higher susceptibility to oxacillin, as detected by disk-diffusion method (zone diameter = 20 mm vs 0 mm, shown in (b)) and broth microdilution method (MIC = 64 mg/L vs 4 mg/L, shown in ). Similarly, knocking out arlRS in MRSA USA500 strain 2395 (GenBank Accession Number: CP007499) resulted in much lower MIC to oxacillin (WT: 64 mg/L; USA500ΔarlRS: 8 mg/L). The altered phenotype was restored by arlRS gene complementation in both USA300ΔarlRS strain and USA500ΔarlRS strain ().
Figure 1. Effect of arlRS knockout on bacterial growth and oxacillin resistance of S. aureus USA300 strain. (a) Growth curves of S. aureus USA300 TCH1516 and its isogenic arlRS mutants. Bacterial strains were cultured in MHB + 2% NaCl at 35°C with shaking and the OD600 values were measured hourly using an automated growth curve detector. The experiments were repeated three times and a representative set of growth curves is shown. ΔarlRS- the arlRS knockout mutant; PCNarlRS −ΔarlRS complemented with plasmid expressing-arlRS; PCN −ΔarlRS transformed with an empty plasmid. (b) Oxacillin susceptibility of S. aureus USA300 TCH1516 and its isogenic arlRS mutants, detected by the Disk-diffusion method according to the CLSI guidelines.
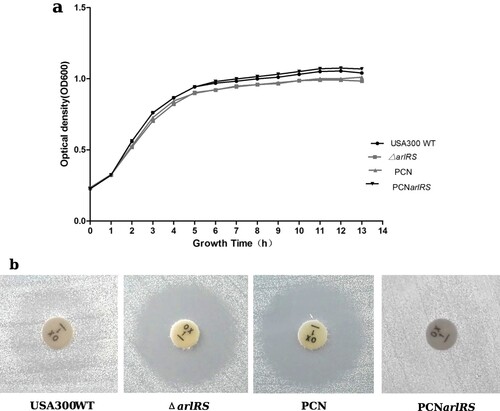
Table 2. The MICs of oxacillin for different S. aureus strains (the broth microdilution method).
Effect of arlRS silence in clinical MRSA isolates on their susceptibility to oxacillin
In order to investigate the influence of ArlRS on oxacillin resistance in other clinical MRSA strains, the anti-sense RNA (asRNA) expression plasmids of arlR and arlS (pMX-arlR, pMX-arlS) were constructed and transformed to 6 clinical MRSA isolates, using the empty vector pMX6 as a negative control. The transformation was succeeded in three isolates with high oxacillin resistance (MICs ranging from 64 mg/L to 128 mg/L). With 250 ng/ml ATc induction, the three strains containing pMX-arlR or pMX-arlS showed in 2–4-fold decrease in MIC to oxacillin, while no obvious change was found in the vector control (Supplementary Table 3).
Influence of arlRS knockout on expression of β-lactam resistance related genes
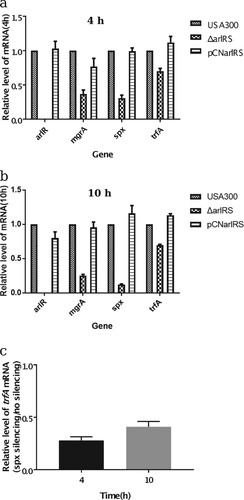
To define the arlRS regulon and the role of ArlRS in oxacillin resistance in MRSA, total RNAs from the wild-type USA300 TCH1516 strain and arlRS knockout mutant USA300ΔarlRS in mid-log growth phase (4 h) and stationary phase (10 h) were extracted, and transcriptional levels of β-lactam resistance related genes were determined by the RNA-Seq (). The mRNA levels of two transcriptional regulator genes, spx (locus tag: USA300HOU_0955) and mgrA (locus tag: USA300HOU_0709), and a cell wall antibiotic resistance-related gene, trfA (locus tag: USA300HOU_0956) in USA300ΔarlRS were found to be down-regulated 4-fold, 3.7-fold and 1.3-fold respectively at 4 h, and 6.3-fold,5-fold and 1.3 fold respectively at 10 h, while no significant change was detected in transcriptional levels of other genes including mecA and blaZ.
Table 3. Comparison of the transcription level of genes related to β-lactam antibiotics resistance in USA300 and ΔarlRS.
