ABSTRACT
Cryptosporidium felis is the major etiologic agent of cryptosporidiosis in felines and has been reported in numerous human cryptosporidiosis cases. Sequence analysis of the 60-kDa glycoprotein (gp60) gene has been developed for subtyping C. felis recently. In this study, 66 C. felis isolates from the United States, Jamaica, Peru, Portugal, Slovakia, Nigeria, Ethiopia, Kenya, China, India and Australia were subtyped using the newly established tool. Forty-four specimens yielded gp60 sequences, generating 23 subtypes clustered in 4 subtype families (XIXa, XIXc, XIXd and XIXe) with high bootstrap support in a phylogenetic analysis of sequence data. Among them, XIXa showed high genetic diversity at the nucleotide level, with the formation of 18 subtypes from both cats and humans with different geographic distribution. In contrast, all 11 XIXd isolates derived from humans from various countries had identical sequences. Results of this study improve our understanding of the genetic diversity, host specificity and transmission dynamics of C. felis.
Introduction
Cryptosporidium spp. are protozoan parasites that can cause significant gastrointestinal disease in humans and animals [Citation1]. Currently, the majority of reported cases of human cryptosporidiosis are caused by several Cryptosporidium species, including C. hominis, C. parvum, C. meleagridis, C. felis, C. canis, C. cuniculus, and C. ubiquitum [Citation2–5]. Among them, C. felis was initially reported in domestic cats from Japan in 1979 and proposed as a new species based on host occurrence and oocyst morphology, which was later supported by genetic characterizations of isolates [Citation6,Citation7]. This species has been reported in numerous human cases subsequently [Citation8], and sometimes in association with the presence of infection in cats in the same household [Citation9,Citation10].
Sequence analysis of the 60-kDa glycoprotein (gp60) gene has been widely used in subtyping C. hominis and C. parvum, with the identification of host-adapted subtype families within both species [Citation11]. Recently, gp60-based subtyping tools have been developed for some other Cryptosporidium spp. such as C. meleagridis, C. ubiquitum, C. viatorum, C. ryanae and Cryptosporidium chipmunk genotype I [Citation12–16]. The use of these subtyping tools has significantly improved our understanding of the transmission of Cryptosporidium spp., especially the role of anthroponotic and zoonotic infections in cryptosporidiosis epidemiology [Citation2,Citation15].
A gp60-based subtyping tool has become available recently for C. felis [Citation10]. Thus far, the genetic characterization of C. felis has been mostly restricted to isolates acquired in three European countries (the United Kingdom, Sweden and Denmark). In this study, additional C. felis isolates mostly from Africa and the Americas were analysed to understand the geographic and host distribution of subtypes within this zoonotic Cryptosporidium species.
Materials and methods
Specimens
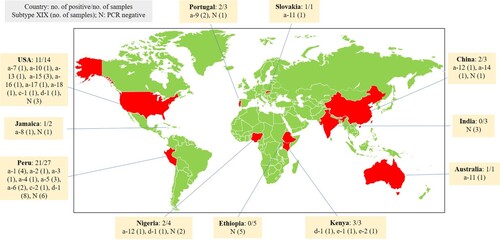
DNA preparations from 66 C. felis-positive samples were included in this study, including 58 from humans, 6 from cats, 1 from a calf, and 1 from a rhesus macaque. Human samples were collected in the United States, Jamaica, Peru, Portugal, Ethiopia, Kenya, Nigeria, India, and China; cat samples were from the United States, Peru, Slovakia, and Australia; while the calf sample was from Portugal and rhesus macaque sample from China (). Genomic DNA samples were stored in –80°C freezer for no more than 20 years prior to PCR analysis in the study. These samples were initially determined to be positive for C. felis by PCR and sequence analysis of the small subunit (SSU) rRNA gene as previously described [Citation17].
Table 1. Cryptosporidium felis isolates used in this study, their subtype identity and copy numbers of major tandem repeats in the gp60 gene.
