ABSTRACT
Vancomycin-resistant enterococci infections are of great public health significance due to limited therapeutic options. We investigated epidemiological trends and risk factors of vancomycin resistance in enterococci isolates from patients with bloodstream infections in the EU/EEA from 2012 to 2018. Routine vancomycin susceptibility data of clinical E. faecium (n = 67,022) and E. faecalis (n = 103,112) blood isolates from the European Antimicrobial Resistance Surveillance Network were analysed using descriptive statistics and multivariable regression analyses. In Europe, proportions of vancomycin-resistant E. faecium (VREFm) increased from 8.1% (95%CI 6.7–9.7%) in 2012 to 19.0% (95%CI 16.8–21.5%) in 2018. Rising VREFm proportions were observed across all European regions, both genders and all age groups except children and adolescents (1–19 years). Adults (20–59 years) and elderly (≥60 years) had an increased likelihood of VREFm compared to children and adolescents (1–19 years) (OR: 1.99 [95%CI 1.42–2.79, p < 0.001] and OR: 1.56 [95%CI 1.09–2.23, p = 0.014], respectively). Inpatients hospital units, including internal medicine and ICUs, were associated with an increased likelihood of VREFm (OR: 2.29 (95%CI 1.58–3.32, p < 0.001) compared to the emergency department which reflects patients with community origin of E. faecium infections. The mean proportion of vancomycin-resistant E. faecalis in Europe was found to be low (1.1% [95%CI 0.9–1.4%]). Local and regional authorities should intensify efforts directed at diagnostic and antimicrobial stewardship for vancomycin and all last resort drugs for the management of VREFm, particularly for hospitalized elderly patients.
Introduction
Enterococcus spp. are Gram-positive bacteria that commonly inhabit the intestinal tracts of healthy humans and animals but have the potential to cause invasive infections if the delicate microbiota balance is disrupted [Citation1,Citation2]. They have adapted to colonizing and persisting in a hospital environment, allowing for easy transmission through multiple routes of cross contamination including invasive medical devices [Citation2–4]. Enterococcus faecium (E. faecium) and Enterococcus faecalis (E. faecalis) are the most frequently isolated species in nosocomial settings [Citation5]. Globally, both species are commonly associated with hospital outbreaks of bacteremia, urinary tract infections, endocarditis amongst others [Citation5,Citation6]. Such outbreaks not only result in significant economic costs to health systems, they also risk exposing vulnerable patients to potentially fatal infection [Citation3,Citation7,Citation8]. This challenge is compounded by treatment difficulties associated with the development of high-level resistance to several antibiotics that are either intrinsic or acquired through horizontal transfer of plasmids and transposons [Citation7–9]. Vancomycin-resistant E. faecium (VREFm) has been identified as the leading multidrug-resistant Enterococcus spp. in healthcare environments [Citation3,Citation10,Citation11]. Due to its clinical and public health significance, the World Health Organization (WHO) and the U.S. Centers for Diseases Control and Prevention listed VREFm as a high priority pathogen in urgent need of drug research and development [Citation12,Citation13]. The European Antimicrobial Resistance Surveillance Network (EARS-Net) reported that the mean proportion of vancomycin-resistant E. faecium in invasive isolates was 17.3% (95% CI 17–18) in 2018 compared to 10.4% (95% CI 10–11) in 2014 in countries of the European Union and European Economic Area (EU/EEA) [Citation9]. The increasing proportion of VREFm has also been documented at the country level (e.g. Germany, Italy, Slovakia, and Norway) within the EU/EEA [Citation9,Citation14,Citation15]. While human infections with vancomycin-resistant E. faecalis have been reported worldwide, E. faecalis has remained generally susceptible to vancomycin compared to E. faecium in these regions, including Europe [Citation16–22].
Despite the existing evidence, a comprehensive epidemiological picture of invasive vancomycin-resistant enterococci in Europe is lacking. In particular, it has not been systematically assessed how patient characteristics (such as age) and healthcare setting (such as intensive care) are associated with the likelihood of vancomycin resistance in E. faecium bloodstream infections. Although a general increase of VREFm is reported for Europe, it is not known which patient groups are affected by rising VREFm proportions, which is crucial for the targeted implementation of infection control and prevention programs. To deepen the understanding of the increasingly problematic enterococci infections, this study aimed to analyse epidemiological trends of vancomycin-resistant E. faecium and E. faecalis and to determine factors that are associated with an increased likelihood of vancomycin resistance in E. faecium blood isolates using EARS-Net data from 2012 to 2018.
Methods
Study design and the European antimicrobial resistance surveillance database
We conducted a retrospective observational study on E. faecium and E. faecalis (2012–2018) using data retrieved from the European Antimicrobial Resistance Surveillance Network (EARS-Net) database (TESSy). EARS-Net is a network of European surveillance systems that collects routine clinical antimicrobial susceptibility (AST) data on invasive isolates (blood and cerebrospinal fluid (CSF)) from the 27 countries in the European Union as well as Norway, Iceland and the United Kingdom [Citation13]. Detailed information about the methodology of EARS-Net is provided in the EARS-Net surveillance reports and protocols [Citation14]. In EARS-Net, E. faecium and E. faecalis isolates are classified as sensitive (S), intermediate (I), or resistant (R) to antimicrobial drugs based on the standards used in the participating laboratories, e.g. guidelines of the European Committee on Antimicrobial Susceptibility Testing (EUCAST), Clinical and Laboratory Standards Institute (CLSI) or other national guidelines. In addition to S-I-R data, individual laboratories provide further epidemiological information, such as date of specimen collection, country of origin, specimen type (i.e. blood and CSF), hospital unit (e.g. intensive care unit [ICU] or general units like internal medicine unit), patient gender and patient age.