To confirm the RNA-Seq results, two different methods were carried out, including quantitative RT–PCR and the promoter-reporter system. The qRT-PCR result showed that in USA300ΔarlRS spx, mgrA and trfA transcription decreased 3.1-fold, 2.6-fold and 1.4-fold respectively at 4 h and decreased 6.1-fold, 4.3-fold and 1.4-fold at 10 h, while in the arlRS complementation strain those genes expression was restored ((a,b)). Besides, by using promoter-GFP reporter assay, the intensity of GFP fluorescence indicating either spx or mgrA expression, was lower in USA300ΔarlRS, compared to that in the wild type strain (Supplementary Figure 1).
Figure 2. Effect of arlRS knockout on transcription of spx, mgrA and trfA (qRT-PCR). The mRNA in the S. aureus wild type strain (USA300), arlRS knockout mutant (ΔarlRS) and complementation strain (PCNarlRS) was extracted from 4 h (a) and 10 h (b) cultures. The transcription levels of arlR, mgrA, spx and trfA were quantified by qRT-PCR with SYBR green PCR reagents and the primers listed in Supplementary Table 3. The mRNA in the S. aureus USA300-pMX-spx was extracted from 4 and 10 h under no ATc induction and 250 ng/ml ATc induction conditions. The transcription levels of trfA was quantified by qRT-PCR (c). The relative expression levels of the genes are represented as mean ± standard deviation.
Effect of spx and mgrA on MRSA susceptibility to oxacillin
To investigate the effect of spx and mgrA on oxacillin resistance in MRSA, a spx silence strain (USA300-pMX-spx) and an mgrA knockout mutant (USA300ΔmgrA) were constructed, using USA300 TCH1516 as a parent strain. In MHB + 2% NaCl medium, both USA300-pMX-spx and USA300-pMX6 (an empty vector control) strains had an oxacillin MIC of 64 mg/L; when 250 ng/ml ATc was added in the medium, the control strain USA300-pMX6 had an oxacillin MIC of 32 mg/L, while USA300-pMX-spx showed a significant decrease in oxacillin MIC (below 0.25 mg/L, ). As a control, when 250 ng/ml ATc was present but oxacillin was absent in the medium, the OD600 value of USA300-pMX-spx culture was the same as that of USA300-pMX6 culture. Similar results were obtained in the spx silence strain of USA500. Meanwhile, the mgrA knockout mutant USA300ΔmgrA showed a 2 two-fold decrease in oxacillin MIC, compared to the wild type strain (32 mg/L vs. 64 mg/L).
To further investigate the role of spx in oxacillin susceptibility in the arlRS mutant, the cadmium chloride (CdCl2) inducible spx gene overexpression plasmid pCN-spx was constructed and introduced into USA300ΔarlRS. The MIC to oxacillin in USA300ΔarlRS containing pCN-spx was similar to that of the mutant when there was no CdCl2 in the culture medium, while it was restored to wild strain level by overexpressing spx when 2 μM CdCl2 was present for induction ().
Regulation of spx expression by ArlRS
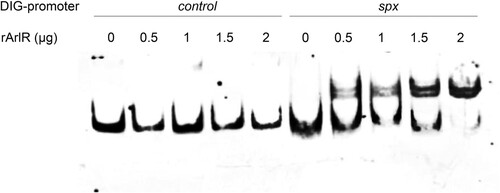
To investigate whether ArlRS regulates spx expression directly, EMSA were performed with a digoxin labelled 146-bp DNA fragment upstream of spx (Pspx, 263-bp to 118-bp upstream of the start codon of spx) and recombinant the DNA binding protein ArlR (rArlR). As shown in , when 0.5 μg to 2 μg rArlR was present, it bound to Pspx and formed a DNA–protein complex, shifting Pspx behind (lane 6 to lane 10) in a dose-dependent manner, compared to Pspx alone (lane 1). However, as a control, up to 2 μg rArlR did not shift a 150-bp DNA fragment upstream of arlR (ParlR) behind (lane 2 to lane 5).
