ABSTRACT
Postoperative endophthalmitis (PE) is the devastating complication that frequently results in vision loss. Recently, enterococcus have emerged as a major cause of PE in several countries and resulted in poor visual outcome. However, the reason remains elusive. We investigate whether selection pressure of fluoroquinolone exerts effects on microorganism profiles isolated from PE. Medical records of patients who were diagnosed with PE at eight resident training institutions between January 2004 and December 2015 were reviewed. The most common isolate was Enterococcus faecalis (28.0%), followed by Staphylococcus epidermidis (18.6%) and other coagulase negative Staphylococci (7.6%). However, the rates of E. faecalis isolated from conjunctival microbes were 6.2% (16/257) and their resistance to fluoroquinolones was higher than those of S. epidermidis. In vitro and in vivo co-culture models of E. faecalis and S. epidermidis were established for survival assays after administration of fourth-generation fluoroquinolone. In in vitro co-culture model, the survival assay of E. faecalis and S. epidermidis against the treatment of moxifloxacin showed that E. faecalis survived significantly better than S. epidermidis in the presence of moxifloxacin 1 µg/mL and more. In in vivo co-culture model, E. faecalis survived significantly better than S. epidermidis after topical treatment of moxifloxacin (5 mg/mL). E. faecalis has been the most common causative strain of PE in Korea. We suggest that the increase of E. faecalis in PE could be associated with the selection pressure of fourth-generation fluoroquinolone.
Summary: Enterococcus spp. have emerged as a leading causative strain of postoperative endophthalmitis in 11-year clinical data. We suggest that the increase of Enterococcus spp. is associated with the selection pressure of fourth-generation fluoroquinolone.
Introduction
Postoperative infectious endophthalmitis (PE) is the most devastating complication after intraocular surgery. Cataract surgery is the most common cause of PE. In a recent large meta-analysis, the incidence of PE ranged from 0.012% to 1.3% [Citation1,Citation2]. Infectious endophthalmitis is initiated by the entrance of microorganisms into the intraocular space, and the type of microorganism is a critical factor in determining visual outcome [Citation3,Citation4].
So far, the most frequent causative strain of PE has been known as coagulase negative staphylococci (CNS), including Staphylococcus epidermidis [Citation5,Citation6]. Recently, Enterococcus spp. have emerged as a major causative strain of PE in Sweden, Korea, and Taiwan [Citation4,Citation7,Citation8]. The reason underlying the increase of Enterococcus spp. has not been fully understood, but several studies speculated that an important contributory factor might be the selection pressure from increased consumption of broad-spectrum antibiotics perioperatively [Citation4,Citation9,Citation10]. Enterococcus spp. are of particular clinical importance because they cause a fulminant and destructive disease course and poor visual outcomes [Citation11–13]. Therefore, the emergence of Enterococcus spp. as a major cause of PE would negatively impact the expectations for visual outcomes.
Enterococcus spp. have an intrinsic resistance to multiple antibiotics such as cephalosporins, aminoglycosides, and fluoroquinolones [Citation14–16]. For the broad-spectrum topical antibiotics used to prevent PE, fourth generation fluoroquinolones are commonly used perioperatively without clear evidence [Citation17,Citation18]. Fluoroquinolones show poor or moderate antimicrobial activity against Enterococcus spp. [Citation14,Citation19].
Despite the increasing importance of Enterococcus spp. in PE, clinical data about the susceptibility of Enterococcus spp. isolated from eye to various fluoroquinolones is scarce. The Clinical and Laboratory Standard Institute (CLSI) does not provide a resistance cut-off value of all generation fluoroquinolones for Enterococcus spp., and the published studies on the antibiotics susceptibility of Enterococcus spp. to fluoroquinolone used various methods depending on each hospital with different cut-off values [Citation20]. Moreover, there has been no clear investigation to address whether the domination of Enterococcus spp. of PE was caused by their intrinsic resistance toward fluoroquinolone.
In this study, the causative isolate of PE was investigated using 11-year clinical data, and their antibiotic susceptibility was analysed. Two main causative bacteria, Enterococcus faecalis and S. epidermidis, were isolated from conjunctival microbes and tested for antibiotics susceptibility on fluoroquinolones. In vitro and in vivo co-culture models of E. faecalis and S. epidermidis were established and used for survival assay to investigate whether the selection pressure of fourth-generation fluoroquinolone has effects on the domination of E. faecalis.
