ABSTRACT
Acinetobacter baumannii causes healthcare-associated infections worldwide. Capsular polysaccharide (CPS) is shown an important virulence factor of A. baumannii both in vitro and in vivo. Capsule locus 2 (KL2) for CPS is the most common KL type and is associated with carbapenem resistance. It is unclear whether KL2 is related to the clinical outcome of invasive A. baumannii infection. Here we had followed patients with A. baumannii bacteraemia prospectively between 2009 and 2014. One-third of the unduplicated blood isolates were randomly selected each year for microbiological and clinical studies. The KL2 gene cluster was identified using polymerase chain reaction. A total of 148 patients were enrolled randomly. Eighteen isolates (12.2%) carried KL2, and 130 isolates (87.8%) didn’t. Compared with non-KL2 isolates, KL2 isolates had significantly higher resistance to imipenem, sulbactam, and tigecycline. Compared with the non-KL group, in the KL2 group, the hospital stay before development of bacteraemia was longer (P < 0.001), a higher percentage had pneumonia (P = 0.004), and the white blood cell count was lower (P = 0.03). Infection with KL2 A. baumannii predicted mortality (adjusted hazard ratio [aHR], 2.03; 95% confidence interval [CI], 1.09–3.78; P = 0.03), independently of the Pitt bacteraemia score (aHR, 1.34; 95% CI, 1.23–1.46; P < 0.001) and leucopenia (aHR, 2.16; 95% CI, 1.30–3.57; P = 0.003). Thrombocytopenia contributed to the effect of KL2 on mortality in bacteraemia (Sobel test P = 0.01). Large-scale studies are warranted to confirm these findings and the underlying mechanisms deserve further investigation.
Introduction
Acinetobacter baumannii is a major pathogen implicated in hospital-acquired infections across the globe over past two decades [Citation1]. Infection with A. baumannii is associated with a high mortality rate [Citation2–4]. Appropriate empirical antimicrobial therapy is important for the treatment of A. baumannii infections [Citation5], but the threat remains because of emerging resistance worldwide. The mortality rate of A. baumannii bacteraemia remains high even after appropriate treatment [Citation5]. Therefore, the mechanisms of A. baumannii virulence warrant investigation.
In A. baumannii bacteraemia, different clones can cause different outcomes [Citation6]. Sequence type (ST) 2 had been reported as the dominant multilocus sequence type of A. baumannii, and exhibits many virulence-related traits, such as high ability of biofilm formation and adherence to lung epithelial cells [Citation7]. Genotype ST2 is associated with worse clinical outcomes in infections [Citation8]. In A. baumannii, capsular polysaccharide (CPS) is a major virulence factor that is crucial for bacterial survival in vitro and in vivo [Citation9]. Specific capsule types are proved to overwhelm mammalian defences in vivo, including K2 [Citation10]. K2 capsular polysaccharide is associated with capsule locus (K locus) 2 gene cluster, which encodes enzymes for biosynthesis and export of CPS [Citation11]. Capsule locus 2 (KL2) is the most common KL type in analysis of posted whole genome sequences [Citation11,Citation12], and most of KL2 strains belong to ST2 [Citation11].
The primary aim of this study was to examine whether KL2 A. baumannii is more virulent and leads to higher mortality in invasive infection. We also aimed to delineate the KL2 distributions among the patients with A. baumannii bacteraemia in Taiwan.
Materials and methods
Hospital setting, bacterial isolates, and patients
This study was conducted at the National Taiwan University Hospital, a 2200-bed medical centre, located in northern Taiwan. Between January 2009 and December 2014, patients who had bacteraemia caused by A. baumannii complex were enrolled prospectively. For patients who experienced multiple episodes of A. baumannii complex bacteraemia, only the first episodes were included. We randomly selected one third of the non-duplicated A. baumannii complex in each year for microbiological studies. Bacterial isolates of genospecies-identified A. baumannii were subjected for KL2 gene cluster identification, and these patients were included for clinical investigation. Only adults older than 18 years were included. This study was approved by the Research Ethics Committee of NTUH (NTUH 201008047R). The isolates were collected as part of clinical routine practice, and the informed consent process was waived by the ethics committee.