Figure 3. Binding of rArlR to the spx promoter region (EMSA). The promoter regions of spx and arlR (as a control) were amplified and labelled with digoxin (DIG). Lanes 1–5 were loaded with 0.4 ng DIG-arlR promoter region and increasing amounts of recombinant ArlR (0, 0.5, 1, 1.5, 2 μg respectively). Lanes 6–10 were loaded with 0.4 ng DIG-spx promoter region and increasing amounts of recombinant ArlR (0, 0.5, 1, 1.5, 2 μg respectively). The DIG-labelled DNA fragments were transferred to positively charged nylon membranes and visualized by an enzyme immunoassay using anti-Digoxigenin-AP, Fab-fragments and the chemiluminescent substrate CSPD. Chemiluminescent signals were recorded on X-ray film.
Inhibition of ArlS’ kinase activity
In order to screen for compounds that can block the histidine kinase ArlS autophosphorylation and subsequent signal transduction, the recombinant cytoplasmic domain of ArlS protein (ArlS’) which contains the HATPase_c domain was purified and its activity to hydrolyze ATP was confirmed by the Kinase-Glo™ Luminescent Kinase Assay (Supplementary Figure 2a). By screening in the FDA-approved drugs library, oritavancin diphosphate, was found to inhibit the kinase activity of ArlS’ (3 μg) by 98% at a concentration of 25 μM. A dose-dependent inhibition was further detected and the IC50 value of oritavancin was 5.47 μΜ, calculated with the logistic regression fit (Supplementary Figure 2b).
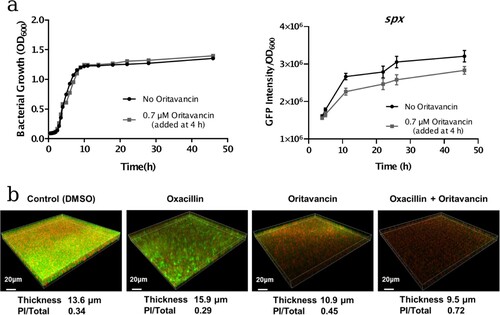
To investigate the effect of oritavancin on spx expression, the spx promoter-GFP reporter assay was carried out. The USA300-Pspx strain was cultivated to mid-log phase (4 h), then oritavancin was added to the culture to a concentration of 0.7 μM and no antibiotic treated culture was used as a control. Afterwards, the cell densities and GFP fluorescence intensities were monitored at different time points during cultivation. It showed that during 42 h cultivation, spx expression (indicated with the intensity of GFP fluorescence normalized with OD600 value) in oritavancin treatment group was lower than that in the control group ((a)).
Figure 4. Effects of oritavancin on spx expression and oxacillin susceptibility of USA300 in mature biofilms. (a) The effect of oritavancin on spx expression was detected with spx promoter-GFP reporter assay: the USA300-Pspx strain was cultivated to mid-log phase (4 h, OD600 ∼0.6), then oritavancin was added to a final concentration of 0.7 μM. The cell densities and GFP fluorescence intensities were monitored at different time points during cultivation. no antibiotic treated culture was used as a control. The spx expression is indicated with the intensity of GFP fluorescence normalized with OD600 value. (b) Biofilms of the MRSA USA300 strain TCH1516 were grown in fluorodishes at 37°C for 24 h. Afterwards, the planktonic cells were removed and fresh TSB containing 0.1% DMSO (control), 32 mg/L oxacillin, 3.1 μM oritavancin, or 32 mg/L oxacillin plus 3.1 μM oritavancin was added and incubated at 37°C for another 24 h. The mature biofilms were stained with SYTO9 and propidium iodide (PI) and observed under a Leica TCS SP8 CLSM with a 63× 1.4-NA oil-immersion objective. The viable cells were stained with green fluorescence while the dead cells with red fluorescence. Images of three-dimensional biofilm structure were constructed using IMARIS 9.0 software and the thicknesses of the biofilms were shown. The fluorescence intensities of SYTO9 and PI were determined by using Image J software, and ratio of PI/Total (PI + SYTO9) were calculated.
Effect of oritavancin on the oxacillin susceptibilities of the MRSA strains in planktonic state and mature biofilms
To detect if oritavancin enhances oxacillin susceptibilities in MRSA strains in planktonic state, a synergism assay was performed using the checkerboard method. Firstly, the MICs of oritavancin and oxacillin were detected and confirmed in USA300, USA500 strains and three clinical MRSA isolates; then the combinational effect of the two drugs was determined and the fractional inhibitory concentration (FIC) index was calculated. The FIC index varied from 0.375 to 0.5 in five MRSA strains, all defined as synergism effects ().