Materials and methods
Clinical data
We reviewed medical records of patients with PE after operation for cataract, glaucoma, or vitreous in seven resident training institutions in Korea from January 2004 to December 2015. Institutional review board (IRB) approval was obtained from Gyeongsang National University, and the protocol of this study adhered to the provisions of the Declaration of Helsinki. Diagnosis was based on the doctor’s discrimination by the clinical manifestations of patients, and microbiologic cultures were performed by aspiration of the aqueous humour or vitreous. For the clinical analysis of PE, the following data were evaluated: case numbers of presumptive and culture-positive endophthalmitis, operation, and isolated microorganisms and their antibiotic susceptibility. Culture and identification method was described in detail in the supplementary data.
Collection of conjunctival microbes
Specimens were obtained from 208 patients (416 eyes) who were scheduled for cataract surgery or intravitreal injection at the Gospel Hospital, Kosin University from April 2014 to January 2018. All contact lens wearers, patients with any inflammatory or infectious ocular surface, or any recent (at least 3 months) users of antibiotics that could alter the status of conjunctival flora were excluded. The patient was asked to look up, and the inferior cul-de sac was swabbed using a sterile cotton tip without touching the eyelid margin or lashes. The specimens were inoculated directly in 5% blood agar plates at the bedside and incubated at 37°C for 3∼7 days. Each colony was collected and cultured on the different culture dishes. Cultured bacteria were identified in the same manner. All isolated strains of E. faecalis and S. epidermidis were further examined for antibiotic susceptibility by broth micro-dilution method which was described in detail in the supplementary method.
Survival assay in in vitro co-culture model
The co-culture bacterial solution of standard strains of S. epidermidis and E. faecalis were prepared to have equal concentration of both bacteria (2.5 × 107 CFU/mL) in TSB media and incubated at 37°C for 14 h. Then, the 100 µL of co-cultured solution was diluted with 900 µL of TSB media containing various concentrations of moxifloxacin (0∼16 µg/mL, Tokyo Chemical Industry, Japan) and further incubated for 6 h. After incubation with moxifloxacin, the bacterial solution was serially diluted and plated on the CDA plate (MB-C1611, MB-cell, Korea), which can differentiate the colonies of E. faecalis and S. epidermidis by the colours of the colonies; the E. faecalis colonies were blue and S. epidermidis colonies were white. Bacterial strain and growth conditions were described in detail in the supplementary method.
Survival assay in in vivo co-culture model
Rabbits (New Zealand white rabbit, 4∼5 kg, Hyochang Science, Korea) were used for the in vivo co-culture model of S. epidermidis and E. faecalis. The animals were divided into three groups: the normal control group (NC), co-culture group (CO), and moxifloxacin-treated co-culture group (MC). Each group consisted of five rabbits and only the right eyes were used for experiment. Neither microorganism nor moxifloxacin was administered to the NC group. For the CO and MC groups, rabbits were anaesthetized by intramuscular injection of by tiletamine-zolazepam (Virbac Korea, France) and xylazine (Bayer, Germany), and 9 µL of S. epidermidis solution (4X109 CFU/mL) and 1 µL of E. faecalis solution (4X109 CFU/mL) were administered into the lower conjunctival sac. To avoid the loss of bacterial solutions, tarsorrhapy was performed using continuous 6–0 nylon (Ethicon, USA). In the MC group, 5 µL of moxifloxacin (0.5%, Tokyo Chemical Industry Co., Japan) was administered to the lower conjunctival sac in similar manner twice at 0 and 1 h after the administration of solutions. In order to compare the number of bacteria among the groups, a sterile cotton swap tip was used to collect the bacteria in the inferior cul-de-sac at 4 h after the administration of bacterial solution. The swapped cotton tips were immediately soaked in 1 mL normal saline in an Eppendorf tube and shaken at 2800 rpm for 60 s using a microtube homogenizer (BeadBug, Benchmark Scientific, USA) to release bacterial cells from cotton tips. Next, the solution was serially diluted and plated on CDA plates for differential colony counting. After overnight incubation at 37°C, the number of colonies of E. faecalis and S. epidermidis were counted as described above. The study was approved by the IRB and Institutional Animal Care and Use Committee of Kosin University College of Medicine (KMAP-18-19). All experiments that involved animal subjects were performed in accordance with the guidelines and regulations of the Association for Research in Vision and Ophthalmology.