Microbiological studies
Blood cultures were processed by the clinical microbiology laboratory at NTUH and the isolates of A. baumannii complex identified using the VITEK 2 system (bioMérieux Inc., La Balme les Grottes, France) were collected. Genospecies were identified according to 16S–23S ribosomal RNA gene intergenic spacer region, as described previously [Citation4].
Antimicrobial susceptibility testing
The minimum inhibitory concentrations (MICs) of colistin, tigecycline and imipenem for A. baumannii isolates were determined using broth microdilution. The MICs of levofloxacin, ampicillin-sulbactam, cefepime, amikacin and minocycline were determined using Sensititre GNX3F (Trek Diagnostics, West Sussex, England), and were interpreted according to the Clinical and Laboratory Standards Institute [Citation13]. Isolates for which tigecycline MIC of ≤2 μg/mL were considered as susceptible [Citation14].
Multilocus sequence typing
Primers for the seven house-keeping genes (cpn60, fusA, gltA, pyrG, recA, rplB, and rpoB) were designed, and sequencing were performed according to the methods of Diancourt et al. [Citation15]. Sequence chromatograms were edited and stored using BioNumerics software (version 6.0). Allele sequences and allelic profiles were compared with those available on the MLST website of the Institute Pasteur (www.pasteur.fr/mlst), and sequence type were assigned.
Capsule locus 2 identification
Two sets of primers were used to detect KL2 gene cluster by polymerase chain reaction according to the method of Kenyon et al. [Citation11]. Firstly, primers psaF_f (5′–TGGTGAAGCAATTCAAGCTG–3′) and psaF_r (5′–AATAAGGCATGCACCCAAAG–3′) were designed to detect any K locus with a psa module responsible for synthesis of pseudaminic acid [Citation16]. Secondly, among psa module-carrying K loci, primers wzy_gtr3_f (5′–CTCTTATCGGGCTCAAAATC–3′) and wzy_gtr3_r (5′–GCCCATTTACTATCAACCCG–3′) were used to detect the region between wzy and gtr3 that was specific for the KL2 [Citation11].
Clinical data collection and definitions
We collected clinical data prospectively for enrolled patients. The data included demographic data, underlying diseases, infectious focus, regimens of antimicrobial therapy, and data of laboratory examination results at the time of bacteraemia onset. The site of infection was classified according to US Centres for Disease Control and Prevention [Citation17]. Patients were classified as having primary bacteraemia when no obvious port of entry identified. The Charlson comorbidity index was used to adjust patient’s underlying diseases [Citation18], and the Pitt bacteraemia score was used to measure the severity of bacteraemia [Citation19]. All-cause in-hospital mortality was recorded.
The onset day of A. baumannii bacteraemia was defined as the day when at least one set of blood culture that was positive for A. baumannii was drawn. Appropriate empirical antimicrobial therapy was defined as using intravenous antimicrobial agent to which A. baumannii isolate was susceptible within 2 days of bacteraemia onset. Tigecycline was considered an appropriate regimen only while treating infections caused by isolates for which tigecycline MIC of <1 μg/mL [Citation20]. Healthcare-associated bloodstream infection was defined as bacteraemia that occurred more than 48 h after hospitalization [Citation21]. Use of immunosuppressive agents was defined as usage of antineoplastic, cytotoxic chemotherapy, or corticosteroids at a dosage of prednisolone ≥20 mg/day for ≥2 weeks or prednisolone 30 mg/day for ≥1 week within 6 weeks before the onset of bacteraemia [Citation4]. The primary outcome was 28-day all-cause mortality following the onset of A. baumannii bacteraemia.
