ABSTRACT
Onychomycosis is most commonly caused by dermatophytes. In this study, we examined the spectrum of phenotypically non-dermatophyte and non-Aspergillus fungal isolates recovered over a 10-year period from nails of patients with onychomycosis in Hong Kong. A total of 24 non-duplicated isolates recovered from 24 patients were included. The median age of the patients was 51 years, and two-thirds of them were males. One-third and two-thirds had finger and toe nail infections respectively. Among these 24 nail isolates, 17 were confidently identified as 13 different known fungal species, using a polyphasic approach. These 13 species belonged to 11 genera and ≥9 families. For the remaining seven isolates, multilocus sequencing did not reveal their definite species identities. These seven potentially novel species belonged to four different known and three potentially novel genera of seven families. 33.3%, 41.7% and 95.8% of the 24 fungal isolates possessed minimum inhibitory concentrations of >1 µg/mL to terbinafine, itraconazole and fluconazole, respectively, the first line treatment of onychomycosis. A high diversity of moulds was associated with onychomycosis. A significant proportion of the isolates were potentially novel fungal species. To guide proper treatment, molecular identification and antifungal susceptibility testing should be performed for these uncommonly isolated fungal species.
Introduction
Onychomycosis is most commonly caused by dermatophytes, such as Trichophyton species and Epidermophyton floccosum. Occasionally, it is associated with yeasts, such as Candida species; as well as other non-dermatophytic moulds, including mainly Scopulariopsis brevicaulis, Hendersonula toruloidea, Aspergillus species, Acremonium species and Fusarium species, which account for a few per cents to around 20% of all cases of onychomycosis in some series [Citation1–5]. Due to the recent use of molecular technologies for identification of fungi, fungal species that have never been reported to be isolated from nails, including novel fungal species, are now recognized to be causes of onychomycosis. For example, in our study on the spectrum of Exophiala infections, we described the first reported cases of onychomycosis caused by E. bergeri, E. oligosperma as well as a novel Exophiala species, E. hongkongensis [Citation6]. In another recent study, we also described another novel, onychomycosis-causing fungal species, Aspergillus hongkongensis [Citation7].
As a result of our experience on the diversity of Exophiala and Aspergillus species associated with nail infections, we hypothesized that there is a previously unrecognized spectrum of fungi associated with onychomycosis. Furthermore, this unrecognized spectrum may also include potentially novel fungal species. To test these hypotheses, we performed genotypic identification on 24 phenotypically non-dermatophyte and non-Aspergillus fungal isolates recovered from finger and toe nails of patients with onychomycosis in four hospitals in Hong Kong. All these 24 fungal isolates were difficult-to-identify by conventional microscopic examination of lactophenol cotton blue stained adhesive tape preparations of the fungal colonies. In vitro susceptibilities of these 24 strains to 11 different antifungal agents were also characterized.
Materials and methods
Patients and fungal isolates
A total of 24 phenotypically non-dermatophyte and non-Aspergillus mould isolates, recovered from nail specimens collected during October 2006 to May 2016, were sent from four different hospitals in Hong Kong. All specimens were collected, transported, handled and processed according to the guidelines by the Clinical Laboratory Standards Institute (CLSI) [Citation8]. Specifically, nails of the patients were cleaned with 70% alcohol prior to sample collection. Surface scrapings of the nails were then discarded, and deep area scrapings and debris were collected and transported in clean envelopes or sterile tubes. All subsequent work involving the processing and inoculation of the specimens was performed in a Class II biosafety cabinet in order to avoid possible environmental contamination. Moulds grown on the primary inoculation sites after direct inoculation of the nail samples on Sabouraud dextrose agar (SDA) (Oxoid, UK) supplemented with chloramphenicol (50 µg/ml) (Calbiochem, La Jolla, CA) were isolated, whereas any other mould present in other area of the culture plates were regarded as contaminants and discarded. These mould strains exhibited unrecognized morphologies and species level identification could not be confidently made by the clinical laboratories. All clinical data of the patients were collected by retrieving and analysing the patients’ hospital records. This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority. The reference strains Aspergillus flavus ATCC 204304, Aspergillus fumigatus ATCC 204305, Candida parapsilosis ATCC 22019T and Pichia kudriavzevii (synonym: Candida krusei) ATCC 6258T were obtained from the American Type Culture Collection (ATCC), USA.
