ABSTRACT
Pediatric tuberculosis (TB) is a serious infectious disease that affects many children worldwide and is more likely to be extrapulmonary than adult TB. However, the clinical and epidemiological profile, and cost burden of pediatric extrapulmonary TB (EPTB) in China remain unknown. Here, we conducted a descriptive, multicenter study of pediatric TB patients from 22 hospitals across all six regions in China from October 2015 to December 2018. Of 4,654 patients, 54.23% (2,524) had pulmonary TB (PTB), 17.76% (827) had EPTB, and 28.00% (1,303) had concurrent extrapulmonary and pulmonary TB (combined TB). Compared with PTB, EPTB and combined TB were associated with lower hospitalization frequency (2.43 and 2.21 vs. 3.16 times), longer length of stay (10.61 and 11.27 vs. 8.56 days), and higher rate of discharge against medical advice (8.46% and 9.44% vs. 5.67%). EPTB was associated with higher mortality (0.97% vs. 0.24% and 0.31%), higher rate of low birth weight (17.69% vs. 6.79% and 6.22%), worse diagnosis at the first visit (21.16% vs. 34.67% and 44.47%), and worse hospitalization plan situation (4.35% vs. 7.81% and 7.44%), compared with PTB and combined TB. EPTB and combined TB had higher financial burdens (17.67% and 16.94% vs. 13.30%) and higher rates of catastrophic expenditure (8.22% and 9.59% vs. 5.03%), compared with PTB. Meningitis TB (34.18%) was the most frequent form of total extrapulmonary infection and had the highest cost burden and rate of catastrophic expenditure. In conclusion, improved screening approaches for pediatric EPTB are needed to reduce diagnostic challenges and financial burden.
Introduction
Globally, tuberculosis (TB) is among the 10 most common causes of death; it is the main cause of infectious disease-related death[Citation1, Citation2]. According to the World Health Organization, there were approximately 10.0 million TB cases worldwide in 2019[Citation1]. Pediatric patients (aged < 15 years) with TB constituted approximately 12% of all cases worldwide; this rate has increased annually[Citation2]. Because of gaps in diagnosis and access to care, pediatric TB is associated with a high mortality rate[Citation3]. A mathematical modelling study showed that one in four pediatric TB patients die from the disease[Citation4]. Therefore, pediatric TB is a serious infectious disease that affects many children worldwide; global TB management approaches should prioritize improvements in diagnosis, treatment, and prevention[Citation5].
In addition to the lungs, TB affects many extrapulmonary sites; this is known as extrapulmonary TB (EPTB)[Citation6]. The Global TB Report 2020[Citation1] found that EPTB constituted 16% of notified TB cases worldwide; its proportion ranged from 8% in the Western Pacific Region to 24% in the Eastern Mediterranean Region. In clinical practice, EPTB at an obscure site is often incidentally detected [Citation7]. Because of nonspecific symptoms and signs, along with a lack of constitutional features, EPTB is often not suspected early in the course of illness and remains a delayed diagnosis. Notably, TB is more likely to cause more severe disease in children than in adults; it is also more likely to be extrapulmonary[Citation8].
To our knowledge, there have been few large-scale epidemiological studies of pediatric TB in China. Data regarding the prevalence of TB in children are often cited from the fourth nationwide random survey of TB, which was reported in 2002[Citation9]. In 2005, the Chinese Centre for Disease Control and Prevention developed a web-based Tuberculosis Information Management System for real-time collection of TB data, including data regarding pediatric TB, in 31 provinces in mainland China[Citation10]. However, centralized reporting is only required for pulmonary TB (PTB), not EPTB, according to the Chinese Law of Preventing and Controlling Infectious Disease. Moreover, children with TB are treated at pediatric or large general hospitals, which are not directly linked to the Tuberculosis Information Management System [Citation11]. Therefore, epidemiological trends regarding forms of pediatric EPTB remain unclear. Thus far, only two single-centre studies have reported EPTB data. A descriptive study of 1212 patients aged 0–18 years with TB (655 cases with extrapulmonary infection, 54%) was conducted from January 2002 to December 2010 in Beijing Children’s Hospital [Citation12]. Another study analyzed 1577 consecutive patients with pediatric TB (1137 cases with extrapulmonary infection, 73%) at a Referral Tuberculosis Hospital in Shandong Province [Citation13]. However, multicenter pediatric EPTB data remain unavailable in China. Here, we retrospectively reviewed the epidemiological data and burden of pediatric EPTB among patients from nine provincial and 13 municipal hospitals in mainland China from October 2015 to December 2018. We aimed to determine patient characteristics, clinical and epidemiological profiles, and cost burden of pediatric EPTB in China.