Selection of enterococci isolates
In October 2019 we extracted 2012–2018 data for E. faecium and E. faecalis from the TESSy database with the approval of the European Centre for Disease Prevention and Control. All enterococci isolates were of bloodstream origin. The TESSy database of EARS-Net only includes the first isolate from a given patient in the respective year. To identify unique isolates, we created a composite identifier comprised of the reporting country, unique laboratory identifier, hospital identifier, patient identifier, date of sample collection and the identified pathogen. Isolates with duplicate composite identifiers and more than one AST against the same antibiotic were excluded. In the next steps, we excluded isolates that were not tested against vancomycin, were from outpatients or were not assigned a hospital ID.
Variables
Patient age was categorized into four age categories (<1, 1–19, 20–59, ≥60 years). Patient gender was classified into female or male. The country of origin of the isolate was grouped into four major regions of Europe (North: Denmark, Finland, Iceland, Ireland, Norway, Sweden, United Kingdom; West: Austria, Belgium, France, Germany, Luxembourg, Netherlands; South: Croatia, Cyprus, Greece, Hungary, Italy, Malta, Portugal, Slovenia, Spain; East: Bulgaria, Czech Republic, Estonia, Latvia, Lithuania, Poland, Romania, Slovakia). Hospital unit types were categorized into emergency department, intensive care unit (ICU), internal medicine, surgery, oncology, and other units. In order to investigate the population-weighted proportion of co- and cross-resistance of vancomycin-resistant E. faecium isolates to ampicillin, amoxicillin, linezolid, gentamicin and teicoplanin, only isolates that were tested separately against each of ampicillin, amoxicillin, linezolid, gentamicin and teicoplanin, respectively, were selected. Isolates were defined as ampicillin-, amoxicillin-, linezolid-, gentamicin- and teicoplanin-resistant if they were tested “resistant” against these antibiotics.
Outcomes and statistical analyses
The primary outcome was the population-weighted proportion of vancomycin-resistant E. faecium and E. faecalis isolates among all E. faecium and E. faecalis isolates, respectively, expressed in percentage (%) and 95% confidence intervals (95% CI). An isolate was defined as vancomycin-resistant if it was tested resistant or intermediate against vancomycin. Importantly, among E. faecium isolates identified as “resistant” with our definition, only 0.7% were tested as intermediate.
The potential association between different variables and vancomycin resistance of E. faecium isolates was analysed using univariable and multivariable logistic regression analyses. For univariable analyses, the following predictors for vancomycin resistance were considered: Year of sampling, gender, age group, European region, and hospital unit type. These variables were selected before the analysis based on the availability of data and our prior hypotheses about variables that may be associated with vancomycin resistance in E. faecium. We included all variables from the univariable analyses in the model for the multivariable analysis. In order to analyse whether the yearly change of VREFm proportions is associated with a particular patient and healthcare characteristics, four distinct multivariable logistic regression analyses were performed, including the interaction of year of sampling with each of the four aforementioned variables separately. Individual adjusted odds ratios for VREFm time trends for (i) European region, (ii) gender, (iii) age and (iv) care type were extracted from the multivariable regression by using linear combinations of the log-odds of the coefficient for year and the respective interaction coefficient.
All statistical analyses were performed using R version 3.6.1 [Citation23] and the “survey” package (version 3.37) [Citation24]. For all analyses in all strata we accounted for clustering at hospital level and applied country population-based weighting. The population data were obtained from the Eurostat database [Citation25]. Country population weighting was used to ensure that the data from each country contributed proportionally (in relation to its population size) to the calculation of resistance proportions. This was done to minimize bias from significant differences in isolate numbers from various countries.
Results
Baseline characteristics of included E. faecium blood isolates
The baseline characteristics of the analysed E. faecium isolates are outlined in . A total of 67,022 blood isolates of E. faecium from 63,459 patients were collected in 2057 hospitals across Europe from 2012 to 2018. The majority of the isolates originated from elderly patients (median age: 69 years, IQR: 59–78 years). For the isolates with a reported patient gender (n = 61,423, 91.6%), the female/male ratio was 0.65. The inpatient hospital units accounted for the vast majority (96.5%) of the isolates, with about a quarter each derived from patients treated in ICUs (25.4%) and internal medicine units (23.2%). 3.5% of E. faecium isolates were recorded among patients seen in the emergency department. About two-thirds of the isolates (64%) were from the Northern and Western regions of Europe, which represented 55% of the total population of the 30 countries included in the study.
Table 1. Baseline characteristics of blood isolates of Enterococcus faecium and Enterococcus faecalis in the EU/EEA
Temporal and spatial trends of VREFm in the Europe
In the study period 2012 through 2018, the population-weighted mean proportion of VREFm in blood isolates in Europe was 13.0% (95% CI 11.4–14.8%). There was an increase from 8.1% (95% CI 6.7–9.7%) in 2012 to 19.0% (95% CI 16.8–21.5%) in 2018 (A). The yearly increase of VREFm was found to be statistically significant in a multivariable regression analysis (OR: 1.28 [95%CI 1.24–1.33, p<0.001]) adjusting for factors that might impact VREFm likelihood, such as European region, hospital unit category, patient age and gender (). Importantly, between 2012 and 2018, rising trends of VREFm proportions were observed in all European regions and in the majority of countries (B and A). In the Northern and Eastern regions, VREFm proportions increased from 11.9% (95% CI 8.0–17.3%) and 7.0% (95% CI 4.2–11.4%) in 2012 to 28.4% (95% CI 22.8–34.8%) and 32.0% (95% CI 27.7–36.7%) in 2018, respectively. In Western and Southern regions VREFm proportions rose from 7.8% (95% CI 5.5–10.9%) and 6.6% (95% CI 5.0–8.7%) to 11.2% (95% CI 8.7–14.3%) and 15.3% (95% CI 12.6–18.5%), respectively. Multivariable analysis including interaction between European region and year while adjusting for other variables potentially affecting resistance proportions confirmed the increase of VREFm proportions in all four European regions studied (). Interestingly, from 2012 to 2018, noticeable regional differences in VREFm proportions were observed in Europe. The population-weighted mean proportion of VREFm was 22.3% (95% CI 19.7–25.2%) and 18.2% (95% CI 15.8–20.8%) in the Eastern and Northern region, respectively, compared to 11.3% (95% CI 9.8–12.9%) and 7.6% (95% CI 6.0–9.6%) in the Southern and Western region, respectively. Univariable and multivariable regression analyses confirmed that E. faecium blood isolates from Eastern and Northern Europe were more likely to be vancomycin-resistant than isolates from Southern and Western European regions (). The geographical differences were also still apparent in 2018. While the Eastern and Northern regions reported VREFm proportions of 32.0% (95%CI 27.7–36.7%) and 28.4% (95%CI 22.8–34.8%), respectively, proportions were 15.3% (95%CI 12.6–18.5%) and 11.2% (95%CI 8.7–14.3%) in the Southern and Western regions, respectively (B and B).