Molecular characterization
The partial 28S nuclear ribosomal DNA (nrDNA) and internal transcribed spacer (ITS) region were used as the primary markers for fungal identification. The partial translation elongation factor 1α gene (tef1a) was also sequenced since it was proposed as the secondary barcode for fungi [Citation9]. In addition, partial actin gene (act) and partial β-tubulin gene (benA) sequencing was performed for Cladosporium and Penicillium strains, respectively. Extraction of fungal DNA, polymerase chain reaction (PCR) and sequencing of the partial 28S nrDNA, ITS, partial tef1a, partial act and/or partial benA for the case isolates were carried out following our previous publication [Citation6] with the primer pairs ITS1/ITS4 [Citation10], NL1/NL4 [Citation11], EF1-1018F/EF1-1620R or Al33_alternative_f/EF1-1620R [Citation9], ACT-512F/ACT-783R [Citation12] and bt2a/bt2b [Citation13], respectively. The DNA sequences obtained were then analysed by local alignment against sequences from the DDBJ/ENA/GenBank databases using BLAST for fungal identification. In addition, these DNA sequences, together with those of other closely related species accessioned in the DDBJ/ENA/GenBank databases, Q-bank [Citation14] or Biological Resource Center, National Institute of Technology and Evaluation (NBRC), Japan, were then analysed by multiple sequence alignment using MUSCLE 3.8 [Citation15]. After end-trimming, divergent or poorly aligned regions of the DNA sequences were removed using Gblocks 0.91b [Citation16,Citation17] with relaxed parameters. Tests for substitution models and phylogenetic tree reconstruction were performed by the maximum likelihood method using MEGA 6.0.6 [Citation18].
Antifungal susceptibility test
The in vitro susceptibilities against amphotericin B (Cayman Chemical, Ann Arbor, MI), anidulafungin (TargetMol, Boston, MA), caspofungin (TargetMol), micafungin (TargetMol), fluconazole (TargetMol), isavuconazole (TargetMol), itraconazole (Sigma-Aldrich), posaconazole (Sigma-Aldrich), voriconazole (TargetMol), flucytosine (TargetMol) and terbinafine (Sigma-Aldrich) (test range: 0.0156–8 mg/L for itraconazole and posaconazole; 0.0312–16 mg/L for other drugs) were determined by the microbroth dilution method according to the guidelines by the European Committee on Antimicrobial Susceptibility Testing [Citation19]. Briefly, All the drugs were dissolved in sterile dimethyl sulphoxide (Sigma-Aldrich) for the preparation of stock solutions (3.2 g/L), which were stored in polypropylene vials (Axygen Scientific, Union City, CA) at −80°C until use. For the preparation of microdilution plates, the antifungal agent stock solutions were diluted using double-strength RPMI 1640 medium (Gibco, Grand Island, NY) buffered with 3-(N-morpholino)propanesulfonic acid (MOPS) (Gibco) supplemented with 2% glucose (BDH Chemicals, UK; w/v) and for each antifungal agent a dilution series at two times the final concentrations was produced and dispensed into the flat-bottomed wells of tissue culture-treated polystyrene 96-well microdilution plates (Wuxi NEST Biotechnology, China). The microdilution plates were sealed and stored at −80°C until use. For the preparation of inoculum, conidia were harvested from fungal cultures on SDA incubated at 25°C for 5–7 days and then resuspended in 0.1% Tween 20 (Sigma-Aldrich). The conidial suspensions were then filtered using cell strainers with a pore size of 10 µm (pluriSelect, Germany) to remove large hyphal fragments. The turbidity of each of the conidial suspension was then adjusted to 0.5 McFarland standard, and the conidial suspensions were then diluted ten times with sterile distilled water before being inoculated into the wells of the microdilution plates. The inoculated plates were incubated at 25°C, 30°C or 35°C, depending on the maximum growth temperature of the strains. Test results were read on days 2, 3 and/or 6 post-inoculation, depending on the growth rate of the strains. For echinocandins, minimum effective concentration (MEC) endpoints were recorded as the lowest drug concentrations in which abnormal, short and branched hyphal clusters were observed; whereas for the other antifungal agents, minimum inhibitory concentration (MIC) endpoints yielding no visible fungal growth by eyes were recorded. Aspergillus flavus ATCC 204304, Aspergillus fumigatus ATCC 204305, Candida parapsilosis ATCC 22019T and Pichia kudriavzevii ATCC 6258T were used as quality controls.