Materials and methods
Data source
This retrospective study obtained discharge data from the Futang Research Centre of Pediatric Development (FRCPD), which is affiliated with the National Centre for Children’s Health, China. The FRCPD was founded and established as a multi-tiered pediatric diagnosis and treatment network; it includes 47 provincial and municipal level member hospitals, along with 3000 primary level pediatric institutions. Detailed information regarding the FRCPD is available at http://www.futang.org/about/fu-tang-jie-shao.htm.
The data in our study were collected from nine provincial and 13 municipal hospitals in 21 provinces across all six regions in China from October 2015 to December 2018. The data were acquired from The FUTang Updating medical REcords (FUTURE) Database. More details and the process of data cleaning have been presented in a previous publication[Citation14].
Diagnostic criteria
The diagnosis of TB was made in accordance with the World Health Organization criteria[Citation15]. All analyses included children aged < 18 years with TB who had either bacteriologically confirmed TB (biological specimen positivity determined via culture, smear microscopy, or World Health Organization-approved rapid diagnostic tests) or clinically diagnosed TB (bacteriological criteria were not fulfilled but a diagnosis of active TB was made by a clinician). This definition included cases that were diagnosed based on X-ray abnormalities or suggestive histology, as well as EPTB cases without laboratory confirmation. PTB was defined as any case of TB that only involved the lung parenchyma or the tracheobronchial tree[Citation15]. EPTB was defined as any case of TB that only involved organs other than the lungs (e.g. pleura, lymph nodes, abdomen, genitourinary tract, skin, joints and bones, and/or meninges). Concurrent extrapulmonary and pulmonary TB (combined TB) was defined as any case of TB that involved both the lungs and organs other than the lungs.
Variables and outcomes
Sociodemographic, TB-related, and outcome variables were extracted from the home page of medical records for each patient. The sociodemographic variables mainly included sex, age, ethnicity, place of residence, and birth weight; the TB-related variables included type of TB, diagnosis during the initial visit, and discharge conditions. The outcome variables were hospitalization frequency, length of hospital stay (LOS), and cost burden of TB. In this study, the total hospitalization expenses were divided into four categories: diagnostic tests, medication, inpatient care, and other expenses. We assessed the cost burden of TB by comparing the simulated out-of-pocket expenditures of TB cases relative to the per capita disposable income. Additionally, we collected data regarding the rural and urban per capita disposable income and family size according to the data from the National Bureau of Statistics (https://data.stats.gov.cn/index.htm). The disposable income was calculated as follows: per capita disposable income (Supplementary Table 1) multiplied by the mean family size (3.17, 3.12, 3.05, and 3.02 members per family from 2015 to 2018). Catastrophic expenditure was defined as ≥ 50% of a family’s disposable income[Citation16] (exchange rate: CNY 7.0 = USD 1.0).
Statistical analysis
Continuous variables were presented as means and standard deviations, while categorical variables were presented as percentages. To examine differences among patients with different types of TB, the Pearson χ2 test or one-way analysis of variance was used as appropriate. Multivariable models were built using “Enter” logistic regression procedures. P-values < 0.05 were considered statistically significant. Data analyses were conducted using Statistical Package for the Social Sciences software, version 19.0 (SPSS Inc., Chicago, IL, USA) and JMP software, version 14.0.0 (SAS Institute Inc., Cary, NC, USA). All TB cases were coded according to their province of residence (geo-coded) and were matched to a 1:100,000 digital map of China using ArcGIS software (ArcGIS 10; ESRI Inc., Redlands, CA, USA). The map was coloured according to the six major regions of China’s geographical divisions: North, Northeast, East, Central South, Southwest, and Northwest.