Figure 1. Time trend of vancomycin-resistant E. faecium from blood isolates in the EU/EEA. Time trend of vancomycin-resistant Enterococcus faecium in (A) 30 countries of the European Union, European Economic Area and the United Kingdom, and in (B) major regions within Europe. Vancomycin resistance proportions are expressed as population-weighted mean proportions (%) among all Enterococcus faecium blood isolates, with corresponding 95% confidence intervals.
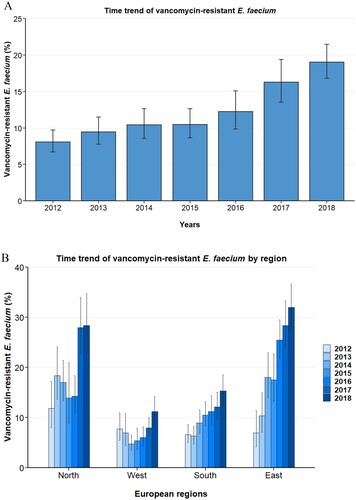
Table 2. Analysis of factors associated with vancomycin resistance in E. faecium blood isolates in the EU/EEA.
Table 3. Adjusted odds ratios (aOR) for time trends (2012–2018) of VREFm proportions based on four distinct multivariable analyses including interaction of year with region, gender, age or hospital unit category, respectively, additionally to the variables included before (see ).
Age and gender
To determine any underlying influence of the age and gender of hospitalized patients on VREFm proportions, we analysed any association between these demographic variables and VREFm. The data displayed in show that isolates from infants (<1 year) exhibited lower VREFm proportions (6.3% [95% CI 4.2–9.4%]) than adults (20–59 years; 15.6% [95% CI 13.2–18.3%]) and elderly patients [≥60 years; 13.0% (95%CI 11.5–14.6%) (A)]. In addition, our data suggest that children and adolescents (1–19 years) also showed lower VREFm proportions (11.1% [95% CI 8.6–14.3%]) than the older age groups, which was confirmed by multivariable analysis ().
Figure 2. Vancomycin-resistant E. faecium from blood isolates stratified into age. (A) Vancomycin-resistant Enterococcus faecium stratified into age, expressed as population-weighted mean proportions (%) among all Enterococcus faecium blood isolates, with corresponding 95% confidence intervals. (B) Time trends of vancomycin-resistant Enterococcus faecium in different age groups.
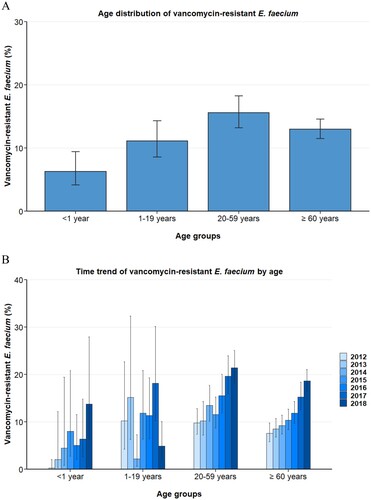
Importantly, the increase of VREFm proportions from 2012 to 2018 was found in blood isolates from infants (<1 year, adjusted OR: 1.53 [95 CI 1.24–1.89, p<0.001]), adults (20–59 years, adjusted OR: 1.25 [95% CI 1.18–1.32, p < 0.001]) and elderly patients (≥60 years, adjusted OR: 1.30 [1.24–1.36, p<0.001]) ( and B). Although a moderate increase was observed for children and adolescent (1–19 years, OR: 1.10 [95% CI 0.96–1.27, p = 0.177]), this rise was not statistically significant.
There were no prominent differences in mean VREFm proportions between female and male gender for the study period 2012–2018 (13.7% [95%CI 11.7–15.8%] and 12.9% [95%CI 11.4–14.6%], respectively) and in 2018 (19.8% [95% CI 17.1–22.9%] and 18.7% [95%CI 16.4–21.2%], respectively). However, multivariable logistic regression analyses showed an odds ratio of 0.91 (95% CI 0.84–1.0, p = 0.041) for vancomycin resistance in male compared to the female patients (). As shown in , the increase in VREFm proportions was observed in both female and male patients (OR: 1.23 [95% CI 1.13–1.36, p<0.001] and 1.27 [95% CI 1.22–1.33, p<0.001], respectively).
Hospital unit type
Analyses of E. faecium blood isolates per hospital unit type reveal substantial differences in vancomycin resistance proportions between isolates drawn in emergency departments and in inpatients hospital units such as internal medicine, ICU and surgical units (). While the population-weighted European mean VREFm proportion (2012–2018) was 6.1% (95%CI 4.0–9.1%) for the emergency department, considerably higher VREFm proportions were observed in internal medicine (12.1% [95% CI 10.5–14.0%]), intensive care (13.2% [95% CI 10.6–16.4%]) and surgical units (11.2% [95% CI 8.9–14.1%]) (). Since no pronounced differences were observed between internal medicine, intensive care, surgical, oncology and other hospital units, these units were aggregated into inpatient hospital units for logistic regression analyses. Univariable and multivariable regression analyses confirmed that the likelihood of VREFm in blood isolates from inpatient hospital units was markedly higher than in isolates from the emergency department (OR: 2.29 [95% CI 1.56–3.37, p < 0.001]) ().