Statistics
All results were obtained from three independent experiments. One-way ANOVA was used to estimate differences among groups. All experiments were repeated five times to improve reliability. All data are presented as mean ± standard deviation. All statistical analyses were performed with GraphPad Prism (Version 6.03). Statistical significance was defined as p < .05.
Results
Profile of microorganisms isolated in PE ()
Two hundred and fifty-three patients of PE were included. The PE was caused by the following surgeries: post-cataract surgery (89.3%, 226 cases), glaucoma operation (4.3%, 11 cases), and vitrectomy (6.3%, 16 cases). The proportion of cataract surgery was highest among the causes of PE. The culture of intraocular specimen was performed in 226 eyes from the total 253 eyes, and the culture positivity rate was 52.2% (118/226 eyes). The most common causative bacterial species was Enterococcus spp. (28.0%, 33 cases), followed by S. epidermidis (18.6%, 22 cases) and other CNS (7.6%, 8 cases) ().
Table 1. Profile of causative microorganisms cultured from postoperative endophthalmitis.
Antibiotic susceptibility test of microorganisms isolated from PE
The second and third-generation fluoroquinolones showed relatively poor antimicrobial activity against E. faecalis, S. epidermidis, and other CNS, showing their susceptibility in the range of 42-67% of isolates (). However, the fourth-generation fluoroquinolone, moxifloxacin, showed excellent activity on S. epidermidis and other CNS (). Unfortunately, moxifloxacin was not included in the antibiotic susceptibility report for E. faecalis using automated diagnostics since moxifloxacin was not normally considered to treat E. faecalis. S. epidermidis and CNS isolates showed no resistance to moxifloxacin, but 57.1% and 54.5% were resistant to ciprofloxacin, 38.5% and 33.3% to ciprofloxacin, and 69.0% and 54.5% to oxacillin, respectively ().
Table 2. Antibiotic susceptibility results of Enterococcus faecalis, Staphylococcus epidermidis, and other coagulase-negative staphylococci (CNS) cultured from postoperative endophthalmitis samples.
Microorganisms of conjunctival microbes
To see if there are changes in the normal conjunctival microbes, 416 conjunctiva samples were cultured and 342 microorganisms were isolated. Among isolated bacteria, 257 isolates were Gram-positive bacteria (75.1%), and 85 isolates were Gram-negative bacteria (24.9%) (). The most common isolate of Gram-positive microorganism was S. epidermidis (33.0%, 113 isolates), followed by Corynebacterium spp., other CNS, and E. faecalis. The number of E. faecalis isolates was 16, that is 4.7% of total isolated microorganisms.
Table 3. Profile of conjunctival microbes. Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS, Bruker Daltonics GmbH, Germany) was used for identification of microorganisms by identification card (VITEK 2 ID Card, bioMérieux, USA).
Antibiotic susceptibility test of E. faecalis and S. epidermidis isolated from conjunctival microbes
Standard strains of E. faecalis and S. epidermidis were tested for the antibiotic susceptibility to be used as references for resistance cut-off values (Supplementary ). Our MIC data of S. epidermidis and E. faecalis match with CLSI data [Citation20]. The CLSI standard cut-off values of antibiotics resistance were used for S. epidermidis and E. faecalis clinical isolates except for the moxifloxacin resistance cut-off value for E. faecalis due to the lack of the CLSI standard data [Citation20]. In our study, we conservatively decided the resistance cut-off value as 2 μg/mL moxifloxacin for E. faecalis because it was four times the highest MIC value of E. faecalis reference strain.