Results
Distribution of the participating hospitals and cases
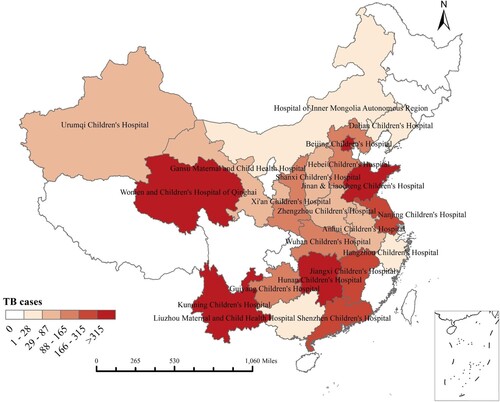
We collected discharge data regarding 4,654 TB patients from nine provincial and 13 municipal hospitals in China from October 2015 to December 2018. Among these patients, 54.23% (2,524) had PTB, 17.77% (827) had EPTB, and 28.00% (1,303) had combined TB. The 22 research centres in this study were located in 21 provinces across all six regions of China, including six centres in East China, five centres in Central South China, five centres in Northwest China, three centres in North China, two centres in Southwest China, and one centre in Northeast China (). The six centres with the largest numbers of cases were Beijing Children’s Hospital (n = 1342), Hunan Children’s Hospital (n = 741), Women and Children’s Hospital of Qinghai (n = 468), Jinan Children’s Hospital (n = 344), Kunming Children’s Hospital (n = 337), and Jiangxi Children’s Hospital (n = 223) (Supplementary Table 2 and ).
Figure 1. Distribution of participating hospitals comprising provincial (orange colour) and municipal hospitals (blue colour).
The overall male-to-female ratio of pediatric TB cases was 1.53 (2813/1841), and the rate of low birth weight was 8.21%. The mean age was 5.15 years; 37.57% and 34.06% of the patients were aged 1–3 and > 6 years, respectively. In total, 1633 (35.09%) patients were diagnosed with TB during the first visit. The mean frequency of hospitalization with TB was 2.77 times, and the mean LOS was 9.68 days. Furthermore, 4,040 (86.81%) patients were discharged based on the physician’s discretion, while 336 (7.22%) were discharged against medical advice and 18 died ( and Supplementary Figure 1).
Table 1. Characteristics of EPTB and combined TB pediatric patients compared with PTB pediatric patients, China, 2015–2018.
According to data from Beijing Children's Hospital, the largest of the 22 research centres, 83.47% (n = 1106) of the pediatric TB cases received the Bacillus Calmette–Guérin vaccine. Bacteriologically confirmed TB cases and clinically diagnosed TB cases constituted 29.88% (n = 401) and 70.12% (n = 941) of the total cases, respectively.
Comparison of pediatric patient characteristics among PTB, EPTB, and combined TB groups
We assessed the characteristics of patients in the PTB, EPTB, and combined TB groups (). The overall male-to-female ratios in the three groups were 1.46 (1500/1024), 1.65 (515/312), and 1.58 (798/505), respectively. There were no significant differences in terms of sex (P = 0.274). EPTB patients were significantly younger than PTB and combined TB patients (4.72 years vs. 4.92 and 5.84 years, P < 0.001). Concerning ethnicity, the proportion of Han people was significantly greater in the EPTB group than in the PTB or combined TB groups (87.55% vs. 83.24% and 78.20%, P < 0.001). Compared with PTB (42, 6.97%) and combined TB (15, 6.22%), EPTB (23, 17.69%) was more commonly observed in children with low birth weight (P < 0.001). However, the proportion of patients diagnosed at the first visit was lower among patients with EPTB (175, 21.16%) than among patients with PTB (875, 34.67%) or combined TB (583, 44.74%) (P < 0.001). Compared with PTB, EPTB and combined TB were associated with lower hospitalization frequency (2.43 and 2.21 vs. 3.16 times, P < 0.001), longer LOS (10.61 and 11.27 vs. 8.56 days, P < 0.001), and higher rate of discharge against medical advice (8.46% and 9.44% vs. 5.67%, P < 0.001). Patients with EPTB had higher mortality (0.97% vs. 0.24% and 0.31%, P < 0.001) and worse hospitalization plan situation (rehospitalization plan within one month after discharge, 4.35% vs. 7.81% and 7.44%, P = 0.003) than did patients with PTB or combined TB.