Figure 3. Vancomycin-resistant E. faecium from blood isolates stratified into hospital units. (A) Vancomycin-resistant Enterococcus faecium stratified into hospital units, expressed as population-weighted mean proportions (%) among all Enterococcus faecium blood isolates, with corresponding 95% confidence intervals. (B) Time trends of vancomycin-resistant Enterococcus faecium in blood isolates from the emgercency department and inpatient hospital units (internal medicine, surgical units, intensive care unit, onocology and other hospital units).
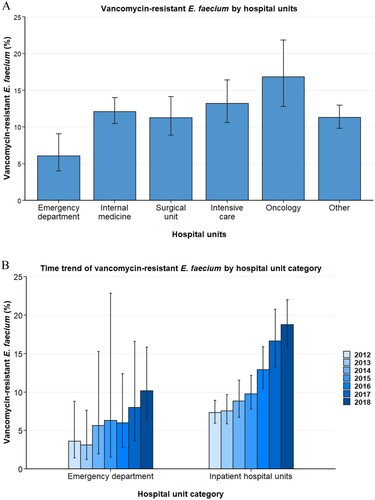
Importantly, the rise of VREFm between 2012 and 2018 was seen in inpatient hospital units, where VREFm proportions rose from 7.3% (95% CI 6.0–8.9%) to 18.7% (95% CI 15.9–22.0%) (B). In line with this, multivariable regression analyses assessing the interaction between year and hospital units showed a statistically significant VREFm increase in inpatient hospital units (OR: 1.29 [95% CI 1.24–1.34, p<0.001]) (). Although an increase of VREFm proportions was found in emergency departments between 2012 (3.6% [95% CI 1.4–8.8%]) and 2018 (10.2% [95% CI 6.4–15.8%]) (B), the rise was not statistically significant in multivariable regression analysis (OR: 1.11 [95% CI 0.97–1.28, p = 0.121]) ().
Co-resistance and cross-resistance
E. faecium blood isolates were evaluated for co- and cross-resistance with a range of antibiotics that are documented as alternative therapy options for vancomycin-resistant E. faecium infections, either as monotherapy or in combination with other drugs. We tested for co-resistance (amoxicillin, ampicillin, linezolid and gentamicin) and cross-resistance (teicoplanin) among vancomycin-sensitive and vancomycin-resistant E. faecium isolates (). The majority of E. faecium isolates that were resistant to vancomycin also exhibited high co-resistance to the penicillin antibiotics: ampicillin [99.4% (95% CI 99.2–100%)] and amoxicillin [99.3% (95%CI 98.2–100%)]. In comparison, vancomycin-sensitive E. faecium isolates (VSEF) also showed high resistance to ampicillin (88.9% [95% CI 88.1–90.0%]) and amoxicillin (84.8% [95% CI 82.5–87.0%]) but co-resistance proportions were lower than those from vancomycin-resistant E. faecium isolates. Considerable co-resistance among vancomycin-resistant and -sensitive E. faecium isolates was also found for gentamicin, a commonly used aminoglycoside (VREFm: 48.5% [95% CI 44.7–52.0%], VSEF: 43.4% [95% CI 41.0–46.0%]). Notably, co-resistance to linezolid, an alternative treatment option for VREFm infections, was very low among vancomycin-resistant E. faecium isolates (1.8% [95%CI 1.5–2.0%]). Cross-resistance to the glycopeptide antibiotic teicoplanin was very low among vancomycin-sensitive E. faecium isolates (0.4% [95%CI 0.2–1.0%]) but high among vancomycin-resistant E. faecium isolates (80.4% [95% CI 77.3–83.0%]) ().
Table 4. Co-resistance and Cross-resistance of vancomycin-resistant and -sensitive blood isolates to selected antibiotics.
Vancomycin-resistant E. faecalis
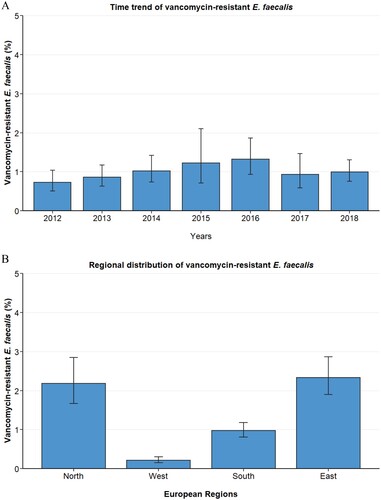
We analysed proportions of vancomycin resistance among 103,112 E. faecalis isolates from patients with bloodstream infections (see for baseline characteristics of the included E. faecalis blood isolates). During the whole study period, the European population-weighted mean proportion of vancomycin resistance in E. faecalis isolates was 1.1% (95% 0.9–1.4%). The proportion of vancomycin-resistant E. faecalis remained relatively stable between 2012 and 2018 (A). Similar to E. faecium, proportions of vancomycin resistance among E. faecalis differed substantially between European regions. While in the North and East, proportions of 2.2% (95% CI 1.7–2.9%) and 2.3% (95% CI 1.9–2.9%) were observed, only 0.21% (95% CI 0.15–0.30%) and 1.0% (95% CI 0.81–1.2%) of all E. faecalis blood isolates exhibited vancomycin resistance in the West and South, respectively (B).