Table 4. Effects of combination of oritavancin and oxacillin against MRSA strains.
As S. aureus biofilm formation is associated with antibiotic resistance, we further investigated the effect of oritavancin on oxacillin susceptibilities of bacterial cells in biofilms. The MRSA strain USA300 was inoculated in the fluorodishes and incubated for 24 h to form mature biofilms. Afterwards, 0.1% DMSO (control), 32 mg/L oxacillin, 3.1 μM oritavancin, 32 mg/L oxacillin plus 3.1 μM oritavancin was added respectively and incubated for another 24 h. After Live/Dead staining with SYTO9 and propidium iodide (PI), the thicknesses of mature biofilms and viability of embedded cells of MRSA were detected by Confocal Laser Scanning Microscopy. It showed that in control group, oxacillin group, oritavancin group and drug combination group, the average thickness of biofilm was 13.6, 15.9, 10.9 and 9.5 μm, respectively, while the ratio of dead cells in the treated mature biofilms indicated with PI/(PI + SYTO9) fluorescence intensity was 0.34, 0.29, 0.45 and 0.72, respectively ((b)).
Discussion
In this work, we find that the two-component signal transduction system ArlRS regulates susceptibility to oxacillin in MRSA strains USA300, USA500 as well as three clinical isolates. Furthermore, oritavancin diphosphate was found to inhibit the kinase activity of ArlS’ and have synergy effect on antibacterial activities of oxacillin against the MRSA strains.
Transposon insertion mutation in USA300 arlR or arlS gene, arlRS knockout in USA300 and USA500, silencing arlR or arlS in MRSA isolates, all result in increased sensitive to oxacillin, suggesting an important role of arlRS in regulating oxacillin resistance in MRSA. Transcriptional profiles analysis of USA300 and its arlRS knockout mutant ΔarlRS shows no significant difference in mRNA levels of the genes that have been reported to be related to β-lactam antibiotics resistance, except mgrA and fmtB. MgrA is a global transcriptional regulator, which has been found to negatively regulate autolysis genes and affect expression of virulence genes in S. aureus [Citation34–36]. MgrA acts as a direct activator for abcA, a gene encoding an ATP-dependent transporter, which is related with cell wall autolysis and β-lactam antibiotics resistance [Citation37]. Besides, mgrA deletion in S. aureus ISP794 strain resulted in a decrease in oxacillin MIC from 4 to 2 mg/L [Citation38]. ArlRS has been reported to modulate mgrA expression. However, the results from previous reports and in the present study indicate mgrA is not the major factor affecting oxacillin susceptibility in the arlRS mutant. First, although mgrA may affect cell wall autolysis, Memmi G et al. have demonstrated that inactivation of arlRS does not play a role in autolysis of MRSA strains including USA300 [Citation16]. Meanwhile, in the present study, abcA expression shows no significant change in arlRS mutant, compared to USA300 wild-type strain. Furthermore, we find that mgrA knockout in USA300 only results in a slight reduction of MIC for oxacillin (from 64 mg/L to 32 mg/L), while knockout arlRS in USA300 leads to a much lower MIC value (4 mg/L). There is limited information about another oxacillin resistance gene fmtB, which codes for a ∼263 kDa cell wall-anchored protein. Komatsuzawa H et al. have found that transposon insertion mutation in fmtB of MRSA strain COL is indirectly linked with loss of oxacillin resistance, which cannot be restored by trans-complementation with fmtB in fmtB mutants but can be restored by overexpressing its downstream glmM gene [Citation39]. In this work, however, the glmM expression showed no difference in the USA300 and ΔarlRS strains. The increased fmtB mRNA levels in ΔarlRS can be explained by the decreased mgrA expression, since MgrA represses fmtB transcription [Citation34].