PCR analysis of the gp60 gene
The recently developed nested PCR targeting the partial C. felis gp60 gene was used in typing the C. felis isolates in the present study [Citation10]. The primers used included GP60-Felis-F1 (5′-TTT CCG TTA TTG TTG CAG TTG CA-3′) and GP60-Felis-R1 (5′-ATC GGA ATC CCA CCA TCG AAC-3′) in primary PCR and GP60-Felis-F2 (5′-GGG CGT TCT GAA GGA TGT AA-3′) and GP60-Felis-R2 (5′-CGG TGG TCT CCT CAG TCT TC-3′) in secondary PCR. The amplicons were approximately 1,200 and 900 bp, respectively. The primary and secondary PCR mixtures contained 1 µl of DNA template (for primary PCR) or 2 µl of primary PCR product (for secondary PCR), 250 nM primary PCR primers or 500 nM secondary PCR primers, 2.5 mM MgCl2, 200 µM deoxynucleotide triphosphates, 1 × PCR buffer (15 mM Tris-HCl, 50 mM KCl and MgCl2; pH=8.0), and 1.5 U Taq polymerase in a total of 50 µl. To reduce PCR inhibition, 400 ng/µl of nonacetylated bovine serum albumin was used in the primary PCR. The amplification was performed on a GeneAmp PCR 9700 thermocycler (Applied Biosystems, Foster City, CA), consisting of an initial denaturation at 94°C for 5 min; 35 cycles at 94°C for 45 s, 52°C for 45 s, and 72°C for 80 s; and a final extension at 72°C for 10 min. Both positive (C. felis DNA from a human sample from Peru) and negative (molecular-grade water) controls were used in each PCR run. The secondary PCR products were visualized under the UV light after 1.5% agarose gel electrophoresis.
Sequence analyses
All positive secondary PCR products were sequenced in both directions on an ABI 3130xl Genetic Analyzer (Applied Biosystems). The sequences were assembled using ChromasPro 2.1.6 (www.technelysium.com.au/ ChromasPro.html), manually cleaned and edited using BioEdit 7.0.5.3 (www.mbio.ncsu.edu/BioEdit/bioedit.html), and aligned with each other and reference sequences downloaded from GenBank using MUSCLE implemented in MEGA 10 (www.megasoftware.net/) with manual adjustments of sequence gaps. Simple tandem repeats presented in nucleotide sequences were identified using Tandem Repeats Finder 4.09 (http://tandem.bu.edu/trf/trf.html). To infer the phylogenetic relationship among subtypes of C. felis, a maximum-likelihood tree was constructed in MEGA 10 using the general time reversible model and gamma distribution in the calculation of substitution rates. The reliability of cluster formation was evaluated using the bootstrap method with 1,000 replicates. Unique nucleotide sequences derived from this study were deposited in GenBank under accession nos. MT458667–MT458684 and MT636067–MT636069.
Results
Amplification efficiency of the gp60 PCR
PCR products of the expected size were obtained from 44 (67%) of the 66 C. felis DNA preparations, including 37/58 from humans, 6/6 from cats, and 1/1 from a rhesus macaque. All 3 Indian and 5 Ethiopian isolates were negative in the gp60 PCR. The success rates for C. felis in other countries were 21/27 for Peru, 11/14 for the United States, 3/3for Kenya, 2/4 for Nigeria, 2/3 for Portugal, 2/2 for China, 1/2 for Jamaica, 1/1 for Slovakia and 1/1 for Australia (). They generated PCR products of visibly different sizes as revealed by agarose gel electrophoresis.
