ABSTRACT
Toxoplasmosis is one of the most common zoonotic diseases in the world. Felines excrete environmentally resistant Toxoplasma gondii oocysts. However, there is no direct evidence to prove tigers are the intermediate host of T. gondii. Here, we show that, IgG antibodies to T. gondii in 80% (8/10) of captive tigers. Two viable T. gondii strains (ToxoDB genotype #9) were isolated by bioassay in mice using striated muscles of two tigers (Tiger#3 and Tiger#8). Additionally, mice were confirmed as T. gondii-positive by bioassay of feces #89–110, but no viable T. gondii strain was isolated successfully. The fecal samples from tigers may contain T. gondii oocysts. This is the first report of T. gondii isolation from tigers. These results provide direct evidence that an extra-intestinal cycle of T. gondii may develop in tigers.
Dear editor: Toxoplasmosis is one of the most common zoonotic diseases in the world. Felines specifically excrete environmentally resistant Toxoplasma gondii oocysts [Citation1]. The tiger (Panthera tigris) was listed as a threatened species by the International Union for Conservation of Nature, as only 3, 200 tigers exist in the world today [Citation2,Citation3]. Captive felids in zoos which are infected with T. gondii could be a possible source of contamination for other animals, animal caring staff and visitors. However, there is currently no direct evidence that an extra-intestinal cycle of T. gondii occurs in tigers.
In this study, from December 2016 to May 2019, fresh tiger tissues (n = 9) or serum samples (n = 2) were collected from zoos by local veterinarians and transported to the Laboratory of Veterinary Pathology of Henan Agricultural University for histopathological diagnosis on tissue samples, serological diagnosis on serum and heart juice samples. Fresh fecal samples (n = 141) from 32 individuals (four to five samples per tiger) were also collected from the individual living houses of tigers in the morning by the authors of this study (, Figure S1). The food of captive tigers included raw beef, pork, and chicken ribs. Histopathological diagnosis revealed that pneumonia, declined immune function, and reproductive disorders were the common causes of tigers death in the majority of cases (Figure S2).
Table 1. Background and isolation of Toxoplasma gondii from tigers.
In order to investigate T. gondii infection in these tigers, T. gondii antibodies were identified by the modified agglutination test (MAT) (cut off = 1:25) [Citation4]. Results revealed that T. gondii IgG antibodies were found in the heart juice or serum of eight tigers (80%) (). This indicated that most of the tigers had been infected with T. gondii. The tigers may have been infected with T. gondii after ingesting viable cysts from raw meat or oocysts from contaminated food and water. T. gondii antibodies were negative in Tiger #5 (stillborn fetus) and Tiger #10 (artificial feeding, 9 months). T. gondii was previously isolated from captive meerkats, and it was thereby speculated that the oocysts shed by captive felids or feral cats contaminated the zoo environment [Citation5].
DNA was extracted by silica membrane from the tissue samples and used to detect T. gondii by PCR using primer Tox5/8 [Citation6]. T. gondii DNA amplified products were found in the heart, tongue, diaphragm, and skeletal muscles of Tigers #1 and #3 (). Striated tissue from six T. gondii seropositive tigers were subjected to acid pepsin digestion and bioassayed in mice [Citation1]. The other three samples were stillborn fetuses or frozen tissues. Two viable T. gondii strains were obtained from seropositive tiger samples (MAT titer ≥ 1:200), this result verified the validity of MAT use on tiger samples. T. gondii antibodies and parasites were detected in mice inoculated with tissues from Tigers #3 and #8 at 61 days post-infection (DPI). Additionally, many brain tissue cysts were observed in mice inoculated with tissues from Tigers #3 (223 ± 224, 249 DPI) and #8 (7640 ± 824, 102 DPI) after euthanasia. The parasites were confirmed to be T. gondii by immunohistochemical staining (). IFN-γ knockout mice died of toxoplasmosis at 14 and 8 DPI after inoculation with samples from Tigers #3 and #8, respectively, and tachyzoites were found in smears of the lungs, mesenteric lymph nodes, and ascites. The two isolates were successfully propagated in Vero cells, TgTigerCHn1 and TgTigerCHn2 (). DNA samples extracted from T. gondii tachyzoites in cell cultures were characterized by PCR-RFLP [Citation7]. They were identified as ToxoDB#9, the predominant genotype found in China [Citation8,Citation9]. The ROP18/ROP5 genotype combination (II/II) predicated that they were non-lethal to mice [Citation10]. However, TgTigerCHn1 and TgTigerCHn2 were found to be of intermediate virulence and virulence to mice, respectively (Table S1). This result might indicate that there are still other factors related to virulence.