Nucleotide sequence accession numbers
The partial 28S nrDNA, ITS, partial tef1a, partial act and/or partial benA sequences of the case isolates have been deposited to the DDBJ/ENA/GenBank databases. The nucleotide accession numbers are listed in Supplementary Table 1.
Results
Patient characteristics
The clinical characteristics of the 24 patients with phenotypically non-dermatophyte and non-Aspergillus mould isolated from nails are shown in . Except for one patient whose demographic information was not available, 15 (65.2%) of the remaining 23 patients were males while eight (34.8%) were females. The ages of the 23 patients ranged from 4 to 75 years, with a median age of 51 years. Out of the 22 patients with traceable clinical histories, 14 (63.6%) possessed predisposing underlying diseases, most frequently diabetes mellitus, hyperlipidaemia and hypertension, which may have made them more prone to the infections. Among the 18 patients with retrievable information on the nails involved, six (33.3%) and 12 (66.7%) patients had their fingernails and toenails involved, respectively. The nails affected in the other four patients were not specified.
Table 1. Cases of onychomycosis caused by non-dermatophytic, non-Aspergillus moulds reported in this study.
Molecular characterization
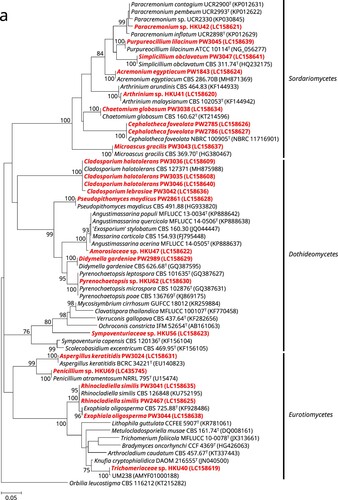
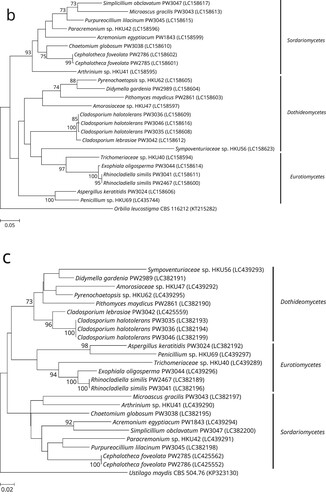
Of the 24 phenotypically non-dermatophyte and non-Aspergillus nail isolates, 16 were identified as 12 different known fungal species, namely Acremonium eqyptiacum, Aspergillus keratitidis, Cephalotheca foveolata, Chaetomium globosum, Cladosporium halotolerans, Didymella gardeniae, Exophiala oligosperma, Microascus gracilis, Pseudopithomyces maydicus, Purpureocillium lilacinum, Rhinocladiella similis and Simplicillium obclavatum, based on partial 28S nrDNA, ITS, and/or partial tef1a sequencing as well as phylogenetic analyses (). These isolates exhibited ≥99.5% (28S nrDNA) and ≥99.0% (ITS) sequence identities with their respective species (). As for the partial tef1a sequences, only six out of these 16 isolates could be successfully identified as their respective species with sequence identities of ≥99.8% (). The tef1a sequences of the other ten isolates could not be matched to their respective species because there was a lack of respective sequence data in the DDBJ/ENA/Genebank databases.
Figure 1. Phylogenetic trees showing the classification and relationship of the 24 nail isolates recovered in this study inferred from (a) partial 28S nuclear ribosomal DNA (nrDNA) (403 nucleotide positions of the trimmed sequence alignments), (b) internal transcribed spacer (ITS) region (468 nucleotide positions of the trimmed sequence alignments) and (c) partial tef1a (524 nucleotide positions of the trimmed sequence alignments) sequence data by the maximum likelihood method using the substitution models TN93 (Tamura-Nei model) + G (gamma-distributed rate variation) (28S nrDNA), K2 (Kimura 2-parameter model) + G + I (estimated proportion of invariable sites) (ITS) or TN93 + G + I (tef1a). The scale bars indicate the estimated numbers of substitutions per base. All names and accession numbers are given as cited in the DDBJ/ENA/GenBank databases. Numbers at nodes indicate levels of bootstrap support calculated from 1,000 trees and are expressed as percentage. Only nodes that were well supported (≥70% bootstrap support) have their bootstrap values shown. The 24 nail isolates were scattered across three different fungal classes (Dothideomycetes, Eurotiomycetes, and Sordariomycetes).