Statistical analysis
The median and inter-quartile range (IQR) were calculated for continuous variables. The percentages were calculated for categorical variables. The Mann–Whitney U test and Fisher’s exact test were used to compare continuous and categorical variables, respectively, between two groups. Multivariate Cox proportional-hazards models were used to analyse the outcome. Variables with a P value of ≤0.2 in univariate regression were added in a stepwise manner and selected for the final model in multivariate analysis by minimizing Akaike's information criterion [Citation22]. Intermediate variables were identified using the Sobel test [Citation23,Citation24]. Because of the potential for overadjustment bias, intermediate variables were not included in outcome analysis in the final model [Citation25]. The data were analysed using Stata software (version 14, StataCorp, College Station, TX, USA). Two-sided P values ≤0.05 were considered to be significant.
Results
During the study period, a total of 148 A. baumannii blood isolates were collected for KL2 identification. Eighteen (12.2%) isolates had CPS associated with KL2. As shown in , KL2 isolates had significantly higher resistance to amikacin, cefepime, imipenem, levofloxacin, sulbactam, and tigecycline. There was no difference in colistin resistance between KL2 and non-KL2 isolates (P = 0.99).
Table 1. Comparison of the minimum inhibitory concentrations and resistance rates of capsule locus 2 (KL2) and non-capsule locus 2 (non-KL2) isolates of Acinetobacter baumannii.
The median (IQR) age of the 148 patients enrolled was 62.5 (22.2) years. The median Pitt bacteraemia score was 4 (7) points, Charlson comorbidity index was 3 (4) points, and hospitalization duration before A. baumannii bacteraemia onset was 18 (28) days. Sixty-three (42.6%) patients had underlying malignancies, and 50 (33.8%) patients had received an immunosuppressive agent. The 28-day all-cause mortality rate was 45.9%.
The baseline characteristics differed between KL2 and non-KL2 groups (). The KL2 patient group had a longer hospital stay before bacteraemia onset (44.5 days vs. 15 days, P < 0.001), higher percentage of pneumonia (72.2% vs. 35.4%, P = 0.004), and lower white blood cell counts and platelet counts at the onset bacteraemia (P = 0.03, and 0.005 respectively). The underlying diseases and Charlson comorbidity index did not differ significantly between the KL2 and non-KL2 groups. Although the percentage of patients receiving appropriate empirical antimicrobial therapy (P = 0.80) and the Pitt bacteraemia scores (P = 0.18) did not differ significantly between groups, the KL2 group had a higher risk of 28-day mortality than the non-KL2 group (P = 0.02).
Table 2. Demographics and clinical characteristics of patients with Acinetobacter baumannii bacteraemia.
Mortality analysis regarding capsule locus 2
Univariate Cox regression identified the patient characteristics associated with mortality as a longer duration of hospitalization before A. baumannii bacteraemia, underlying leukaemia, ventilator-associated pneumonia, higher Pitt bacteraemia score, leucopenia, thrombocytopenia, and hyperbilirubinaemia. KL2 was significantly associated with a higher risk of mortality than non-KL2 (hazard ratio [HR], 2.09; 95% confidence interval [CI], 1.14–3.84; P = 0.02).
KL2 isolates were associated with high antimicrobial resistance to levofloxacin, imipenem, sulbactam and tigecycline. However, the percentage of patients receiving appropriate empirical antimicrobial therapy was not significantly lower in the KL2 than in the non-KL2 group (50% vs. 44.6%, P = 0.80). We used stratification analysis to identify interactions between KL2 and the appropriateness of empirical antimicrobial therapy. After stratification, the mortality rates did not differ significantly between patients who received appropriate empirical antimicrobial therapy and those who received inappropriate therapy within the KL2 patient group (66.7% vs. 77.8%; P = 0.99), and within the non-KL2 group (36.2% vs. 47.2%; P = 0.22). Even when receiving appropriate empirical antimicrobial therapy, the KL2 patient group still had a higher mortality rate than the non-KL group (HR, 2.51; 95% CI, 1.01–6.24; P = 0.048).
