Article title: Chromatin remodelling factor BAF155 protects hepatitis B virus X protein (HBx) from ubiquitin-independent proteasomal degradation
Authors: Chen, H., Zhang, Y., Ye, S., Wu, Q., Lin, Y., Shen, K., Chen, W., Lin, X., & Lin, X.
Journal: Emerging Microbes & Infections
DOI: https://doi.org/10.1080/22221751.2019.1666661
This article was originally published with an error in . The correct version of the Figure 5 is shown below:
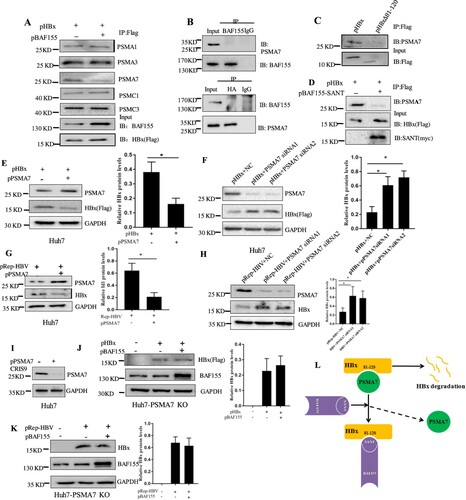
Figure 5. BAF155 stabilizes HBx through inhibition of proteasome-mediated protein degradation. (A) Coimmunoprecipitation of HBx and proteasome in the presence of proteasome inhibitor. Huh7 cells were transfected with pHBx in combination with pBAF155. 24 h after transfection, cells were treated with 20 μM MG132 for 6 h. Total cell extracts were first subjected to immunoprecipitation using anti-Flag antibody and then the immune complex was assayed by western blotting with respective antibodies. (B) Coimmunoprecipitation analysis of interaction between the BAF155 and PSMA7. (C) Coimmunoprecipitation analysis of interaction between the HBx mutant lacking aa81-120 and PSMA7. (D) Coimmunoprecipitation analysis of interaction between BAF155 SANT domain and PSMA7 in the presence of HBx. (E) Overexpression of PSMA7 decreased HBx protein levels in pHBx-transfected Huh7 cells. (F) Knockdown of endogenous PSMA7 increased HBx protein levels in the Huh7 cells transfected with pHBx. (G) Overexpression of PSMA7 decreased HBx protein levels in the Huh7 cells transfected with pRep-HBV. (H) Knockdown of endogenous PSMA7 increased HBx protein levels in the Huh7 cells transfected with pRep-HBV. (I) Knockout of PSMA7 in Huh7 cells by CRISPR/Cas9 system as examined by western blot analysis. (J and K) The protein levels of HBx in the PSMA7-knockout Huh7 (Huh7-PSMA7 KO) cells transfected with pBAF155 in combination with pHBx (J) or pRep-HBV (K). (L) Schematic models for the mechanism by which BAF155 functions to compete with PSMA7 for binding to HBx thus disrupting PSMA7 native association with HBx for HBx degradation. Values are mean ± SD, n = 3. *p < 0.05