From the isolates of human conjunctiva, 82 isolates of S. epidermidis and 12 isolates of E. faecalis were available for antibiotic susceptibility test. The proportion of resistant S. epidermidis isolates to ciprofloxacin, levofloxacin, and moxifloxacin was 23.2%, 24.4%, and 17.1% and that of resistant E. faecalis was 33.3%, 33.3%, and 33.3%, respectively (). The proportion of resistant E. faecalis to moxifloxacin was approximately two times higher than that of S. epidermidis. All breakpoints used here were based on systemic breakpoints provided by CLSI or derived from the method suggested by CLSI. However, the breakpoint for topical therapy has not been established so far, so we conducted survival tests of S. epidermidis and E. faecalis in vitro and in vivo co-culture model.
Table 4. Antibiotic susceptibility test of S. epidermidis and E. faecalis isolated from human normal conjunctiva to ciprofloxacin, levofloxacin, and moxifloxacin.
Survival assay in in vitro co-culture model
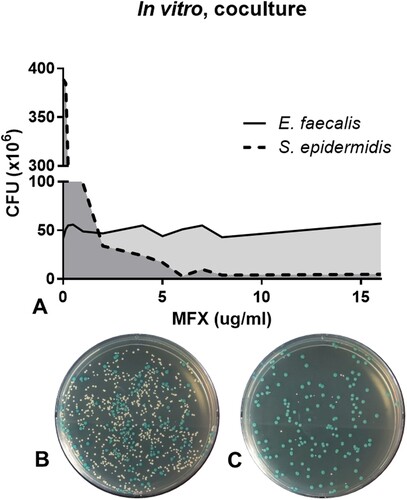
In vitro co-culture of E. faecalis and S. epidermidis was performed to understand the growth effect between two bacterial species and the effect of antibiotics on both species. Even though the equal number of bacterial cells of two species were co-cultured without antibiotics, S. epidermidis outgrew E. faecalis with approximately 8:1 ratio (A, data on 0 µg/mL moxifloxacin). When moxifloxacin was added to the co-culture, the colony numbers of S. epidermidis started to decrease from 0.25 µg/mL moxifloxacin and reached less than 10 CFU at 6.0 µg/mL moxifloxacin (, Supplementary ). On the contrary, the colony number E. faecalis was not changed even at 16.0 µg/mL moxifloxacin treatment, which was 64 times higher than E. faecalis MIC of moxifloxacin. These data show that the administration of moxifloxacin has an effect on the selective survival of E. faecalis in the in vitro co-culture setting at higher moxifloxacin concentrations.
Figure 1. Survival assay of S. epidermidis and E. faecalis in in vitro co-culture under various concentrations of moxifloxacin (A). Representative bacterial culture plate images showing the distribution of the bacterial colonies on 0.25 μg/mL moxifloxacin (B) and 16 μg/mL moxifloxacin (C). Colour detection culture plates were used; E. faecalis are shown as blue colonies and S. epidermidis are shown as white colonies. CFU, colony forming unit; MFX, moxifloxacin.
Survival assay in in vivo co-culture model
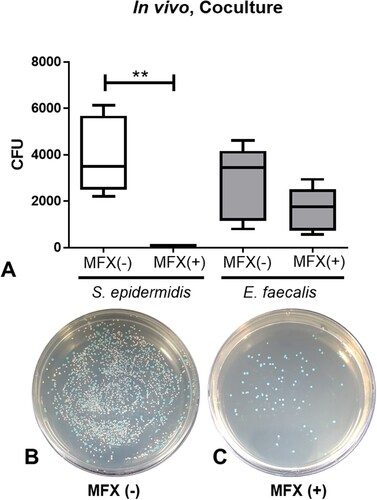
Here we further investigated the selective survival of E. faecalis under moxifloxacin exposure in the in vivo co-culture model. The number of colonies of S. epidermidis and E. faecalis in the antibiotics untreated co-culture (CO) group were 369 and 281.9 CFU, respectively, and that in moxifloxacin treated co-culture (MC) group were 62 and 164.7 CFU, respectively (). The number of colonies of S. epidermidis in the CM group was significantly lower than that of the CO group (p = 0.0008), but the number of colonies of E. faecalis did not show a significant difference between the two groups (p = 0.1973). Administration of moxifloxacin drops (twice, 5 µL, 5 mg/mL) revealed antibiotic-selective survival of E. faecalis in comparison to S. epidermidis in vivo.