Discussion
Improved understanding of the interplay of factors that drive antimicrobial drug-resistant infections provides opportunities to address the associated clinical and public health burden on individuals, health systems, and society [Citation26,Citation27]. Therefore, we analysed isolates of E. faecium and E. faecalis collected from patients with bloodstream infections in hospitals across Europe between 2012 and 2018 using EARS-Net data.
Our analysis showed that there was a profound increase of vancomycin resistance in E. faecium blood isolates between 2012 (8.1%) and 2018 (19.0%) in EU/EEA countries (including the United Kingdom). Despite this increase, the 2018 mean VREFm proportion in Europe is still lower than current data reported from other parts of the world, such as the United States (66%) [Citation28], Australia (47%) [Citation7] and countries from the Eastern Mediterranean region such as Iran [Citation29–31]. However, VREFm proportions reported in this study are significantly higher than those observed in Chinese hospitals, where VRE rates of lower than 2% were observed [Citation32–34]. Similar to the situation in the EU/EEA, increasing VRE proportions have also been described in countries of the Eastern Mediterranean region [Citation29]. Contrary to the trend in Europe, a large multicentre study showed that VREFm proportions in blood isolates reduced from 80.7% in 2010 to 66% in 2016 in the United States [Citation28].
The rising trend in the EU/EEA countries is concerning since E. faecium infections are not only a major cause of nosocomial bloodstream infection but are also associated with a considerable disease burden. A recent study estimated that vancomycin-resistant enterococcus infections accounted for approximately 16,000 infections and 1000 attributable deaths in 2015 in EU/EEA countries [Citation35]. In line with the rise of vancomycin resistance observed in our study, the study of Cassini et al. found that the number of vancomycin-resistant enterococci infections and attributable deaths almost doubled between 2007 and 2015 [Citation35]. Compared to the situation in Europe, the burden of vancomycin-resistant enterococci is steadily decreasing in the Unites States, where the estimated number of cases in hospitalized patients declined from 85.000 in 2012–54,500 in 2017, most likely explained by increased infection control efforts and appropriate antibiotic use [Citation13].
Importantly, our study shows that VREFm proportions in blood isolates increased in all EU/EEA regions and the majority of countries recorded a relative increase of VREFm rates. In addition, regional analyses showed that in 2018, VREFm was more pronounced (about two-fold higher) in the Northern and Eastern regions compared to the Southern and Western region of Europe. This is a contrast to the usual North/West - South/East gradient of antibiotic resistance for many pathogens observed in the EU/EEA including Acinetobacter spp., Pseudomonas aeruginosa and Klebsiella pneumoniae [Citation9]. However, a considerable intra-regional heterogeneity was observed for the four major European regions at country level. For example, Ireland and the United Kingdom had considerably higher VREFm proportions than other northern countries, such as Norway and Sweden. Moreover, previous studies have shown that VREFm proportions can also significantly differ within individual countries as described for Germany, where a strong north–south disparity was observed [Citation15]. The wide regional differences suggest that peculiar local factors might be driving the differences in vancomycin resistance among E. faecium isolates, such as VRE(Fm) diagnostics, infection control measures including active surveillance and varying antibiotic use. Local studies that incorporate demographic, treatment and clinical outcome data can delineate these unique factors that drive vancomycin resistance especially among hospitalized patients.
Our study found that adult and elderly patients with E. faecium bloodstream infections have a higher risk for VREFm than younger patients. Higher vancomycin resistance proportions in adults and elderly patients with enterococci infections have also been described in other studies [Citation15,Citation36]. In addition, similar age trends have been reported for other bacterial pathogens, including Escherichia coli, Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa, Helicobacter pylori and Klebsiella pneumonia [Citation37–40]. As shown for enterococci, older patients are more likely to be colonized (and subsequently infected) with drug-resistant organisms due to more frequent exposure to antibiotics throughout their lives, thereby promoting the selection of drug-resistant bacteria [Citation41]. Another possible reason is the more frequent exposure of older patients to long-term care facilities or other healthcare facilities, a known “reservoirs of resistance” due to poor infection control and prevalent use of broad-spectrum antibiotics [Citation42,Citation43]. In addition, the increasing trend in VREFm proportions was seen across all age groups except children and adolescents between 1 and 19 years. This may be explained by the lower frequency of hospital admission and/or lower consumption of antibiotics among children and adolescents in Europe compared to other age groups [Citation44]. Increasing VREFm proportions in infants not neonates and elderly patients is of concern since ineffective antibiotic therapies are associated with increased mortality and morbidity in these vulnerable patient groups [Citation45–47].
With respect to the origin of isolates, our study revealed that E. faecium isolates from inpatient hospital units, including ICUs and internal medicine, have a substantially higher proportion of vancomycin resistance compared to isolates from patients in the emergency department. A plausible explanation for this is the fact that patients treated in emergency departments most likely acquired the E. faecium infection in the community, rather than in the hospital. This interpretation is consistent with evidence from previous studies that reported a lower prevalence of vancomycin resistance among E. faecium isolates from emergency and outpatient hospital clinics compared to isolates from hospital inpatients [Citation30,Citation36,Citation48]. In addition, we found that VREFm proportions significantly increased in inpatient hospital units over the years. This might be explained by increased use of invasive medical devices among inpatients, since the role of medical devices in the transmission of multidrug-resistant E. faecium infections is well documented [Citation20]. Moreover, these inpatient hospital units are also characterized by the use of broad-spectrum antibiotics and patient populations that have serious pathologies and are more likely immunocompromised, which lowers their colonization resistance to known opportunistic pathogens like VREFm [Citation49].