For the first time, we demonstrate that spx, which has not been reported to be associated with oxacillin susceptibility before, plays a great role in ArlRS mediated oxacillin resistance. First, the mRNA level of spx gene is dramatically decreased in the ΔarlRS, compared to that in USA300. Besides, overexpressing spx in ΔarlRS restored the oxacillin resistance to a similar level in USA300 (MIC = 64 mg/L). Furthermore, the response regulator ArlR can direct regulate spx transcription by binding to its promoter region ( and Supplementary Figure 3). More importantly, silencing spx results in a dramatic increase of oxacillin susceptibility in both USA300 and USA500 strains (MIC <0.25 and 0.125 mg/L respectively). Spx is first reported in Bacillus subtilis as a global regulator of genes that is induced by disulfide stress, through a unique mechanism that requires direct interaction with the subunit of RNA polymerase but not with DNA [Citation40]. Spx is highly conserved in low G + C Gram-positive bacteria such as Staphylococcus, Listeria, Enterococcus, and Streptococcus [Citation41–43]. However, the function of spx in Staphylococcus has not been intensively studied. Recent works tend to recognize spx as an essential gene in both S. aureus and S. epidermidis [Citation41,Citation42], although it can be knocked out in B. subtilis. Pamp SJ et al. have reported construction of a spx deletion mutant in the S. aureus 8325-4 strain, and find inactivation of spx makes the cells highly susceptible to multiple stresses including high and low temperature, salt stress, and hydrogen peroxide [Citation42]. Villanueva M et al. have discovered recently by deep sequencing that the Δspx strain harbours suppressor mutations that allowed it to grow without spx [Citation44]. Our group has tried to construct a spx deletion mutant of USA300 but cannot achieve. Thus, we use antisense RNA technique to conditionally silence spx expression in USA300 and USA500 with ATc. Compared to no ATc induction condition, 250 ng/ml ATc induced spx antisense RNA expression results in delayed bacterial growth in lag phase and log phase, and similar levels in stationary phase. Meanwhile, spx silencing leads to an over 256-fold increase in susceptibility to the oxacillin and spx overexpression can restore loss of oxacillin resistance in ΔarlRS, suggesting its important role in MRSA. There are very limited reports about the relation of Spx and antibiotic resistances. Jousselin A et al. have found Spx modulates the expression of trfA gene involved in glycopeptide resistance in S. aureus [Citation45]. The trfA encodes teicoplanin resistance factor A, which closely resembles an adaptor protein of Listeria monocytogenes and B. subtilis. Deletion of trfA and trfB (ΔtrfAB) in MRSA strain NRS3 leads to increased susceptibility to oxacillin [Citation46]. Our qRT-PCR data is consistent with the finding, for the transcription level of trfA is decreased in the ΔarlRS by ∼1.4 fold and is restored in arlRS complementation strain. In the spx silencing strain, the mRNA levels of trfA at 4 and 10 h were decreased by 3.6-fold and 2.5-fold ((c)). Furthermore, the CdCl2 inducible trfA gene overexpression plasmid pCN-trfA was constructed and transformed to USA300ΔarlRS. The MIC to oxacillin in USA300ΔarlRS containing pCN-trfA was similar to that in the mutant when there was no CdCl2, while it was partially restored by overexpressing trfA when 2 μM CdCl2 was present (). These results indicate that trfA plays a role in arlRS and spx mediated oxacillin susceptibility. Besides, Göhring N et al. show that inactivation of yjbH, which encodes a Spx-interacting protein governing Spx proteolytic degradation, led to moderate resistance to oxacillin in S. aureus [Citation47]. However, no change in yjbH mRNA level was found in ΔarlRS in our work. Thus, the role of spx in regulating oxacillin resistance warrants further investigations, e.g. whether spx mediates oxidative stress responses to hydroxyl radicals produced by bactericidal antibiotics.