Figure 2. Survival assay of S. epidermidis and E. faecalis in in vivo co-culture after administration of 0.5% moxifloxacin (A). Representative bacterial culture plate images showing the distribution of the bacterial colonies on the absence (B) and presence (C) of moxifloxacin topical drops. Colour detection culture plates were used. E. faecalis are shown as blue colonies and S. epidermidis are shown as white colonies. **, p < 0.005, CFU, colony forming unit; MFX, moxifloxacin; MFX (+), moxifloxacin-treated; MFX (−), moxifloxacin-untreated.
Discussion
This study showed that Enterococcus spp. has been one of the major causative microorganism of PE. To find out the reason for the increase of Enterococcus spp., we investigated if there were changes in the normal conjunctival microbes and established in vitro and in vivo co-culture model of E. faecalis and S. epidermidis. There was no domination of Enterococcus spp. in conjunctival microbes and E. faecalis selectively survived under the pressure of fourth-generation fluoroquinolone in the survival assays. This selective survival under fluoroquinolone could induce the domination of Enterococcus spp. on conjunctiva perioperatively, enabling Enterococcus spp. to enter the intraocular space and causing PE.
In the past, the incidence of Enterococcus spp. in PE was reported to be only about 2.2% in The Endophthalmitis Vitrectomy Study (EVS), showing much lower incidence than that of CNS (70.0%) [Citation5]. However, in addition to South Korea, the incidence of Enterococcus spp. has been increasing in Sweden, Japan, and Taiwan [Citation4,Citation7,Citation8,Citation21]. A recent report in Taiwan showed that Enterococcus spp. was the most common isolate (38.1%) of PE, followed by S. epidermidis (28.6%) [Citation8]. Kim et al. and Friling and Montan speculated that the intrinsic resistance of Enterococcus spp. to broad spectrum antibiotics used perioperatively might be associated with the emergence based on the antibiotic susceptibility results of microorganisms cultured from PE [Citation4,Citation10]. However, whether these approaches provide a valid explanation for the emergence of Enterococcus spp. as a major causative microorganism of PE has been uncertain for the following reasons. The clinical antibiotic susceptibility reports collected from many clinical laboratory departments of individual institutes were varied, often not including antibiotics that are commonly used in eye clinic such as fluoroquinolone to be tested on the Enterococcus spp. isolates. Moreover, the CLSI standard for recent fluoroquinolone drugs for Enterococcus spp. has not been defined. Therefore, in this study, we tried to test the hypothesis that the selection pressure of perioperative antibiotics is the cause of the rising E. faecalis as dominant causative microorganisms of PE by investigating the effect of fourth generation fluoroquinolone in in vitro and in vivo co-culture model of E. faecalis and S. epidermidis.
Because the source of bacteria causing PE mostly comes from the patient’s own conjunctival microbes [Citation20,Citation22], we investigated microbes distribution on conjunctiva before cataract operation or intravitreal injection. The proportions of S. epidermidis and E. faecalis in conjunctival microbes were 33.0% and 4.7% in this study. In other studies, CNS was the most common isolate, with a proportion of 28.6-72.4%, and Enterococcus spp. was rarely isolated from normal conjunctiva, while several studies isolated Enterococcus spp. from 2.5% to 5.2% in their cases [Citation23–27]. We observed similar pattern of microorganisms population isolated from normal conjunctiva to the previous studies.
Next, we examined fluoroquinolones susceptibility of E. faecalis and S. epidermidis isolated from the conjunctiva. The MIC of E. faecalis standard strain on fluoroquinolones was two to four times higher than that of S. epidermidis standard strain in the different generations of fluoroquinolones. In clinical isolates, the proportion of resistant E. faecalis to fluoroquinolones was much higher than that of S. epidermidis.