The presence of KL2 significantly predicted a lower platelet count (10,000/μL) (β-coefficient, –64.5; P = 0.01), and the Sobel test showed that the presence of KL2 had an indirect effect on mortality via platelet count (P = 0.02). In multivariate Cox regression model (), the presence of KL2 predicted mortality (adjusted HR [aHR], 2.03; 95% CI, 1.09–3.78; P = 0.03, (A)), independently of the Pitt bacteraemia score and leucopenia at the onset of bacteraemia.
Figure 1. Kaplan–Meier survival curves in patients with Acinetobacter baumannii bacteraemia. (A) Comparison between the capsule locus 2 (KL2) and non-capsule locus 2 (non-KL2) groups. (B) Comparison between the multilocus sequence type 2 (ST2), non-sequence type 2 (non-ST2), capsule locus 2 (KL2) and non-capsule locus 2 (non-KL2) groups at 28 days. aHR adjusted hazard ratio.
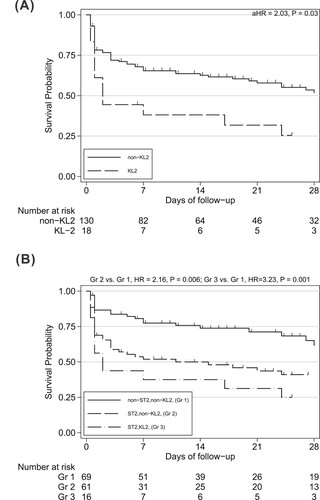
Table 3. Multivariate Cox proportional hazard model analysis of the factors predicting mortality of patients with A. baumannii bacteraemia.
Mortality analysis regarding sequence type 2 and capsule locus 2
Because ST2 was the most dominant sequence type, we further analysed the association between different capsule locus type and sequence type. Among 148 A. baumannii blood isolates, 69 (46.6%) were non-ST2/non-KL2, two (1.4%) were non-ST2/KL2, 61 (41.2%) were ST2/non-KL2, and 16 (10.8%) were ST2/KL2. Most of the KL2 isolates belonged to ST2 (16/18, 88.9%), only 16/77 (20.8%) of the ST2 isolates were KL2. Differences in mortality were analysed in patients with bacteraemia caused by ST2, non-ST2, KL2 or non-KL2 isolates. The mortality rate was least in patients with bacteraemia caused by non-ST2/non-KL2 isolates (30.4%), followed by those with bacteraemia caused by non-ST2/KL2 isolates (50%), and by ST2/non-KL2 isolates (55.7%). Patients with bacteraemia caused by ST2/KL2 isolates had the highest mortality rate (75%).
In multivariable Cox analysis, with ST2/KL2 as the reference, non-ST2/non-KL2 (aHR, 0.46; 95% CI, 0.22–0.95; P = 0.04) and ST2/non-KL2 (aHR, 0.46; 95% CI, 0.23–0.91; P = 0.03) were associated with lower mortality (). Within the ST2 group, KL2 independently predicted mortality (aHR, 2.26; 95%, CI 1.11–4.57; P = 0.02). However, the association with mortality did not differ significantly between the non-ST2/KL2 group and non-ST2/non-KL2 group (HR, 2.85; 95% CI, 0.37–21.87; P = 0.31). Survival differences between the groups with non-ST2/non-KL2, ST2/non-KL2 and ST2/KL2 are shown in the Kaplan–Meier curves in (B).
Discussion
To our knowledge, this is one of only a few studies describe the epidemiology of KL type of invasive A. baumannii infection. In this study, we found that the KL2 is associated with higher antimicrobial resistance. We found associations between the clinical outcome and the presence KL2 A. baumannii isolates in patients with bacteraemia. Independent to leucopenia and the Pitt bacteraemia score, KL2 is associated with higher mortality. We also found KL2 significantly predicted lower platelet count which contributed to the effect of KL2 on mortality in bacteraemia.