One (strain PW3042) of the 24 nail isolates could only be identified as a member of the Cladosporium sphaerospermum species complex using partial 28S nrDNA and ITS sequencing. BLAST analysis showed that this strain possessed ≥98.0% 28S nrDNA sequence identities to members of the Cladosporium sphaerospermum species complex, while the ITS sequence of this strain exhibited 99.6%, 99.2% and 98.6% identities to those of the ex-type strains of Cladosporium dominicanum, Cladosporium lebrasiae and Cladosporium cycadicola, respectively. In order to better resolve the species identity of strain PW3042, sequencing of an additional gene locus, partial act, was performed and the result showed that this strain possessed 97.9%, 94.8% and 89.2% identities to those of the ex-type strains of Cladosporium lebrasiae, Cladosporium dominicanum and Cladosporium cycadicola, respectively. Phylogenetic analysis using the concatenated ITS and partial act sequence showed that strain PW3042 was clustered with the ex-type strain of Cladosporium lebrasiae with high bootstrap support (Supplementary Figure 1), suggesting that it was a member of this species. Similar to the previous 16 nail isolates, the tef1a sequence of strain PW3042 could also not be matched to Cladosporium lebrasiae which was due to the lack of corresponding sequence data in the DDBJ/ENA/Genebank databases.
For the remaining seven nail isolates, their partial 28S nrDNA, ITS and tef1a sequences could not be used to reveal their definite species identities. BLAST analysis showed that strain HKU69 possessed 100% partial 28S nrDNA sequence identity to the ex-type strain of Penicillium atramentosum as well as 99.8%, 99.4% and 99.3% ITS sequence identities to the ex-type strains of Penicillium mexicanum, Penicillium magnielliptisporum and Penicillium atramentosum, respectively. This suggested that strain HKU69 is a member of Penicillium section Paradoxa; although tef1a sequencing showed that HKU69 possessed a 95.8% sequence identity to Penicillium chrysogenum strain MOS731, which belongs to section Chrysogena instead, and this might have been due to the fact that tef1a sequence data for section Paradoxa are not available in the DDBJ/ENA/GenBank databases. To further resolve the identity of strain HKU69, sequencing of an additional gene locus, partial benA, was performed and the result showed that there were 97.9%, 96.5% and 94.9% identities between the benA sequence of strain HKU69 and those of the ex-type strains of Penicillium mexicanum, Penicillium magnielliptisporum and Penicillium atramentosum, respectively. Phylogenetic analysis based on the benA sequences showed that strain HKU69 was clustered with, but distinct from Penicillium mexicanum (Supplementary Figure 2), suggesting that this strain may represent a novel Penicillium species in section Paradoxa. Similarly, phylogenetic characterization based on the ITS, partial 28S nrDNA and/or partial tef1a sequences showed that strains HKU41, HKU42 and HKU62 stood out as distinct branches in three different genera, namely Arthrinium, Paracremonium and Pyrenochaetopsis, respectively; and the three strains were the most closely related to, but distinct from, Arthrinium malaysianum, Paracremonium contagium/Paracremonium pembeum and Pyrenochaetopsis microspora, respectively (Supplementary Figures 3–5). This suggested that strains HKU41, HKU42 and HKU62 may represent novel species in these three genera. As for strains HKU40, HKU47 and HKU56, phylogenetic analyses showed that they were clustered with genera of the families Trichomeriaceae, Amorosiaceae and Sympoventuriaceae, respectively; and the three strains were the most closely related to, but distinct from, the genera Knufia, Angustimassarina and Ochroconis, respectively (Supplementary Figures 6–8). This suggested that strain HKU40, HKU47 and HKU56 may represent novel genera and species with these three families.