The difference of antibiotic susceptibility between E. faecalis and S. epidermidis to fluoroquinolones could be a clue implying the selective survival of E. faecalis under broad spectrum antibiotics. In vitro co-culture of E. faecalis and S. epidermidis data showed that E. faecalis can survive in response to antibiotics (from 2 to 16 µg/mL) more than S. epidermidis. Surprisingly, under an extremely high concentration of moxifloxacin (16 µg/mL), the colony number of E. faecalis was maintained in contrast to that of S. epidermidis. Without moxifloxacin, S. epidermidis is about eight times more populated than E. faecalis in in vitro co-culture model. However, E. faecalis started to populate more than S. epidermidis at the moxifloxacin concentration of 1–2 µg/mL. Considering that the concentration of moxifloxacin on the market is 0.5%, which corresponds to 5 × 103 µg/mL, one drop of moxifloxacin is about 0.05 mL containing 250 µg moxifloxacin. This phenomenon was also observed in the in vivo animal experiment. When the colony number of E. faecalis and S. epidermidis in conjunctiva were compared after moxifloxacin drops, the colony number of E. faecalis was significantly greater than that of S. epidermidis. Our data suggest the selective survival of E. faecalis in the in vivo co-culture rabbit model under the pressure of moxifloxacin. According to an existing animal study, when a single drop of 0.3% moxifloxacin was topically administrated in rabbit eyes, the concentration in the tear film dropped to less than 10 µg/mL in a short time but remained above 1.0 μg/mL upto the six hour after the administration [Citation28]. These conditions might be sufficient for E. faecalis to selectively survive on conjunctiva over other Gram positive microorganisms, including S. epidermidis.
In conclusion, we propose that the increase in Enterococcus spp. as a cause of PE may result from selective pressure of perioperative broad spectrum antibiotics such as fourth-generataion fluoroquinolones. Currently, the fourth-generation fluoroquinolone is the most commonly used antibiotic for the prevention of infection after cataract operation globally including Korea. These changes are very likely to be observed worldwide. Of concern, the increase of Enterococcus spp. isolate in PE has poor impact on the visual outcome of endophthalmitis [Citation3,Citation4,Citation11,Citation12,Citation29]. To address this challenge, various efforts will be needed in the near future.
Supplemental Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cao H, Zhang L, Li L, et al. Risk factors for acute endophthalmitis following cataract surgery: a systematic review and meta-analysis. PLoS One. 2013;8:e71731. doi: 10.1371/journal.pone.0071731
- Nam KY, Lee JEJE, Lee JEJE, et al. Clinical features of infectious endophthalmitis in South Korea: a five-year multicenter study. BMC Infect Dis. 2015;15:177. doi: 10.1186/s12879-015-0900-5
- Han DP, Vine AK, Blodi BA, et al. Microbiologic factors and visual outcome in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:830–846. doi: 10.1016/S0002-9394(14)71959-2
- Kim HW, Kim SY, Chung IY, et al. Emergence of Enterococcus species in the infectious microorganisms cultured from patients with endophthalmitis in South Korea. Infection. 2014;42:113–118. doi: 10.1007/s15010-013-0530-z
- HAN DP, Wisniewski SR, Wilson LA, et al. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy study. Am J Ophthalmol. 1996;122:1–17. doi: 10.1016/S0002-9394(14)71959-2
- Benz MS, Scott IU, Flynn HW, et al. Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/S0002-9394(03)00896-1
- Friling E, Lundström M, Stenevi U, et al. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15–21. doi: 10.1016/j.jcrs.2012.10.037
- Teng YT, Teng MC, Kuo HK, et al. Isolates and antibiotic susceptibilities of endophthalmitis in postcataract surgery: a 12-year review of culture-proven cases. Int Ophthalmol. 2017;37:513–518. doi: 10.1007/s10792-016-0288-2
- Lundström M, Friling E, Montan P. Risk factors for endophthalmitis after cataract surgery: Predictors for causative organisms and visual outcomes. J Cataract Refract Surg. 2015;41:2410–2416. doi: 10.1016/j.jcrs.2015.05.027
- Friling E, Montan P. Bacteriology and cefuroxime resistance in endophthalmitis following cataract surgery before and after the introduction of prophylactic intracameral cefuroxime: a retrospective single-centre study. J Hosp Infect. 2019;101:88–92. doi: 10.1016/j.jhin.2018.02.005
- Scott IU, Loo RH, Flynn HW, et al. Endophthalmitis caused by Enterococcus faecalis: antibiotic selection and treatment outcomes. Ophthalmology. 2003;110:1573–1577. doi: 10.1016/S0161-6420(03)00502-5
- Todokoro D, Suzuki T, Kobayakawa S, et al. Postoperative Enterococcus faecalis endophthalmitis: virulence factors leading to poor visual outcome. Jpn J Ophthalmol. 2017;61:408–414. doi: 10.1007/s10384-017-0527-8
- Kuriyan AE, Sridhar J, Flynn HW, et al. Endophthalmitis caused by enterococcus faecalis: clinical features, antibiotic sensitivities, and outcomes. Am J Ophthalmol. 2014;158:1018–1023. e1. doi: 10.1016/j.ajo.2014.07.038
- Miller WR, Munita JM, Arias CA. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti. Infect. Ther. 2014. doi:10.1586/14787210.2014.956092.