Sequence characteristics of the gp60 gene of C. felis
The multiple-sequence alignment generated showed the presence of 23 sequence types in four major groups (). Among the four groups, numerous nucleotide substitutions were present over the partial gp60 gene. In addition to the differences in nucleotide differences, there were significant length differences in nucleotide sequences due mostly to the presence of repetitive sequences. Within the most variable group with 29 sequences, the presence of indels and single nucleotide substitutions (SNPs) led to the formation of 18 subtypes. Among them, the 6 subtypes from Peru differed from each other mostly in indels, while the 7 subtypes from the United States differed from each other in indels as well as SNPs. In contrast, the most conserved sequence group with 11 sequences had no nucleotide differences, whereas the other two minor sequence groups contained two sequences with only one SNP each. Significant nucleotide differences were found among the sequence groups.
Table 2. Differences in the number and nature of various tandem repeats among Cryptosporidium felis subtype families.
The difference in the size of PCR products was due to the presence of sequence repeats in the gp60 gene. A 33-bp repeat sequence 5′-CCA CCT AGT GGC GGT AGT GGC GTG TCC CCT GCT-3′ was found at the nucleotides ∼450–530 of the sequence alignment. The sequences generated had 1–3 copies of this repeat (the last copy was incomplete with nearly half of the length). Likewise, a 39-bp repeat sequence 5′-AGC ACA ACT ACG GCT ACA GCG AGC ACT GCG AGT TCG ACA-3′ was also observed at nucleotides ∼770–910 of the alignment. Sequences from the study had 1–4 copies of this repeat. These sequences also contained different copies of the trinucleotide repeat GTT at the 3′ end ( and ).
Phylogenetic relationship among C. felis subtypes
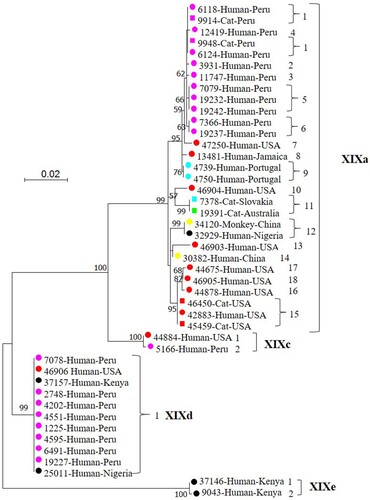
In the maximum likelihood tree, the 44 DNA sequences formed four distinct clusters as expected, with 99–100% bootstrap support (). Three of the four clusters were found in the previous study [Citation10], while one cluster seen in the previous study, B, was absent from the present study. In accordance with the established nomenclature system for Cryptosporidium subtype families [Citation11], the four clusters found in the present study were named as XIXa, XIXc, XIXd and XIXe (newly identified in the present study) subtype families, while the previous Cluster B was named as XIXb subtype family. In the present study, the dominant subtype families XIXa and XIXd contained 29 and 11 isolates, respectively, whereas the other two minor ones contained two isolates each. Among the four subtype families of C. felis, subtype families XIXd and XIXe were more distant from subtype families XIXa and XIXc (). Within the subtype family XIXa, the 6 subtypes from Peru clustered together in the phylogenetic tree, while only 4 of the 7 subtypes from the United States formed a country-associated clade. In the former, two isolates from cats in Lima, Peru had the same subtype (XIXa-1) as two isolates from HIV+ patients in the same city ().
Figure 1. Phylogenetic relationship among four Cryptosporidium felis subtype families identified in the present study based on a maximum likelihood analysis of the partial gp60 gene. Substitution rates were calculated by using the general time reversible model. Numbers on branches are percent bootstrapping values over 50 using 1,000 replicates. Round and square labels indicate samples from humans (including one from a monkey) and cats, respectively. Red, pink, blue, black, yellow and green labels indicate samples from North America, South America, Europe, Africa, Asia and Oceania, respectively.
Distribution of C. felis subtypes by host
Sequences obtained from all 6 feline isolates clustered within the subtype family XIXa, while human-derived isolates could be found in all four clusters. Among the 25 C. felis isolates from humans in Lima, Peru, there were no obvious differences in subtype distribution between children and HIV+ adults. The XIXa subtype family contained one nonhuman primate isolate from China, which generated a sequence identical to the one from a human in Nigeria. With the exception of a calf sample, all PCR failures (21 in 66) occurred with human isolates ().