In vitro antifungal susceptibilities
The MICs or MECs of the 24 nail isolates against 11 antifungal agents are listed in . Excluding strain HKU56 of which the results were only read on day 3 post-inoculation as well as strains HKU40 and PW3024 of which the results were read only on day 6 post-inoculation due to their slow growth, results for the remaining 21 nail isolates were examined on both days 2 and 3 post-inoculation. Results obtained from days 2 and 3 post-inoculation were generally in congruence (≤2 log2 difference) to each other, except for strain HKU69 against isavuconazole, strains PW2861 and PW3035 against itraconazole, strains HKU47, PW2785 and PW2786 against posaconazole, strain PW2785 against voriconazole, strains PW3044 against micafungin as well as strain PW2467 against flucytosine where there were ≥3 log2 difference between the MIC/MEC results obtained on days 2 and 3 post-inoculation. Despite these, it was observed that 66.7% of all the nail isolates tested possessed low MICs against posaconaozle (<1 µg/mL); whereas 95.8% and 87.5% of the nail isolates possessed high MICs against fluconazole (≥8 µg/mL) and flucytosine (≥4 µg/mL), respectively. It was also noted that two (strains HKU42 and PW3043) of the nail isolates possessed high MICs/MECs against all the antifungal agents tested.
Table 2. Minimum inhibitory concentrations or minimum effective concentrations of the phenotypically non-dermatophyte and non-Aspergillus moulds isolated in this study against 11 antifungal agents.
Discussion
In this study, we showed that a high diversity of moulds was associated with onychomycosis. The most affected age group was 40–49 years (34.8%), followed by 50–59 years (26.1%) and 60–69 years (21.7%). This is similar to previous studies that nail infections due to non-dermatophytic moulds are the most prevalent in patients who were 40–69 years old [Citation5,Citation20]. It has been well reported that a large proportion of non-dermatophyte mould onychomycoses involve the big toes of the patients [Citation5,Citation21]. In the present study, 47.4% of the cases with information on the nails involved affected the big toes, in line with the literature. Among the 24 isolates in this study, 17 (71%) could be confidently identified to the species level using a combination of microscopic examination and DNA sequencing. These 17 isolates, representing 13 species, belonged to 11 different genera (Acremonium, Cephalotheca, Chaetomium, Cladosporium, Didymella, Exophiala, Microascus, Rhinocladiella, Pseudopithomyces, Purpureocillium and Simplicillium) and at least nine families (Cephalothecaceae, Chaetomiaceae, Cladosporiaceae, Cordycipitaceae, Didymellaceae, Didymosphaeriaceae, Herpotrichiellaceae, Microascaceae and Ophiocordycipitaceae; Acremonium egyptiacum is currently classified under Hypocreales incertae sedis with no familial assignment yet) (). It is of note that nine of these 13 species have been reported to cause nail infections [Citation6,Citation22–39] and most were also identified by DNA sequencing, although quite a number of the isolates were included in molecular epidemiology studies on specific fungal genera. However, these species in causing nail infections might have been under reported before the use of molecular identification methods. For example, four of the 17 cases in the present study were due to Cladosporium species (Cladosporium halotolerans and Cladosporium lebrasiae), which were usually reported as Cladosporium sphaerospermum species complex when the mould was identified morphologically. Similarly, one of the nail isolates recovered in this study were molecularly identified as Exophiala oligosperma and two as Rhinocladiella similis. These two species might have also been identified as Exophiala jeanselmei–Exophiala spinifera complex in previous reports. Interestingly, molecular identification revealed that one of the phenotypically non-dermatophyte and non-Aspergillus nail isolate recovered in this study (PW3024) was actually an aspergillus (Aspergillus keratitidis [synonym = Sagenomella keratitidis]). Phenotypically, Aspergillus keratitidis is a “Phialosimplex”-like fungus with simple monophialidic conidiogenous structures where conidia are produced in chains [Citation40,Citation41], which are very different from the vesiculate Aspergillus conidiophores. This fungus is also genetically related to Phialosimplex/Polypaecilum [Citation40]. Recent phylogenetic analyses demonstrated that Phialosimplex/Polypaecilum were included in the monophyletic Aspergillus sensus stricto clade [Citation42–44] and so were transferred as Aspergillus subgenus Polypaecilum [Citation44]. Following this change “Sagenomella” keratitidis, despite lacking typical Aspergillus micromorphologies, was also renamed as Aspergillus keratitidis [Citation45].