Data from Beijing Children's Hospital showed that 81.21% of PTB cases, 90.99% of EPTB cases, and 83.11% of combined TB cases received the Bacillus Calmette–Guérin vaccine (P = 0.002). Bacteriologically confirmed TB cases constituted 35.55%, 19.41% and 25.65% of the PTB, EPTB, and combined TB groups, respectively (P < 0.001).
Demographic and clinical characteristics according to forms of EPTB
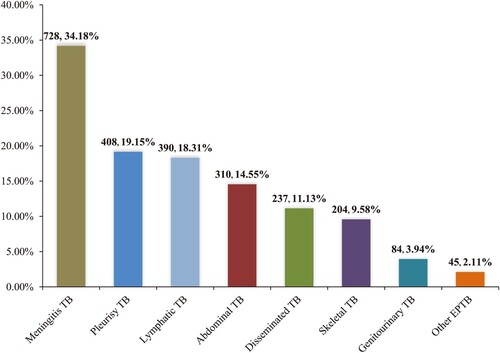
Among the 2,130 patients with extrapulmonary infection (including 827 EPTB and 1303 combined TB cases) in our study, the most frequent form was meningitis TB (n = 728, 34.18%). Additional forms were pleurisy TB (n = 408, 19.15%), lymphatic TB (n = 390, 18.31%), abdominal TB (n = 310, 14.55%), disseminated TB (n = 237, 11.13%), skeletal TB (n = 204, 9.58%), genitourinary TB (n = 84, 3.94%), and other EPTB (n = 45, 2.11%) ().
We investigated the associations of pediatric EPTB factors with various demographic and clinical characteristics ( and ). Multivariate logistic modelling analysis showed that children aged 1–3 years had a higher risk of meningitis TB (aOR: 3.13, 95% CI: 1.56–6.26); children aged 4–6 years had higher risks of meningitis TB (aOR: 4.30, 95% CI: 1.98–9.37) and pleurisy TB (aOR: 22.79, 95% CI: 4.23–122.75); and children aged > 6 years had higher risks of meningitis TB (aOR: 2.72, 95% CI: 1.30–5.71), pleurisy TB (aOR: 24.31, 95% CI: 5.20–113.57), and abdominal TB (aOR: 2.86, 95% CI: 1.17–7.00). Tibetan children had a higher risk of meningitis TB (aOR: 3.76, 95% CI: 2.31–6.13) but lower risks of pleurisy TB (aOR: 0.12, 95% CI: 0.05–0.28) and genitourinary TB (aOR: 0.17, 95% CI: 0.03–0.82); Uighur children had a higher risk of abdominal TB (aOR: 73.36, 95% CI: 6.40–840.90). Abdominal TB was more commonly observed in children with low birth weight (aOR: 8.37, 95% CI: 3.78–18.51). Meningitis TB patients had a shorter LOS [7–14 days (aOR: 0.29, 95% CI: 0.15–0.57) and > 14 days (aOR: 0.56, 95% CI: 0.34–0.91)], whereas pleurisy TB patients had a longer LOS [> 14 days (aOR: 2.60, 95% CI: 1.06–6.38)]. Pleurisy TB patients (aOR: 3.28, 95% CI: 1.08–10.02) and skeletal TB patients (aOR: 4.68, 95% CI: 1.27–17.23) had higher rates of discharge against medical advice. Meningitis TB patients (aOR: 8.19, 95% CI: 1.41–47.59) and abdominal TB patients (aOR: 7.38, 95% CI: 1.04–52.45) had higher rates of mortality. Unexpected discharge conditions were observed more frequently among meningitis TB patients (aOR: 15.08, 95% CI: 3.54–64.20) and disseminated TB patients (aOR: 71.82, 95% CI: 11.44–450.84). Meningitis TB patients had a higher rate of catastrophic expenditure (aOR: 5.59, 95% CI: 3.02–10.35).