The K2 CPS is proved to overwhelm mammalian defences in vivo that administration of K2 capsule depolymerase protects mice from A. baumannii sepsis [Citation10]. The short and branched tetrasaccharide unit of K2 CPS contains a complex uncommon negatively-charged aminosugar, pseudaminic acid [Citation11,Citation26], and fulfils the properties of glycation that A. baumannii own to protect against host immunity [Citation1]. Pseudaminic acid, belonging to the nonulosonic acids superfamily, is found an important glycan present in cell-surface glycoconjugates of bacteria, such as the lipopolysaccharide (LPS) of Pseudomonas aeruginosa [Citation27], and the glycosylated flagellins of Campylobacter jejuni [Citation28]. The role of pseudaminic acid in K2 CPS of A. baumannii may be that pseudaminic acid contributes to bacterial immune evasion by molecular mimicry of human sialic acid [Citation29], or modules host immune responses as it functions in C. jejuni [Citation30].
In present study, thrombocytopenia contributed the effect of KL2 A. baumannii on mortality. Decrease in platelet counts is expected to weaken the important functions of platelet in sepsis, for example, helping to ensnare bacteria in septic blood [Citation31], or inhibiting inflammatory cytokines [Citation32]. In addition, in sepsis induced by gram-negative bacteria, it is well known that LPSs can cause thrombocytopenia [Citation33]. In A. baumannii, LPS triggers sepsis by interaction with host toll-like receptor 4 (TLR4), which lead to host lethality shown in murine sepsis model [Citation34]. Besides, evasion of host innate immune defence is a key driver of A. baumannii virulence [Citation35]. Therefore, the association of KL2 with mortality suggested that K2 CPS may contribute to escape from clearance by innate immune system, which would further enable a higher bacterial density that triggers more severe sepsis medicated by LPS and TLR4 [Citation35] and causes higher mortality.
In one analysis of A. baumannii whole genome sequences, KL2 was the most common KL type (24.2%) and accounted for a substantial percentage (32.2%) of total 2016 ST2 A. baumannii genomes [Citation12]. In another analysis, most of the KL2-carrying strains (92.5%) belonged to ST2 [Citation11]. Similar to the findings reported by Kenyon et al [Citation11], most of the KL2 isolates (88.9%, 16/18) in our study belonged to ST2. A small but substantial percentage (20.8%, 16/77) of the ST2 isolates belonged to KL2. In the study by Kenyon et al., the clinical significance of KL2 in invasive A. baumannii infection was not confirmed because only three blood isolates were included [Citation11]. In our study, KL2 isolates accounted for 12.2% of all A. baumannii blood isolates during the study period (2009–2014). A recent study reported that KL2 was the most common KL type (12.2%) of the A. baumannii blood isolates in two regions of Taiwan (2015–2017) [Citation36]. The prevalence rate of KL2 was 19.3% among the carbapenem-resistant A. baumannii (CRAB) isolates in our study. This rate was comparable to those of other studies focusing on CRAB, ranging between 19.0% and 20.4% [Citation36,Citation37].
Therefore, KL2 may have clinical impact in invasive A. baumannii infection in Taiwan. In addition, we found that non-ST2/KL2 seemed to cause higher mortality than ST2/non-KL2 isolates did. However, because there were only two isolates belonging to non-ST2/KL2, we didn’t demonstrate significant association with mortality compared with that of non-ST2/non-KL2 isolates (HR, 2.85; 95% CI, 0.37–21.87; P = 0.31).
We showed that KL2 A. baumannii was associated higher antimicrobial resistance and greater mortality in bacteraemia. KL identification might be important to understand the virulence of A. baumannii and serve as an outcome predictor of A. baumannii bacteraemia. The K2 capsular polysaccharide might be a potential target in treating A. baumannii bacteraemia via passive immunization, as the role of K1 CPS [Citation38]. Pseudaminic acid-based antibacterial vaccine was proved to confer effective protection against A. baumannii infection in an animal model [Citation39]. In addition, other novel therapeutics targeting the bacteria surface glycoconjugates are under development, e.g. small-molecule inhibitors of the pseudaminic acid biosynthetic pathway enzymes [Citation28,Citation40].