Distribution among C. felis subtypes by country
Among the four subtype families generated from this study, XIXa was found in the United States (9), Jamaica (1), Peru (12), Portugal (2), Slovakia (1), China (1), and Australia (1); XIXc was found in the United States (1) and Peru (1); XIXd was found in the United States (1), Peru (8), Kenya (1), and Nigeria (1); and XIXe was only found in Kenya (2) (, ).
Among the 18 C. felis XIXa subtypes, XIXa-1 (4), XIXa-2 (1), XIXa-3 (1), XIXa-4 (1), XIXa-5 (3), and XIXa-6 (1) were found in Peru; XIXa-7 (1), XIXa-10 (1), XIXa-13 (1), XIXa-15 (2), XIXa-16 (1), XIXa-17 (1), and XIXa-18 (1) in the United States; XIXa-8 (1) in Jamaica; XIXa-9 (2) in Portugal; XIXa-11 (1) in Slovakia; XIXa-12 (1) in Nigeria; XIXa-12 (1) and XIXa-14 (1) in China; and XIXa-11 (1) in Australia (, ).
Discussion
Studies on the transmission of C. felis in humans have been hampered for a long time by the lack of subtyping tools until the recent development of a gp60-based PCR for this zoonotic species [Citation10,Citation11,Citation18]. In this study, the newly developed subtyping tool was utilized in genetic characterization of diverse isolates from several hosts and countries and a nomenclature system was developed in naming the subtype families of C. felis identified thus far. Results of this analysis show a high genetic diversity of this parasite and, together with the data from the published study, the possible occurrence of human-adapted populations of C. felis.
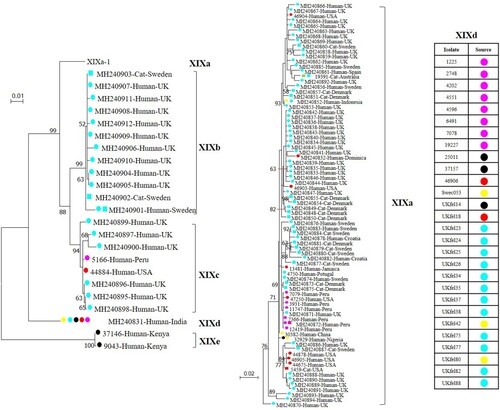
The gp60 gene of C. felis appears to be highly polymorphic compared to its orthologs in other Cryptosporidium spp. This is reflected by the existence of four subtype families among the small number of samples characterized in the present study, and the extensive sequence differences among subtype families and subtypes in both SNPs and indels. This finding is in agreement with recent subtyping data generated by gp60 sequence analysis of 128 C. felis isolates [Citation10]. Subtyping data from the two studies have shown that C. felis consists of at least five subtype families in two large clades in a maximum-likelihood tree (). The first clade consists of subtype families XIXa, XIXb, and XIXc with extensive sequence variations within each subtype family, while the second one consists of XIXd and XIXe with relatively conserved sequences.
Figure 3. Phylogenetic relationship of known Cryptosporidium felis subtypes based on a maximum likelihood analysis of the partial gp60 gene. Substitution rates were calculated using the general time reversible model. Numbers on branches are percent bootstrap values over 50 from analysis with 1000 replicates. Round and square labels indicate samples from humans (including one from a monkey) and cats, respectively. Red, pink, blue, black, yellow and green labels indicate samples from North America, South America, Europe, Africa, Asia and Oceania, respectively. Phylogenetic relationships of five subtype families and the XIXa subtypes are shown in the left tree and the right tree, respectively. The sources of subtype XIXd-1 isolates are indicated in the right table.