Table 2. Demographic and clinical characteristics according to forms of EPTB, China, 2015–2018.
Table 3. Multivariate analysis of associated factors for different forms of EPTB, China, 2015–2018.
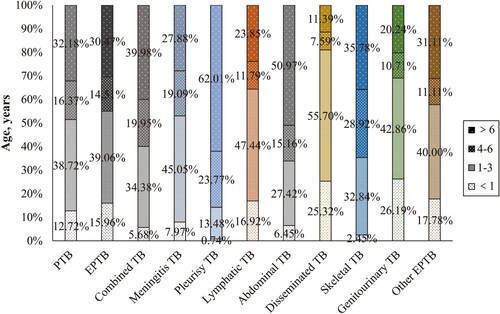
The forms of EPTB differed among age groups. The ages of patients with meningitis TB (4.58 years), lymphatic TB (4.03 years), disseminated TB (2.43 years), and genitourinary TB (3.28 years) were significantly younger than the mean age (5.15 years) of patients with pediatric TB (P < 0.001), and pleurisy TB (8.16 years) and abdominal TB (7.05 years) were comparatively older (P < 0.001) (Supplementary Table 3). Among children aged < 3 years, disseminated TB, genitourinary TB, lymphatic TB, other EPTB, and meningitis TB (81.01%, 69.05%, 64.36%, 57.78%, and 53.02%) were more common than PTB (51.45%) ().
Medical expenses and cost burden of PTB, EPTB, and combined TB
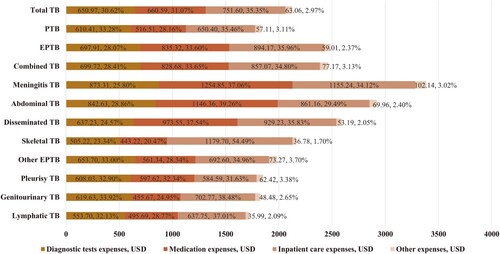
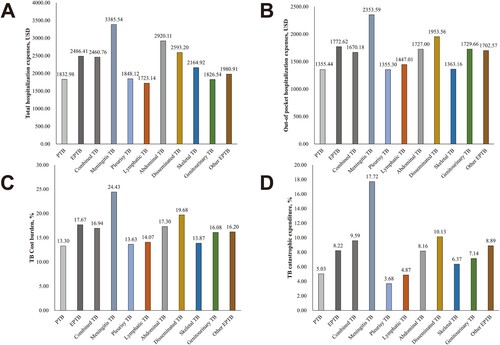
Among all pediatric TB cases in our study, the mean total and out-of-pocket medical expenses were $2124.85 and $1517.69, respectively. The mean diagnostic test expenses, medication expenses, inpatient care expenses, and other expenses were $650.97, $660.59, $751.60, and $63.06, respectively. According to the per capita disposable income of each province, the overall cost burden of TB was 15.09%; the rate of catastrophic expenditure was 6.88% (320/4654). The total ($2486.41 and $2460.76 vs. $1832.98, P < 0.001) and out-of-pocket ($1772.62 and $1670.18 vs. $1355.44, P < 0.001) hospitalization expenses were significantly higher for EPTB and combined TB than for PTB. EPTB and combined TB also had higher financial burdens (17.67% and 16.94% vs. 13.30%, P < 0.001) and higher rates of catastrophic expenditure (8.22% and 9.59% vs. 5.03%, P < 0.001), compared with PTB (). Furthermore, EPTB and combined TB patients had higher expenses in terms of diagnosis ($697.91 and $699.72 vs. $610.41, P = 0.001), medication ($835.32 and $828.68 vs. $516.51, P < 0.001), inpatient care ($894.17 and $857.07 vs. $650.40, P < 0.001), and other expenses ($59.01 and $77.17 vs. $57.11, P = 0.019), compared with PTB patients. However, the proportions of diagnostic expenses (compared to overall expenses) were lower for EPTB and combined TB patients than for PTB patients (28.07% and 28.41% vs. 33.28%) ( and ).