The very high cross-resistance of vancomycin-resistant E. faecium isolates to teicoplanin (80.4%) suggests that the VanA resistance gene is the main driver of glycopeptide resistance in E. faecium in the EU/EEA. Our finding of significant co-resistance of VREFm to ampicillin (>99%) and gentamicin (48.5%) underlines the progressively limited value of these antibiotics in the empirical management of VREFm infections, even when used in synergistic combinations [Citation20,Citation50]. However, it is encouraging that less than 2% of all VREFm blood isolates in this study exhibited co-resistance to linezolid, a last line drug used to treat vancomycin-resistant enterococci infections [Citation51–53]. This is much lower than co-resistance proportions among vancomycin-resistant E. faecium enteric isolates in single centre studies in US (17.1%) and Italy (10.7%) [Citation54,Citation55]. A recent review of surveillance data by Bender et al. calls for caution since an increasing trend - albeit at low level – of linezolid resistance in VREFm, and enterococci in general, is emerging across Europe [Citation56]. Such co-resistance presents additional challenges for patient treatment and infection control measures [Citation50]. Therefore, there is a need to delineate the genomics of linezolid resistance determinants in E. faecium due to its increasing occurrence and the need for early implementation of adequate empiric VREFm treatment [Citation54,Citation57,Citation58]. Molecular methods with high discriminatory power like wide genome sequencing will help to better understand the resistance mechanisms of emerging linezolid-vancomycin-resistant E. faecium. Such understanding is necessary to preserve its effectiveness – considering the diminishing antibiotic pipeline [Citation59] – through stewardship activities and epidemiological surveillance of linezolid resistance across the EU/EEA countries.
In contrast to the results for E. faecium, we found that only 1.1% of E. faecalis blood isolates were vancomycin-resistant and no apparent increase was recorded between 2012 and 2018. In comparison, the proportion of vancomycin-resistant E. faecalis in blood isolates is 1.9–5.3% in the United States [Citation28], whereas in China proportions are lower than 1% among isolates from different specimen material [Citation32,Citation34]. Our findings indicate the necessity to analyse vancomycin resistance patterns distinctively for E. faecium and E. faecalis in order fully understand the extent of the vancomycin resistance in enterococci infections. However, many epidemiological studies and surveillance programs do not differentiate enterococci to the species level. The proportion of vancomycin-resistant isolates in these studies is heavily influenced by the ratio of E. faecium and E. faecalis. Evidence from previous studies showed that the ratio of E. faecium and E. faecalis recorded in enterococci infection greatly differed among several individual studies [Citation15,Citation28,Citation32,Citation33].
Strengths and limitations
This study included over 67,000 and 103,000 clinical blood isolates of E. faecium and E. faecalis, respectively, and is to our knowledge the largest and most comprehensive analysis of the vancomycin resistance profile of enterococci bloodstream infections in the EU/EEA. The analysed dataset is derived from routine clinical antimicrobial susceptibility data from national surveillance programs. The continuous collection of these AMR data allows for a longitudinal analysis; a very important indicator of trend. In addition, the regular external quality assessments of participating laboratories have demonstrated the validity of these AMR data [Citation60]. While participating national laboratories and hospitals might not be fully representative for individual countries, over half of the countries reported a national coverage greater than 80% while population, hospital, and isolate sample representativeness was assessed as high in 25 countries [Citation9]. Another limitation is the wide variation in population coverage among reporting countries. To minimize possible bias from differences in population size and isolate numbers from various countries, all statistical analyses used weighting based on the population sizes of the individual countries. Lastly, different sampling routine and admission characteristics (e.g. stay duration, bed space density) in different healthcare settings can result in biased estimates of VREFm proportions. However, the inclusion of only clinical bloodstream specimen limits the bias that may result from some of the inconsistencies in sampling, even though the frequency of blood sampling varies between hospitals and countries.
Conclusion
This study demonstrated that vancomycin resistance in enterococci blood isolates is mainly reported for E. faecium isolates. The rising trend of vancomycin-resistant E. faecium is pervasive across the EU/EEA and particularly among hospitalized adult and elderly patients. These findings have implications for patient care and justify the need to analyse the available data more rapidly at country level and also identify specific regions with high VREFm within the countries. National and regional authorities should intensify efforts directed at diagnostic and antimicrobial stewardship for vancomycin and all last resort drugs for the control of nosocomial enterococci infections.
Geolocation information
Europe
Acknowledgements
We thank all EARS-Net participating laboratories and hospitals for providing data. We wish to thank the European Centre for Disease Prevention and Control for providing the data set for our paper. We thank our colleagues at the Robert Koch Institute for their input during this study, specifically Angelina Taylor and Sebastian Haller. Disclaimer: The views and opinions of the authors expressed herein do not necessarily state or reflect those of ECDC. The accuracy of the authors’ statistical analysis and the findings they report are not the responsibility of ECDC. ECDC is not responsible for conclusions or opinions drawn from the data provided. ECDC is not responsible for the correctness of the data and for data management, data merging and data collation after provision of the data. ECDC shall not be held liable for improper or incorrect use of the data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155(Pt 6):1749–1757.
- Lebreton F, van Schaik W, Manson McGuire A, et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio. 2013;4(4):e00534-13.
- Lee T, Pang S, Abraham S, et al. Antimicrobial-resistant CC17 Enterococcus faecium: The past, the present and the future. J Glob Antimicrob Resist. 2019;16:36–47.
- Wittea RW W, Klarea I. Enterococcus. Chemotherapy. 1999;45:135–145.
- Zhang Y, Du M, Chang Y, et al. Incidence, clinical characteristics, and outcomes of nosocomial Enterococcus spp. bloodstream infections in a tertiary-care hospital in Beijing, China: a four-year retrospective study. Antimicrobial Resistance & Infection Control. 2017;6(1):73.
- Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–278.
- Coombs GW, Daley DA, Lee YT, et al. Australian group on antimicrobial resistance (AGAR) Australian Enterococcal Sepsis outcome Programme (AESOP) Annual report 2017. Commun Dis Intell (2018). 2019;43.
- Willems RJL, Top J, van Santen M, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerging Infect Dis. 2005;11(6):821–828.