A significant proportion of the isolates were potentially novel fungal species. During our previous studies on the epidemiology of Exophiala and Aspergillus species, through sequencing multiple gene loci, in addition to the high species diversity observed, we also discovered two novel pathogenic fungi, Exophiala hongkongensis and Aspergillus hongkongensis, both recovered from patients with onychomycosis. As for the present study, among the 24 isolates recovered from nail infections, seven (29%) were potentially novel species. DNA sequencing showed that these isolates formed distinct branches on phylogenetic trees. Interestingly, these seven potentially novel species belong to four different known and three potentially novel genera of seven different families, in line with the high diversity of fungal species observed. Remarkably, a number of species closely related to these potentially novel genera and/or species are human and/or animal pathogens. For example, strains HKU41, HKU42 and HKU69 are potentially novel Arthrinium, Paracremonium and Penicillium species, respectively; and members of all these three genera have been reported as agents of mycoses [Citation46–49] In particular, Arthrinium arundinis [Citation50] and Penicillium species [Citation2,Citation51–53] have been associated with onychomycosis. Moreover, strains HKU40 and HKU56 are potentially novel genera and species in the families Trichomeriaceae and Sympoventuriaceae, respectively. Members of both these families are black yeast-like fungi and are well recognized agents of nail infections [Citation54]. It is also of note that strain HKU40 (Trichomeriaceae sp.) is closely related to a Malaysian strain UM238, which was isolated from skin scraping [Citation33]. As for strains HKU47 and HKU62, although Amorosiaceae and Pyrenochaetopsis species have not been reported to be associated with human infection, they are Phoma-like dematiaceous moulds under the order Pleosporales. Members of Pleosporales, especially Phoma species, are also known to cause nail/skin infections [Citation33,Citation55–57]. All these highlighted the potential of these novel strains in causing superficial infections, including onychomycosis.
To guide proper treatment, molecular identification and antifungal susceptibility testing should be performed for these uncommonly isolated fungal species. The first line treatment of finger or toe nail onychomycosis is oral terbinafine, itraconazole or fluconazole for months. In general, dermatophytes are susceptible to these antifungal agents. However, in the present study, it was observed that 12.5% (day 2 results [day 6 for strains HKU40 and PW3024]) or 33.3% (day 3 results [day 6 for strains HKU40 and PW3024]), 41.7% and 95.8% of the fungal isolates possessed MICs of >1 µg/mL to terbinafine, itraconazole and fluconazole, respectively. In addition, it is of note that two (HKU42 and PW1843) of the nail isolates characterized in this study possessed high MICs/MECs for most of the drugs tested. While the susceptibility results obtained in the current study for strain PW1843 was in line with those for Acremonium egyptiacum reported in a previous study where this species only possessed low MICs against terbinafine [Citation30]; susceptibility data for Paracremonium species is not available in the literature. The lack of antifungal susceptibility testing results is also the case for most of the other mould species identified in the present study. Since these fungal species are uncommonly recovered from clinical samples and data of antifungal susceptibility testing results on these species are highly limited in the literature, it would be difficult to predict the susceptibility profile of individual species even when a particular strain is identified to the species level. Unfortunately, the details of the antifungal regimen and clinical response of the patients in this study could not be retrieved for further analysis.
Declaration
Part of this work has contributed to the PhD thesis by Chi-Ching Tsang submitted to The University of Hong Kong, Hong Kong.
Supplemental Material
Download Zip (198 KB)Disclosure statement
Patrick C. Y. Woo has provided scientific advisory/laboratory services for Gilead Sciences, Incorporated; International Health Management Associates, Incorporated; Merck & Corporation, Incorporated and Pfizer, Incorporated. The other authors report no conflicts of interest. The funding sources had no role in study design, data collection, analysis, interpretation, or writing of the report. The authors alone are responsible for the content and the writing of the manuscript.
Additional information
Funding
References
- Clayton YM. Clinical and mycological diagnostic aspects of onychomycoses and dermatomycoses. Clin Exp Dermatol. 1992;17(Supplement 1):37–40.
- Ramani R, Srinivas CR, Ramani A, et al. Molds in onychomycosis. Int J Dermatol. 1993;32(12):877–878.
- Ng KP, Saw TL, Madasamy M, et al. Onychomycosis in Malaysia. Mycopathologia. 1999;147(1):29–32.
- Kaur R, Kashyap B, Bhalla P. Onychomycosis – epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26(2):108–116.
- Hwang SM, Suh MK, Ha GY. Onychomycosis due to nondermatophytic molds. Ann Dermatol. 2012;24(2):175–180.