- Arsene S, Leclercq R. Role of a qnr-like gene in the intrinsic resistance of Enterococcus faecalis to fluoroquinolones. Antimicrob Agents Chemother. 2007;51:3254–3258. doi: 10.1128/AAC.00274-07
- Choi S, Hahn TW, Osterhout G, et al. Comparative intravitreal antibiotic therapy for experimental Enterococcus faecalis endophthalmitis. Arch Ophthalmol. 1996;114:61. doi: 10.1001/archopht.1996.01100130057009
- Behnding A, Ravindran R, Flynn H, et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns around the world. Curr Pharm Des. 2017;23:565–573. doi: 10.2174/1381612822666161216122230
- Chang DF, Braga-Mele R, Henderson BA, et al. ASCRS cataract Clinical Committee. antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300–1305. doi: 10.1016/j.jcrs.2015.01.014
- Hoogkamp-Korstanje JA. In-vitro activities of ciprofloxacin, levofloxacin, lomefloxacin, ofloxacin, pefloxacin, sparfloxacin and trovafloxacin against gram-positive and gram-negative pathogens from respiratory tract infections. J Antimicrob Chemother. 1997;40:427–431. doi: 10.1093/jac/40.3.427
- Segreti J, Jones RN, Bertino JS. Challenges in assessing microbial susceptibility and predicting clinical response to newer-generation fluoroquinolones. J Ocul Pharmacol Ther. 2012;28:3–11. doi: 10.1089/jop.2011.0072
- Suzuki T, Todokoro D, Kobayakawa S, et al. [Postcataract endophthalmitis caused by Enterococcus faecalis]. Nihon Ganka Gakkai Zasshi. 2014;118:22–27.
- Bannerman TL, Rhoden DL, McAllister SK, et al. The source of coagulase-negative staphylococci in the endophthalmitis vitrectomy study. Arch Ophthalmol (Chicago, Ill 1960). 1997;115:357. doi: 10.1001/archopht.1997.01100150359008
- Park SH, Lim J-A, Choi J-S, et al. The resistance patterns of normal ocular bacterial flora to 4 fluoroquinolone antibiotics. Cornea. 2009;28:68–72. doi: 10.1097/ICO.0b013e318182259b
- Capriotti JA, Pelletier JS, Shah M, et al. Normal ocular flora in healthy eyes from a rural population in Sierra Leone. Int Ophthalmol. 2009;29:81–84. doi: 10.1007/s10792-008-9196-4
- Ratnumnoi R, Keorochana N, Sontisombat C. Normal flora of conjunctiva and lid margin, as well as its antibiotic sensitivity, in patients undergoing cataract surgery at Phramongkutklao Hospital. Clin Ophthalmol. 2017;11:237–241. doi: 10.2147/OPTH.S109247
- Hoshi S, Hashida M, Urabe K. Risk factors for aerobic bacterial conjunctival flora in preoperative cataract patients. Eye. 2016;30:1439–1446. doi: 10.1038/eye.2016.143
- Huang Y, Yang B, Li W. Defining the normal core microbiome of conjunctival microbial communities. Clin Microbiol Infect. 2016;22:643.e7–643.e12. doi: 10.1016/j.cmi.2016.04.008
- Robertson SM, Curtis MA, Schlech BA, et al. Ocular pharmacokinetics of moxifloxacin after topical treatment of animals and humans. Surv Ophthalmol. 2005;50; doi:10.1016/j.survophthal.2005.07.001.
- Nam KY, Kim HW, Jeung WJ, et al. Comparison of the most common isolates of postoperative endophthalmitis in South Korea; Enterococcus species vs coagulase-negative staphylococci. BMC Infect Dis. 2016;16:706. doi: 10.1186/s12879-016-2038-5