There were several limitations in our study. Firstly, the sample size was small, especially the group of patients infected with KL2 isolates, which was smaller than expected. Although the presence of KL2 predicted mortality independent of the severity of bacteraemia, we failed to show statistically significant effect of KL2 in non-ST2 patient group. Secondly, other CPS-typing methods should be used to examine the epidemiology and the clinical impact of different KL types. Thirdly, we found a tendency for lower mortality in patients who had received appropriate empirical antimicrobial therapy than those who did not. However, because of the small sample size, the effect of appropriate antimicrobial therapy did not achieve statistical significance. Clinicians should pay attention to appropriate use of antimicrobial therapy as well as bacterial factors. Lastly, this study was conducted a few years ago. A recent study in Taiwan showed similar results that the KL2 prevalence rate was around 12.2% in A. baumannii blood isolates and might be associated with the high mortality of CRAB [Citation36]. Nevertheless, whether the KL2 prevalence rate and clinical impact remain similarly in these years warrants further surveillance.
In conclusion, we found that most of the KL2 A. baumannii isolates identified in our study belonged to genotype ST2. The presence of KL2 was associated with high antimicrobial resistance. Infection with KL2 A. baumannii predicted mortality in patients with bacteraemia independent of the bacteraemia severity measured by the Pitt bacteraemia score. Large-scale studies are warranted to confirm these findings and the underlying mechanisms deserve further investigation.
Acknowledgements
The authors thank the Third Core Facility at the NTUH for technical assistance and facility support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wong D, Nielsen TB, Bonomo RA, et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev. 2017 Jan;30(1):409–447.
- Manikal VM, Landman D, Saurina G, et al. Endemic carbapenem-resistant Acinetobacter species in Brooklyn, New York: citywide prevalence, interinstitutional spread, and relation to antibiotic usage. Clin Infect Dis. 2000 Jul;31(1):101–106.
- Garcia-Garmendia J-L, Ortiz-Leyba C, Garnacho-Montero J, et al. Mortality and the increase in length of stay attributable to the acquisition of Acinetobacter in critically ill patients. Crit Care Med. 1999 Sep;27(9):1794–1799.
- Chuang Y-C, Sheng W-H, Li S-Y, et al. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with Acinetobacter bacteremia. Clin Infect Dis. 2011 Feb 1;52(3):352–360.
- Lee Y-T, Kuo S-C, Yang S-P, et al. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: relation to severity of infection. Clin Infect Dis. 2012 Jul;55(2):209–215.
- Jones CL, Clancy M, Honnold C, et al. Fatal outbreak of an emerging clone of extensively drug-resistant Acinetobacter baumannii with enhanced virulence. Clin Infect Dis. 2015 Jul 15;61(2):145–154.
- Giannouli M, Antunes LCS, Marchetti V, et al. Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect Dis. 2013 Jun 20;13:282.
- Chuang Y-C, Cheng A, Sun H-Y, et al. Microbiological and clinical characteristics of Acinetobacter baumannii bacteremia: implications of sequence type for prognosis. J Infect. 2019 Feb;78(2):106–112.
- Russo TA, Luke NR, Beanan JM, et al. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun. 2010 Sep;78(9):3993–4000.
- Oliveira H, Mendes A, Fraga AG, et al. K2 capsule depolymerase is highly stable, is refractory to resistance, and protects larvae and mice from Acinetobacter baumannii sepsis. Appl Environ Microbiol. 2019 Sep 1;85(17):e00934–19.
- Kenyon JJ, Marzaioli AM, Hall RM, et al. Structure of the K2 capsule associated with the KL2 gene cluster of Acinetobacter baumannii. Glycobiology. 2014 Jun;24(6):554–563.