The important role of ArlRS in regulating oxacillin resistance in MRSA strains indicates that this two-component system is a potential target for antimicrobial resistance breaker drugs, which may enhance the antibacterial activity of oxacillin. Bacterial two-component systems (TCSs) have been demonstrated to be promising targets [Citation48–50], not only because they are vital to bacterial survival, virulence and antibiotic resistance, but also because their homologues have not been identified in humans. Novel inhibitors have been developed against several bacterial TCSs, including Enterococcus faecium VanSR (vancomycin resistance) [Citation51], Staphylococcus epidermidis WalKR (bacterial growth and biofilm formation) [Citation52], E. coli QseCB (virulence) [Citation53] and Mycobacterium tuberculosis DosRST (persistence) [Citation54]. Most of the inhibitors are screened or designed against histidine kinase (HK) CA (HATPase_c) domain because of its conserved features and its essential role in TCS signal transduction [Citation55]. In this work, by screening in the FDA approved drugs library, we have found that oritavancin (diphosphate) can inhibit the ATPase activity of the recombinant histidine kinase ArlS CA domain. Oritavancin is a semisynthetic lipoglycopeptide analogue of vancomycin, which has multiple mechanisms of action against exponentially growing S. aureus cells, including the inhibition of cell wall synthesis and RNA synthesis, disruption of membrane potential and increasing membrane permeability [Citation56,Citation57]. Our finding suggests that inhibition of ArlS kinase activity by oritavancin thereby interfering the signal transduction may be another mechanism of action against MRSA, because ArlRS modulates oxacillin resistance. This may be one of the reasons for the fact that oritavancin has synergy effect on antibacterial activities of oxacillin against the MRSA strains (). Furthermore, CLSM observation showed that ArlRS regulated MRSA biofilm formation (Supplementary Figure 4): compared to the parent strain USA300, USA300ΔarlRS formed thinner biofilm layers (10.32 μm VS. 17.83 μm), which was restored by arlRS complementation (PCNarlRS, 17.8 μm) but not by the empty vector (PCN, 11.26 μm). This may give a possible explanation for that oritavancin treatment results in disruption of mature MRSA biofilms and facilitates bactericidal activity of oritavancin and oxacillin against embedded S. aureus cells (). Our findings are consistent with the report by Belly A et al. that oritavancin is active against biofilm S. aureus cells in vitro [Citation58].
In conclusion, our work demonstrates the two-component system ArlRS plays vital roles in regulating oxacillin susceptibility via direct modulation of spx expression and in regulating biofilm formation in MRSA strains. ArlRS is an attractive target for breaking oxacillin resistance of MRSA in planktonic state and in mature biofilms.
Supplemental Material
Download PDF (760.3 KB)Acknowledgements
We thank Prof. Ying Zhang (John Hopkins University, USA) for providing the MRSA USA300 strain. We thank Prof. J.M. van Dijl (University of Groningen, Netherlands) for the gift of the plasmid pCN51.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Tong SYC, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi: 10.1128/cmr.00134-14
- Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115:642–648. doi: 10.1542/peds.2004-2300
- Roberts RB, Chung M, De Lencastre H, et al. Distribution of methicillin-resistant Staphylococcus aureus clones among health care facilities in Connecticut, New Jersey, and Pennsylvania. Microb Drug Resist. 2000;6:245–251. doi: 10.1089/mdr.2000.6.245
- Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol. 2013;303:324–330. doi: 10.1016/j.ijmm.2013.02.007
- Rybak MJ, Akins RL. Emergence of methicillin-resistant Staphylococcus aureus with intermediate glycopeptide resistance: clinical significance and treatment options. Drugs. 2001;61:1–7. doi: 10.2165/00003495-200161010-00001
- Sakoulas G, Gold HS, Venkataraman L, et al. Methicillin-resistant Staphylococcus aureus: comparison of susceptibility testing methods and analysis of mecA-positive susceptible strains. J Clin Microbiol. 2001;39:3946–3951. doi: 10.1128/jcm.39.11.3946-3951.2001
- Tesch W, Ryffel C, Strassle A, et al. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2′. Antimicrob Agents Chemother. 1990;34:1703–1706. doi: 10.1128/AAC.34.9.1703