- Woo PCY, Ngan AHY, Tsang CCC, et al. Clinical spectrum of Exophiala infections and a novel Exophiala species, Exophiala hongkongensis. J Clin Microbiol. 2013;51(1):260–267.
- Tsang C-C, Hui TWS, Lee K-C, et al. Genetic diversity of Aspergillus species isolated from onychomycosis and Aspergillus hongkongensis sp. nov., with implications to antifungal susceptibility testing. Diagn Microbiol Infect Dis. 2016;84(2):125–134.
- Clinical and Laboratory Standards Institute (CLSI). Principles and procedures for detection of fungi in clinical specimens—direct examination and culture; approved guideline. CLSI document M54-A. Wayne (IL): Clinical and Laboratory Standards Institute; 2012.
- Stielow JB, Lévesque CA, Seifert KA, et al. One fungus, which genes? Development and assessment of universal primers for potential secondary fungal DNA barcodes. Persoonia. 2015;35:242–263.
- White T, Bruns T, Lee S, et al. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Shinsky J, et al., editors. PCR protocols: a guide to methods and applications. San Diego (CA): Academic Press; 1990. p. 315–322.
- O’Donnell K. Fusarium and its near relatives. In: Reynolds DR, Taylor JW, editor. The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. Wallingford (CT): CAB International; 1993. p. 225–233.
- Carbone I, Kohn LM. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91(3):553–556.
- Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61(4):1323–1330.
- Q-bank Fungi. Q-bank Fungi Database 2017. Available from: http://www.q-bank.eu/Fungi/.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797.
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17(4):540–552.
- Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56(4):564–577.
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729.
- Arendrup MC, Meletiadis J, Mouton JW, et al. EUCAST definitive document E.Def 9.3.1. Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds 2017. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_9_3_1_Mould_testing__definitive.pdf.
- Martínez-Herrera EO, Arroyo-Camarena S, Tejada-García DL, et al. Onychomycosis due to opportunistic molds. An Bras Dermatol. 2015;90(3):334–337.
- Morales-Cardona CA, Valbuena-Mesa MC, Alvarado Z, et al. Non-dermatophyte mould onychomycosis: a clinical and epidemiological study at a dermatology referral centre in Bogota, Colombia. Mycoses. 2014;57(5):284–293.
- Naidu J, Singh SM, Pouranik M. Onychomycosis caused by Chaetomium globosum Kunze. Mycopathologia. 1991;113(1):31–34.
- Stiller MJ, Rosenthal S, Summerbell RC, et al. Onychomycosis of the toenails caused by Chaetomium globosum. J Am Acad Dermatol. 1992;26(5):775–776.
- Naidu J. Growing incidence of cutaneous and ungual infections by non-dermatophyte fungi at Jabalpur (M.P.). Indian J Pathol Microbiol. 1993;36(2):113–118.
- Hattori N, Adachi M, Kaneko T, et al. Case report. Onychomycosis due to Chaetomium globosum successfully treated with itraconazole. Mycoses. 2000;43(1–2):89–92.
- Zare R, Gams W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov.). Nova Hedwigia. 2001;73(1–2):1–50.
- Aspiroz C, Gené J, Rezusta A, et al. First Spanish case of onychomycosis caused by Chaetomium globosum. Med Mycol. 2007;45(3):279–282.
- Zeng JS, Sutton DA, Fothergill AW, et al. Spectrum of clinically relevant Exophiala species in the United States. J Clin Microbiol. 2007;45(11):3713–3720.
- Hubka V, Mencl K, Skorepova M, et al. Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Med Mycol. 2011;49(7):724–733.
- Perdomo H, Sutton DA, García D, et al. Spectrum of clinically relevant Acremonium species in the United States. J Clin Microbiol. 2011;49(1):243–256.
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25(2):232–236.
- da Cunha KC, Sutton DA, Gené J, et al. Pithomyces species (Montagnulaceae) from clinical specimens: identification and antifungal susceptibility profiles. Med Mycol. 2014;52(7):748–757.
- Yew SM, Chan CL, Lee KW, et al. A five-year survey of dematiaceous fungi in a tropical hospital reveals potential opportunistic species. PLoS ONE. 2014;9(8):e104352.
- Evans JM, Wang AL, Elewski BE. Successful treatment of Paecilomyces lilacinus onychomycosis with efinaconazole and tavaborole. Skin Appendage Disorders. 2016;1(4):169–171.