- ECDC. Surveillance of antimicrobial resistance in Europe 2018. Stockholm: European Centre for Disease Prevention and Control; 2019.
- Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med. 2000;342(10):710–721.
- Weigel LM, Clewell DB, Gill SR, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302(5650):1569–1571.
- Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327.
- CDC. Antibiotic resistance threats in the United States, 2019. Atlanta (GA): Antibiotic Resistance Coordination and Strategy Unit within the Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention; 2019.
- Peta M, Carretto E, Barbarini D, et al. Outbreak of vancomycin-resistant Enterococcus spp. in an Italian general intensive care unit. Clin Microbiol Infect. 2006;12(2):163–169.
- Markwart R, Willrich N, Haller S, et al. The rise in vancomycin-resistant Enterococcus faecium in Germany: data from the German antimicrobial resistance surveillance (ARS). Antimicrob Resist Infect Control. 2019;8:147.
- Sun H, Wang H, Xu Y, et al. Molecular characterization of vancomycin-resistant Enterococcus spp. clinical isolates recovered from hospitalized patients among several medical institutions in China. Diagn Microbiol Infect Dis. 2012;74(4):399–403.
- Conceição N, Oliveira C, Silva P, et al. Trends in antimicrobial resistance among clinical isolates of enterococci in a Brazilian tertiary hospital: a 4-year study. Rev Soc Bras Med Trop. 2011;44:177–181.
- Mendes RE, Castanheira M, Farrell DJ, et al. Longitudinal (2001–14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010–13) analysis of oritavancin in vitro potency. J Antimicrob Chemother. 2016;71(12):3453–3458.
- Tripathi A, Shukla S, Singh A, et al. Prevalence, outcome and risk factor associated with vancomycin-resistant Enterococcus faecalis and Enterococcus faecium at a Tertiary care hospital in Northern India. Indian J Med Microbiol. 2016;34(1):38–45.
- Nelson I, Agudelo Higuita MMH. Enterococcal disease, epidemiology, and implications for treatment. In: Enterococci: from commensals to leading causes of drug resistant infection [Internet]. Boston: Massachusetts Eye and Ear Infirmary; 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK190429/
- Saeidi S, Mirnejad R, Masoumi Zavaryani S, et al. Molecular epidemiology and antibiotic resistance patterns of Enterococcus faecalis isolates from hospitals in Tehran. Infez Med. 2017;25(2):116–122.
- Dai D, Wang H, Xu X, et al. The emergence of multi-resistant Enterococcus faecalis clonal complex, CC4, causing nosocomial infections. J Med Microbiol. 2018;67(8):1069–1077.
- Team RC. R: A language and environment for statistical computing; 2019.
- Lumley T. Package ‘survey': analysis of complex survey samples; 2020.
- European Commission. Eurostat database 2020. Available from: https://ec.europa.eu/eurostat/data/database.
- Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis. 2019;19(1):4–6.
- Jonas OB, Irwin A, Berthe FCJ, et al. Drug-resistant infections: a threat to our economic future (Vol. 2): final report (English). Washington (DC): World Bank Group; 2017.
- Mendes RE, Sader HS, Castanheira M, et al. Distribution of main Gram-positive pathogens causing bloodstream infections in United States and European hospitals during the SENTRY antimicrobial surveillance Program (2010-2016): concomitant analysis of oritavancin in vitro activity. J Chemother. 2018;30(5):280–289.
- Jabbari SM S, Pormohammad A, Hashemi A, et al. Global prevalence of antibiotic resistance in blood-isolated enterococcus faecalis and enterococcus faecium: A systematic review and meta-analysis. Infect Drug Resist. 2019;12:2713–2725.
- Moghimbeigi A, Moghimbeygi M, Dousti M, et al. Prevalence of vancomycin resistance among isolates of enterococci in Iran: a systematic review and meta-analysis. Adolesc Health Med Ther. 2018;9:177–188.
- Armin S, Zahedani SS, Rahbar M, et al. Prevalence and resistance profiles of vancomycin-resistant enterococcal isolates in Iran; an eight-month report from nine major cities. Infect Disord Drug Targets. 2019.
- Huang L, Zhang R, Hu Y, et al. Epidemiology and risk factors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci infections in Zhejiang China from 2015 to 2017. Antimicrob Resist Infect Control. 2019;8:90.
- Zhi ZYXZ. [Surveillance of bacterial resistance in children and newborns across China from 2014 to 2017]. Zhonghua yi xue za zhi. 2018;98(40):3279–3287.
- Hu F, Zhu D, Wang F, et al. Current status and trends of antibacterial resistance in China. Clin Infect Dis. 2018;67(suppl_2):S128–Ss34.
- Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66.
- Simner PJ, Adam H, Baxter M, et al. Epidemiology of vancomycin-resistant enterococci in Canadian hospitals (CANWARD study, 2007 to 2013). Antimicrob Agents Chemother. 2015;59(7):4315–4317.
- Koppe U, von Laer A, Kroll LE, et al. Carbapenem non-susceptibility of Klebsiella pneumoniae isolates in hospitals from 2011 to 2016, data from the German antimicrobial resistance surveillance (ARS). Antimicrob Resist Infect Control. 2018;7:71.
- Adam HJ, Baxter MR, Davidson RJ, et al. Comparison of pathogens and their antimicrobial resistance patterns in paediatric, adult and elderly patients in Canadian hospitals. J Antimicrob Chemother. 2013;68(Suppl 1):i31–i37.
- Ji Z, Han F, Meng F, et al. The association of Age and antibiotic resistance of Helicobacter pylori: A study in Jiaxing City, Zhejiang Province, China. Medicine (Baltimore). 2016;95(8):e2831.
- Garcia A, Delorme T, Nasr P. Patient age as a factor of antibiotic resistance in methicillin-resistant Staphylococcus aureus. J Med Microbiol. 2017;66(12):1782–1789.