- Ryffel C, Kayser FH, Berger-Bachi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992;36:25–31. doi: 10.1128/AAC.36.1.25
- Hackbarth CJ, Chambers HF. Blai and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1144–1149. doi: 10.1128/AAC.37.5.1144
- Rosato AE, Kreiswirth BN, Craig WA, et al. mecA-blaZ corepressors in clinical Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2003;47:1460–1463. doi: 10.1128/AAC.47.4.1460-1463.2003
- Goldstein F, Perutka J, Cuirolo A, et al. Identification and phenotypic characterization of a beta-lactam-dependent, methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 2007;51:2514–2522. doi: 10.1128/aac.00040-07
- Stranden AM, Ehlert K, Labischinski H, et al. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997
- Piriz Duran S, Kayser FH, Berger-Bachi B. Impact of sar and agr on methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:255–260. doi: 10.1111/j.1574-6968.1996.tb08394.x
- Li L, Cheung A, Bayer AS, et al. The global regulon sarA regulates beta-lactam antibiotic resistance in methicillin-resistant Staphylococcus aureus In vitro and in endovascular infections. J Infect Dis. 2016;214:1421–1429. doi: 10.1093/infdis/jiw386
- Singh VK, Schmidt JL, Jayaswal RK, et al. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int J Antimicrob Agents. 2003;21:256–261. doi: 10.1016/S0924-8579(02)00359-X
- Memmi G, Nair DR, Cheung A. Role of ArlRS in autolysis in methicillin-sensitive and methicillin-resistant Staphylococcus aureus strains. J Bacteriol. 2012;194:759–767. doi: 10.1128/JB.06261-11
- Ji Y, Yu C, Liang X. Transcriptomic analysis of ArlRS two-component signaling regulon, a global regulator, in Staphylococcus aureus. Methods Enzymol. 2007;423:502–513. DOI:S0076-6879(07)23024-1 [pii]. doi: 10.1016/S0076-6879(07)23024-1
- Luong TT, Lee CY. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology. 2006;152:3123–3131. doi: 10.1099/mic.0.29177-0
- Liang X, Zheng L, Landwehr C, et al. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J Bacteriol. 2005;187:5486–5492. DOI: 187/15/5486-a [pii]. DOI:10.1128/JB.187.15.5486-5492.2005
- Fournier B, Klier A. Protein A gene expression is regulated by DNA supercoiling which is modified by the ArlS-ArlR two-component system of Staphylococcus aureus. Microbiology. 2004;150:3807–3819. doi: 10.1099/mic.0.27194-0
- Fournier B, Hooper DC. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J Bacteriol. 2000;182:3955–3964. doi: 10.1128/JB.182.14.3955-3964.2000
- Fournier B, Klier A, Rapoport G. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol. 2001;41:247–261. DOI:mmi2515 [pii]. doi: 10.1046/j.1365-2958.2001.02515.x
- Burgui S, Gil C, Solano C, et al. A systematic evaluation of the two-component systems network reveals that ArlRS is a key regulator of catheter colonization by Staphylococcus aureus. Front Microbiol. 2018;9:342. doi: 10.3389/fmicb.2018.00342
- Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/s0140-6736(06)68231-7
- Charpentier E, Anton AI, Barry P, et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microbiol. 2004;70:6076–6085. doi: 10.1128/aem.70.10.6076-6085.2004
- Xu T, Wu Y, Lin Z, et al. Identification of genes controlled by the essential YycFG two-component system reveals a role for biofilm modulation in Staphylococcus epidermidis. Front Microbiol. 2017;8:724, doi: 10.3389/fmicb.2017.00724
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005
- Pang YY, Schwartz J, Thoendel M, et al. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J Innate Immun. 2010;2:546–559. doi: 10.1159/000319855
- Dubendorff JW, Studier FW. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J Mol Biol. 1991;219:45–59. doi: 10.1016/0022-2836(91)90856-2
- Bruckner R. Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett. 1997;151:1–8. DOI:S0378-1097(97)00116-X [pii]. doi: 10.1016/S0378-1097(97)00116-X
- Vuong C, Gotz F, Otto M. Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis. Infect Immun. 2000;68:1048–1053. doi: 10.1128/IAI.68.3.1048-1053.2000
- Martinez-Irujo JJ, Villahermosa ML, Alberdi E, et al. A checkerboard method to evaluate interactions between drugs. Biochem Pharmacol. 1996;51:635–644. doi: 10.1016/S0006-2952(95)02230-9
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262
- Crosby HA, Schlievert PM, Merriman JA, et al. The Staphylococcus aureus global regulator MgrA modulates clumping and virulence by controlling surface protein expression. PLoS Pathog. 2016;12:e1005604. doi: 10.1371/journal.ppat.1005604