Although C. felis is considered a feline-specific parasite, it has been reported in significant numbers of humans (both immunocompromised and immunocompetent persons) and a few nonhuman primates, calves, horses, foxes [Citation19–24]. Data generated in this study suggest the immune status of patients is irrelevant to the human infections with C. felis subtypes. This is supported by finding subtype families XIXa, XIXc and XIXd in both immunocompromised and immunocompetent patients, including reasonable number of isolates examined from children and AIDS patients in Lima, Peru (). As all gp60 PCR failures occurred with non-feline isolates in this study, some of these hosts could have only light infections of C. felis. The identification of C. felis in faecal specimens was based on nested PCR analysis of the SSU rRNA gene, which has five copies per genome, compared with one copy of the gp60 gene.
Data generated from this study suggest that there could be host adaptation within C. felis. This is supported by the observation of all 11 investigated subtype family XIXd isolates were derived from humans, whereas both human and feline subtypes could be observed in subtype families XIXa and XIXb. Hence, C. felis subtype family XIXd might be adapted to humans. This finding is supported by the published data; in which all 28 XIXd isolates were from humans, while the subtype families XIXa and XIXb contained isolates from both humans and cats (, ) [Citation10]. Notably, no feline isolates were found in the XIXc, XIXd and XIXe subtype families, but only a small number of isolates were characterized for each of them, especially XIXd and XIXe. Therefore, further epidemiological and experimental data are needed to assess the prevalence and infectivity of the subtype families XIXc, XIXd and XIXe in felines. Host adaption is common in some zoonotic Cryptosporidium spp. at the gp60 subtype family level [Citation25], such as C. parvum subtype family IIc in humans, C. hominis subtype family Ik in equine animals, and C. ubiquitum subtype family XIIa in small ruminants [Citation8,Citation13].
Table 3. Distribution of Cryptosporidium felis subtypes and copy numbers of major tandem repeats in published studies.Table Footnotea
Potential geographic segregation exists in the distribution of some C. felis subtypes. This is supported by the presence of genetically related clusters of C. felis subtypes in the same geographic area. For example, subtypes XIXa-1–XIXa-6 were only detected in Peru, and XIXa-13–XIXa-18 were only found in the United States, whereas subtypes XIXa-8, XIXa-9 and XIXe were found in Jamaica, Portugal and Kenya, respectively. Similarly, subtypes in the XIXb subtype family have been only detected in Europe (the United Kingdom and Sweden) thus far () [Citation10]. Moreover, due likely to the presence of geographically unique subtypes, all five Indian and three Ethiopian C. felis isolates were PCR negative. However, data generated in this and previous studies also indicate that C. felis subtype XIXd-1 has a world-wide distribution, as it has been detected in geographically distant areas, such as the United States, United Kingdom, Peru and several African and Asian countries () [Citation10]. Nevertheless, these suggestions are based on the characterization of a small number of samples from limited areas. More data are needed to substantiate the presence of geographic differences in the distribution of C. felis subtypes.
In conclusion, 44 C. felis isolates were successfully characterized using the newly developed gp60 subtyping tool and a nomenclature system was developed for naming C. felis subtypes. The data generated from the present and published studies both suggest potential host-adaptation and geographic isolation within C. felis at the subtype level. Further studies involving more samples from diverse areas, especially those from cats and other animals, are needed to confirm these observations and to improve our understanding of the transmission of this important zoonotic pathogen.
Acknowledgments
We thank our collaborators of earlier studies for providing some C. felis specimens used in the present study. The findings and conclusions in this report are those of the authors and do not necessarily represent the represent of the official position of the U.S. Centers for Disease Control and Prevention.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Checkley W, White Jr AC, Jaganath D, et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect Dis. 2015 Jan;15(1):85–94.
- Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitol. 2014 Nov;141(13):1667–1685.
- Martinez-Ruiz R, de Lucio A, Fuentes I, et al. Autochthonous Cryptosporidium cuniculus infection in Spain: first report in a symptomatic paediatric patient from Madrid. Enferm Infecc Microbiol Clin. 2016 Oct;34(8):532–534.