- Karki S, Houston L, Land G, et al. Prevalence and risk factors for VRE colonisation in a tertiary hospital in Melbourne, Australia: a cross sectional study. Antimicrob Resist Infect Control. 2012;1(1):31.
- Augustine S, Bonomo RA. Taking stock of infections and antibiotic resistance in the elderly and long-term care facilities: A survey of existing and upcoming challenges. Eur J Microbiol Immunol (Bp. 2011;1(3):190–197.
- Laudisio A, Marinosci F, Gemma A, et al. The burden of comorbidity is associated with antibiotic resistance among institutionalized elderly with urinary infection: A retrospective Cohort study in a single Italian Nursing Home between 2009 and 2014. Microb Drug Resist. 2017;23(4):500–506.
- Eurostat Statistics Explained. Hospital discharge and length of stay2019. 2020. Available from: https://ec.europa.eu/eurostat/statistics-explained/index.php/Hospital_discharges_and_length_of_stay_statistics.
- Ramirez CB, Cantey JB. Antibiotic resistance in the neonatal intensive care unit. NeoReviews. 2019;20(3):e135–ee44.
- Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antibiotic era? J Am Geriatr Soc. 2002;50–57(Suppl):S226 -S229.
- Beckett CL, Harbarth S, Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect. 2015;21(1):3–9.
- Whelton E, Lynch C, O'Reilly B, et al. Vancomycin-resistant enterococci carriage in an acute Irish hospital. J Hosp Infect. 2016;93(2):175–180.
- Donskey CJ. The role of the intestinal tract as a Reservoir and Source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39(2):219–226.
- Abbo L, Shukla BS, Giles A, et al. Linezolid- and vancomycin-resistant Enterococcus faecium in Solid Organ Transplant Recipients: infection control and antimicrobial stewardship using whole genome sequencing. Clin Infect Dis. 2018;69(2):259–265.
- Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart association. Circulation. 2015;132(15):1435–1486.
- Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–1767.
- O'Driscoll T, Crank CW. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist. 2015;8:217–230.
- Dilworth TJ, Beck ET, Pedersen RA, et al. High rate of linezolid intermediate susceptibility and resistance among enteric vancomycin-resistant Enterococcus (VRE) recovered from hospitalized patients actively screened for VRE colonization. Infection Control & Hospital Epidemiology. 2019;40(7):821–822.
- Bonora MG, Solbiati M, Stepan E, et al. Emergence of linezolid resistance in the vancomycin-resistant Enterococcus faecium multilocus sequence typing C1 epidemic lineage. J Clin Microbiol. 2006;44(3):1153–1155.
- Bender JK, Cattoir V, Hegstad K, et al. Update on prevalence and mechanisms of resistance to linezolid, tigecycline and daptomycin in enterococci in Europe: towards a common nomenclature. Drug Resist Updates. 2018;40:25–39.
- Wardenburg KE, Potter RF, D'Souza AW, et al. Phenotypic and genotypic characterization of linezolid-resistant Enterococcus faecium from the USA and Pakistan. J Antimicrob Chemother. 2019;74(12):3445–3452.
- Davis HR, Brown RM, Ashcraft DS, et al. In vitro synergy of fosfomycin plus doxycycline against linezolid and vancomycin resistant Enterococcus faecium using a rapid test method and time-kill assay. J Invest Med. 2019;67(2):597.
- Allan Coukell HB. The antibiotic market is broken—and won’t fix itself. The Pew Charitable Trusts; 2019.
- ECDC. External quality assessment of laboratory performance - European antimicrobial resistance surveillance Network (EARS-Net), 2017. Stockholm: European Centre for Disease Prevention and Control; 2018.
Appendix
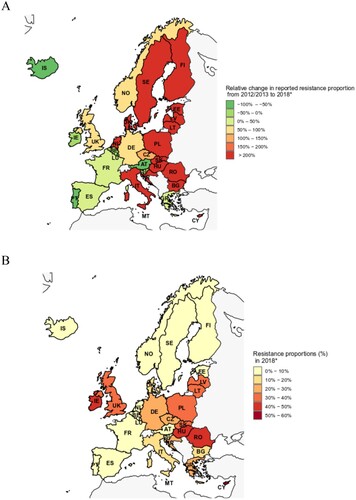
Additional Figure 1. Vancomycin-resistant E. faecium blood isolates in the EU/EEA. (A) Relative changes (in %) of proportions of vancomycin-resistant Enterococcus faecium (VREFm) among all E. faecium blood isolates between 2012/2013 (aggregated) and 2018* in European Union (EU) and Euroepan Economic Area (EEA). (B) Absolute VREFm proportions in 2018 (in %, VREFm isolates among all Enterococcus faecium blood isolates) in EU/EEA countries. * Since for Slovenia no data for 2018 were available, 2017 data were used. AT: Austria, BE: Belgium, BG: Bulgaria, CY: Cyprus, CZ: Czech Republic, DE: Germany, DK: Denmark, EE: Estonia, ES: Spain, FI: Finland, FR: France, GR: Greece, HR: Croatia, HU: Hungary, IE: Irleland, IS: Iceland, IT: Italia, LT: Lithunia, LU: Luxembourg, LV: Latvia, MT: Malta, NL: Netherlands, NO: Norway, PL: Poland, PT: Portugal, RO: Romania, SK: Slovakia, SL: Slovenia, UK: United Kingdom.
Additional Figure 2. Vancomycin-resistant E. faecalis blood isolates in the EU/EAA. (A) Time trend of vancomycin-resistant Enterococcus faecalis in 30 countries of the European Union, Euroepan Economic Area and the United Kingdom, expressed as population-weighted mean proportions (%) among all Enterococcus faecalis blood isolates, with corresponding 95% confidence intervals. (B) Proportions of vancomycin-resistant Enterococcus faecalis in major regions of Europe.