- Sun F, Zhou L, Zhao B-C, et al. Targeting MgrA-mediated virulence regulation in Staphylococcus aureus. Chem Biol. 2011;18:1032–1041. doi: 10.1016/j.chembiol.2011.05.014
- Trotonda MP, Xiong YQ, Memmi G, et al. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis. 2009;199:209–218. doi: 10.1086/595740
- Villet RA, Truong-Bolduc QC, Wang Y, et al. Regulation of expression of abcA and its response to environmental conditions. J Bacteriol. 2014;196:1532–1539. doi: 10.1128/jb.01406-13
- Truong-Bolduc QC, Hooper DC. The transcriptional regulators NorG and MgrA modulate resistance to both quinolones and beta-lactams in Staphylococcus aureus. J Bacteriol. 2007;189:2996–3005. doi: 10.1128/jb.01819-06
- Komatsuzawa H, Ohta K, Sugai M, et al. Tn551-mediated insertional inactivation of the fmtB gene encoding a cell wall-associated protein abolishes methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 2000;45:421–431. doi: 10.1093/jac/45.4.421
- Zuber P. Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol. 2004;186:1911–1918. doi: 10.1128/JB.186.7.1911-1918.2004
- Kajfasz JK, Mendoza JE, Gaca AO, et al. The Spx regulator modulates stress responses and virulence in Enterococcus faecalis. Infect Immun. 2012;80:2265–2275. doi: 10.1128/IAI.00026-12
- Pamp SJ, Frees D, Engelmann S, et al. Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J Bacteriol. 2006;188:4861–4870. doi: 10.1128/JB.00194-06
- Kajfasz JK, Rivera-Ramos I, Abranches J, et al. Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol. 2010;192:2546–2556. doi: 10.1128/JB.00028-10
- Villanueva M, Jousselin A, Baek KT, et al. Rifampin resistance rpoB Alleles or multicopy thioredoxin/thioredoxin reductase suppresses the lethality of disruption of the global stress regulator spx in Staphylococcus aureus. J Bacteriol. 2016;198:2719–2731. doi: 10.1128/JB.00261-16
- Jousselin A, Kelley WL, Barras C, et al. The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob Agents Chemother. 2013;57:3283–3292. doi: 10.1128/AAC.00220-13
- Renzoni A, Kelley WL, Barras C, et al. Identification by genomic and genetic analysis of two new genes playing a key role in intermediate glycopeptide resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:903–911. doi: 10.1128/aac.01287-08
- Gohring N, Fedtke I, Xia G, et al. New role of the disulfide stress effector YjbH in beta-lactam susceptibility of Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:5452–5458. doi: 10.1128/AAC.00286-11
- Okada A, Gotoh Y, Watanabe T, et al. Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol. 2007;422:386–395. doi: 10.1016/S0076-6879(06)22019-6
- Stephenson K, Hoch JA. Developing inhibitors to selectively target two-component and phosphorelay signal transduction systems of pathogenic microorganisms. Curr Med Chem. 2004;11:765–773. doi: 10.2174/0929867043455765
- Stephenson K, Hoch JA. Two-component and phosphorelay signal-transduction systems as therapeutic targets. Curr Opin Pharmacol. 2002;2:507–512. DOI:S1471489202001947 [pii]. doi: 10.1016/S1471-4892(02)00194-7
- Macielag MJ, Demers JP, Fraga-Spano SA, et al. Substituted salicylanilides as inhibitors of two-component regulatory systems in bacteria. J Med Chem. 1998;41:2939–2945. doi: 10.1021/jm9803572
- Liu H, Zhao D, Chang J, et al. Efficacy of novel antibacterial compounds targeting histidine kinase YycG protein. Appl Microbiol Biotechnol. 2014;98:6003–6013. doi: 10.1007/s00253-014-5685-8
- Rasko DA, Moreira CG, Li D. R, et al. Targeting QseC signaling and virulence for antibiotic development. Science. 2008;321:1078–1080. doi: 10.1126/science.1160354
- Gupta RK, Thakur TS, Desiraju GR, et al. Structure-based design of DevR inhibitor active against nonreplicating Mycobacterium tuberculosis. J Med Chem. 2009;52:6324–6334. doi: 10.1021/jm900358q
- Bem AE, Velikova N, Pellicer MT, et al. Bacterial histidine kinases as novel antibacterial drug targets. ACS Chem Biol. 2015;10:213–224. doi: 10.1021/cb5007135
- Zhanel GG, Schweizer F, Karlowsky JA. Oritavancin: mechanism of action. Clin Infect Dis. 2012;54(Suppl 3):S214–S219. doi: 10.1093/cid/cir920
- Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180–2190. doi: 10.1056/NEJMoa1310422
- Belley A, Neesham-Grenon E, McKay G, et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother. 2009;53:918–925. doi: 10.1128/AAC.00766-08