- Sandoval-Denis M, Sutton DA, Martin-Vicente A, et al. Cladosporium species recovered from clinical samples in the United States. J Clin Microbiol. 2015;53(9):2990–3000.
- Yao L, Wan Z, Li R, et al. In vitro triple combination of antifungal drugs against clinical Scopulariopsis and Microascus species. Antimicrob Agents Chemother. 2015;59(8):5040–5043.
- Shi D, Lu G, Mei H, et al. Onychomycosis due to Chaetomium globosum with yellowish black discoloration and periungual inflammation. Med Mycol Case Rep. 2016;13:12–16.
- Wen Y-M, Rajendran RK, Lin Y-F, et al. Onychomycosis associated with Exophiala oligosperma in Taiwan. Mycopathologia. 2016;181(1):83–88.
- Gherbawy YAMH, Maghraby TA, El-Sharony HM, et al. Diversity of keratinophilic fungi on human hairs and nails at four governorates in upper Egypt. Mycobiology. 2006;34(4):180–184.
- Hsieh H-M, Ju Y-M, Hsueh P-R, et al. Fungal keratitis caused by a new filamentous hyphomycete Sagenomella keratitidis. Botanical Studies. 2009;50:331–335.
- Sigler L, Sutton DA, Gibas CFC, et al. Phialosimplex, a new anamorphic genus associated with infections in dogs and having phylogenetic affinity to the Trichocomaceae. Med Mycol. 2010;48(2):335–345.
- Houbraken J, Samson R. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol. 2011;70(1):1–51.
- Samson RA, Visagie CM, Houbraken J, et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 2014;78:141–173.
- Kocsubé S, Perrone G, Magistà D, et al. Aspergillus is monophyletic: evidence from multiple gene phylogenies and extrolites profiles. Stud Mycol. 2016;85(Supplement C):199–213.
- Martinelli L, Zalar P, Gunde-Cimerman N, et al. Aspergillus atacamensis and A. salisburgensis: two new halophilic species from hypersaline/arid habitats with a phialosimplex-like morphology. Extremophiles. 2017;21(4):755–773.
- Rai MK. Mycosis in man due to Arthrinium phaeospermum var. indicum. First case report. Mycoses. 1989;32(9):472–475.
- Schinabeck MK, Ghannoum MA. Human hyalohyphomycoses: a review of human infections due to Acremonium spp., Paecilomyces spp., Penicillium spp., and Scopulariopsis spp. J Chemother. 2003;15(sup2):5–15.
- Viegas C, Sabino R, Parada H, et al. Diagnosis of Tinea pedis and onychomycosis in patients from Portuguese National Institute of Health: a four-year study. Saúde & Tecnologia. 2013;10:36–41.
- Lombard L, van der Merwe NA, Groenewald JZ, et al. Generic concepts in Nectriaceae. Stud Mycol. 2015;80:189–245.
- Dyląg M, Hryncewicz-Gwóźdź A, Jagielski T. Onychomycosis due to Arthrinium arundinis: a case report. Acta Derm Venereol. 2017;97(7):860–861.
- Lim JT-E, Chua HC, Goh CL. Dermatophyte and non-dermatophyte onychomycosis in Singapore. Australas J Dermatol. 1992;33(3):159–163.
- Hajoui FZM, Zeroual Z, Ghfir B, et al. The mould onychomycosis in Morocco: about 150 isolated cases in 20 years. J Mycol Med. 2012;22(3):221–224.
- Hainsworth S, Hamblin JF, Vanniasinkam T. Isolation of dermatophytes (and other fungi) from human nail and skin dust produced by podiatric medical treatments in Australia. J Am Podiatr Med Assoc. 2015;105(2):111–120.
- Saunte DM, Tarazooie B, Arendrup MC, et al. Black yeast-like fungi in skin and nail: it probably matters. Mycoses. 2012;55(2):161–167.
- Tullio V, Banche G, Allizond V, et al. Non-dermatophyte moulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fungal Biol. 2010;114(4):345–349.
- Ahmed SA, Hofmüller W, Seibold M, et al. Tintelnotia, a new genus in Phaeosphaeriaceae harbouring agents of cornea and nail infections in humans. Mycoses. 2017;60(4):244–253.
- Bennett A, Ponder M, Garcia-Diaz J. Phoma infections: classification, potential food sources, and their clinical impact. Microorganisms. 2018;6(3):58.