- Wyres KL, Cahill SM, Holt KE, et al. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive. Microb Genom. 2020 Mar;6(3):e000339.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 29th informational supplement, M100S. Wayne (PA): CLSI; 2019.
- Fernandez-Mazarrasa C, Mazarrasa O, Calvo J, et al. High concentrations of manganese in Mueller-Hinton agar increase MICs of tigecycline determined by Etest. J Clin Microbiol. 2009 Mar;47(3):827–829.
- Diancourt L, Passet V, Nemec A, et al. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5(4):e10034.
- Adams MD, Goglin K, Molyneaux N, et al. Comparative genome sequence analysis of multidrug-resistant Acinetobacter baumannii. J Bacteriol. 2008 Dec;190(24):8053–8064.
- Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008 Jun;36(5):309–332.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999 Jan;11(1):7–12.
- Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010 Jul 1;51(1):79–84.
- Trevor CME. Healthcare-associated bloodstream infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. Philadelphia: Lippincott Williams & Wilkins; 2012. p. 258.
- Bozdogan H. Model selection and Akaike's information criterion (AIC): the general theory and its analytical extensions. Psychometrika. 1987 Sep;52(3):345–370.
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986 Dec;51(6):1173–1182.
- Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009 Jul;20(4):488–495.
- Senchenkova SN, Shashkov AS, Shneider MM, et al. Structure of the capsular polysaccharide of Acinetobacter baumannii ACICU containing di-N-acetylpseudaminic acid. Carbohydr Res. 2014 Jun 4;391:89–92.
- Knirel YA, Vinogradov EV, L'Vov VL, et al. Sialic acids of a new type from the lipopolysaccharides of Pseudomonas aeruginosa and Shigella boydii. Carbohydr Res. 1984 Oct 15;133(2):C5–C8.
- Salah Ud-Din AIM, Roujeinikova A. Flagellin glycosylation with pseudaminic acid in Campylobacter and helicobacter: prospects for development of novel therapeutics. Cell Mol Life Sci. 2018 Apr;75(7):1163–1178.
- Carlin AF, Uchiyama S, Chang Y-C, et al. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009 Apr 2;113(14):3333–3336.
- Stephenson HN, Mills DC, Jones H, et al. Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis. 2014 Nov 1;210(9):1487–1498.
- Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007 Apr;13(4):463–469.
- Xiang B, Zhang G, Guo L, et al. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun. 2013;4:2657.
- Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009 Jun 15;182(12):7997–8004.
- Lin L, Tan B, Pantapalangkoor P, et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio. 2012;3(5):e00312–12.
- Bruhn KW, Pantapalangkoor P, Nielsen T, et al. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis. 2015 Apr 15;211(8):1296–1305.
- Hsieh Y-C, Wang S-H, Chen Y-Y, et al. Association of capsular types with carbapenem resistance, disease severity, and mortality in Acinetobacter baumannii. Emerg Microbes Infect. 2020 Dec;9(1):2094–2104.
- Silva L, Grosso F, Rodrigues C, et al. The success of particular Acinetobacter baumannii clones: accumulating resistance and virulence inside a sugary shield. J Antimicrob Chemother. 2021 Jan 19;76(2):305–311.
- Russo TA, Beanan JM, Olson R, et al. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect Immun. 2013 Mar;81(3):915–922.
- Wei R, Yang X, Liu H, et al. Synthetic pseudaminic-acid-based antibacterial vaccine confers effective protection against Acinetobacter baumannii infection. ACS Cent Sci. 2021 Sep 22;7(9):1535–1542.
- Menard R, Schoenhofen IC, Tao L, et al. Small-molecule inhibitors of the pseudaminic acid biosynthetic pathway: targeting motility as a key bacterial virulence factor. Antimicrob Agents Chemother. 2014 Dec;58(12):7430–7440.