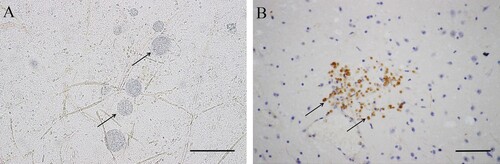
Figure 1. Morphology of T. gondii cysts and tachyzoites in brains of BALB/C mice. A. T. gondii cysts (TgTigerCHn1), 61 DPI, squash, unstained. B. T. gondii tachyzoites (TgTigerCHn2), 10 DPI, IHC stained (rabbit anti-T. gondii antibody). Bar = 50 μm.
The 141 fecal samples were divided into five groups. A bioassay was conducted on BALB/c mice. One group of mice (pool feces #89–110) was T. gondii positive (MAT ≥ 1:200). Unfortunately, this strain was not isolated successfully (). In the natural environment, felids shed T. gondii oocysts for a short period of time, and oocysts were found in only 1% of cats at any given time, according to fecal surveys conducted from 1988–2008 [Citation1]. Seropositive samples gave negative results in mice, which may be explained by the relatively low density, low cyst formation rate, or avirulence of T. gondii. Little is known about the isolation of viable T. gondii from the feces of feral felids. Two T. gondii strains were previously isolated from the feces of cougars [Citation11]. Inactivating (burn or heat) oocysts and cleaning the feces from captive felids are important strategies for controlling T. gondii infection.
To our knowledge, this is the first report of T. gondii isolation in tigers. The tigers in this study were captive and bred in zoos. Tiger blood samples were not collected at the time of capture and were not checked for T. gondii. The infection source of T. gondii may be from the meat provided by the zoos or after the ingestion of oocysts from felid feces. Eating pre-frozen meat to break down the transmission route may be the most effective method for preventing infection. These results provide direct evidence that an extra-intestinal cycle of T. gondii occurs in tigers.
Supplemental Material
Download MS Word (12 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Dubey JP. Toxoplasmosis of animals and humans. Boca Raton (Florida): CRC Press, Taylor & Francis Group; 2010; 1–313.
- Goodrich J, Lynam A, Miquelle D, et al. Panthera tigris. The IUCN Red List of Threatened Species 2015; e.T15955A50659951.
- Forrest JL, Bomhard B, Budiman A, et al. Single species conservation in a multipluse landscape: current protection of the tiger range. Anim Conserv. 2011;14:283–294. doi: 10.1111/j.1469-1795.2010.00428.x
- Dubey JP, Desmonts G. Serological responses of equids fed Toxoplasma gondii oocysts. Equine Vet J. 1987;19:337–339. doi: 10.1111/j.2042-3306.1987.tb01426.x
- Basso W, Moré G, Quiroga MA, et al. Isolation and molecular characterization of Toxoplasma gondii from captive slender-tailed meerkats (Suricata suricatta) with fatal toxoplasmosis in Argentina. Vet Parasitol. 2009;161:201–206. doi: 10.1016/j.vetpar.2009.01.006
- Reischl U, Bretagne S, Krüger D, et al. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect Dis. 2003;3:7. doi: 10.1186/1471-2334-3-7
- Su C, Shwab EK, Zhou P, et al. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137:1–11. doi: 10.1017/S0031182009991065
- Dong H, Su RJ, Lu YY, et al. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000-2017) from China. Front Microbiol. 2018;9:2108. doi: 10.3389/fmicb.2018.02108
- Chaichan P, Mercier A, Galal L, et al. Geographical distribution of Toxoplasma gondii genotypes in Asia: A link with neighboring continents. Infect Genet Evol. 2017;53:227–238. doi: 10.1016/j.meegid.2017.06.002
- Shwab EK, Jiang T, Pena HFJ, et al. The ROP18 and ROP5 gene allele types are highly predictive of virulence in mice across globally distributed strains of Toxoplasma gondii. Int J Parasitol. 2016;46:141–146. doi: 10.1016/j.ijpara.2015.10.005
- Aramini JJ, Stephen C, Dubey JP. Toxoplasma gondii in Vancouver Island cougars (Felis concolor vancouverensis): serology and oocyst shedding. J Parasitol. 1998;84:438–440. doi: 10.2307/3284508