- Garcia RJ, Cox MP, Hayman DTS. Comparative genetic diversity of Cryptosporidium species causing human infections. Parasitology. 2020 Aug 10: 1–6.
- Elwin K, Hadfield SJ, Robinson G, et al. The epidemiology of sporadic human infections with unusual cryptosporidia detected during routine typing in England and Wales, 2000–2008. Epidemiol Infect. 2012 Apr;140(4):673–683.
- Iseki M. Cryptosporidium felis sp. n. (Protozoa Eimeriorina) from the domestic cat. Jap J Parasitol. 1979;28:285–307.
- Morgan UM, Sargent K D, Elliot A, et al. Cryptosporidium in cats – additional evidence for C. felis. Vet J. 1998 Sep;156(2):159–161.
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010 Jan;124(1):80–89.
- Beser J, Toresson L, Eitrem R, et al. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect Ecol and Epidemiol. 2015;5:28463.
- Rojas-Lopez L, Elwin K, Chalmers R M, et al. Development of a gp60-subtyping method for Cryptosporidium felis. Parasit Vectors. 2020 Jan 23;13(1):39–46.
- Xiao L, Feng Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017;8–9:14–32.
- Guo Y, Cebelinski E, Matusevich C, et al. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J Clin Microbiol. 2015 May;53(5):1648–1654.
- Li N, Xiao L, Alderisio K, et al. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg Infect Dis. 2014 Feb;20(2):217–224.
- Stensvold C R, Elwin K, Winiecka-Krusnell J, et al. Development and application of a gp60-based typing assay for Cryptosporidium viatorum. J Clin Microbiol. 2015 Jun;53(6):1891–1897.
- Stensvold C R, Beser J, Axen C, et al. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J Clin Microbiol. 2014 Jul;52(7):2311–2319.
- Yang X, Huang N, Jiang W, et al. Subtyping Cryptosporidium ryanae: a common pathogen in bovine animals. Microorganisms. 2020 Jul 24;8(8):1107.
- Xiao L, Sulaiman I M, Ryan U M, et al. Host adaptation and host-parasite co-evolution in Cryptosporidium: implications for taxonomy and public health. Int J Parasitol. 2002 Dec 19;32(14):1773–1785.
- Lucio-Forster A, Griffiths JK, Cama VA, et al. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010 Apr;26(4):174–179.
- Matos O, Alves M, Xiao L, et al. Cryptosporidium felis and C. meleagridis in persons with HIV, Portugal. Emerg Infect Dis. 2004 Dec;10(12):2256–2257.
- Cama V A, Bern C, Sulaiman I M, et al. Cryptosporidium species and genotypes in HIV-positive patients in Lima, Peru. J Eukaryot Microbiol. 2003;50(Suppl):531–533.
- Bornay-Llinares FJ, da Silva AJ, Moura IN, et al. Identification of Cryptosporidium felis in a cow by morphologic and molecular methods. Appl Environ Microbiol. 1999 Apr;65(4):1455–1458.
- Cardona G A, de Lucio A, Bailo B, et al. Unexpected finding of feline-specific Giardia duodenalis assemblage F and Cryptosporidium felis in asymptomatic adult cattle in Northern Spain. Vet Parasitol. 2015 Apr 30;209(3–4):258–263.
- Guo PF, Chen TT, Tsaihong JC, et al. Prevalence and species identification of Cryptosporidium from fecal samples of horses in Taiwan. Southeast Asian J Trop Med Public Health. 2014 Jan;45(1):6–12.
- Ye J, Xiao L, Ma J, et al. Anthroponotic enteric parasites in monkeys in public park, China. Emerg Infect Dis. 2012 Oct;18(10):1640–1643.
- Feng Y, Ryan U, Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018 Nov;34(11):997–1011.