Table 4. Distribution of hospitalization expenses by different TB types, China, 2015–2018.
Analysis of hospitalization expenses among forms of EPTB showed that the three types of EPTB with the highest total hospitalization expenses were meningitis TB ($3385.54), abdominal TB ($2920.11), and disseminated TB ($2593.20). Additionally, meningitis TB ($2353.59), disseminated TB ($1953.56), and genitourinary TB ($1729.66) had the highest out-of-pocket hospitalization expenses. According to the per capita disposable income of each province, the cost burden and the rate of catastrophic expenditure among different EPTB forms ranged from 24.43% to 13.63% and 17.72% to 3.68%, respectively; both values were highest for meningitis TB ( and ). Concerning the forms of hospitalization expenses, meningitis TB had the highest diagnostic test expenses ($873.31), medication expenses ($1254.85), and other expenses ($102.14); it had the second highest ($1155.24) inpatient care expenses after skeletal TB ($1179.70) (). Among the three types of EPTB with the greatest cost burdens, medication constituted the greatest proportion of expenses (meningitis TB, 37.06%; disseminated TB, 39.26%; and genitourinary TB, 37.54%). Inpatient care constituted the greatest proportion of expenses among skeletal TB (54.49%), genitourinary TB (38.48%), lymphatic TB (37.01%), and other EPTB (34.96%). For pleurisy TB, diagnostic tests constituted the greatest proportion of expenses (32.90%) ( and ).
Figure 5. Hospitalization expenses among 4,654 pediatric patients in China, 2015–2018. A) Total hospitalization expenses of PTB and forms of EPTB. B) Out-of-pocket hospitalization expenses of PTB and forms of EPTB. C) Cost burdens of PTB and forms of EPTB. D) Catastrophic expenditures of PTB and forms of EPTB.
Discussion
We described the epidemiological characteristics of pediatric patients with EPTB in China based on multicenter data obtained from 22 pediatric hospitals throughout mainland China. This representative study contained the largest number of hospitalized pediatric patients with EPTB.
To our knowledge, this is the first study to demonstrate the severe epidemic of pediatric EPTB in China. Nearly 46% of children in this study exhibited EPTB or combined TB; this was higher than the proportions in the UK (38.11%)[Citation17], Colombia (34.41%)[Citation18], Italy (30.87%)[Citation19], Turkey (30.50%)[Citation20], the USA (24.52%)[Citation21], and India (17.68%)[Citation22]. Previous studies of Chinese pediatric TB patients from Beijing Children’s Hospital and Shandong Chest Hospital showed that the overall proportions of pediatric TB patients with extrapulmonary infection were 54% and 73%, respectively[Citation12, Citation13]. A plausible explanation for the differences between the present and previous studies was that both previous studies used single-centre designs and were conducted at high-level hospitals in the eastern part of China which had relatively better medical diagnosis and treatment capabilities countrywide. Thus, these hospitals might diagnose and treat more EPTB patients, compared with other centres.
Additionally, our data showed that meningitis TB was the predominant type of extrapulmonary infection among inpatients in China, constituting nearly 34.18% of all EPTB and combined TB cases. This result was consistent with a previous finding at Beijing Children’s Hospital (38.8%)[Citation12], although it differed from the finding in Shandong Province that pleural TB (29.0%) was the most common type of pediatric EPTB[Citation13]. A possible explanation for this difference is that the mean age of EPTB cases was older (9.26 years) in the study from Shandong Province, compared with the mean ages of EPTB cases in our study (5.15 years) and in the study from Beijing Children's Hospital (5.5 years). Our study indicated that pleural TB mainly occurred in children aged > 6 years (62.01%), while meningitis TB mainly occurred in children aged < 6 years (72.12%). Therefore, differing age distributions among patients in the above studies may have led to distinct distributions of EPTB forms.
Significant differences have been observed between pediatric and adult EPTB in China. Specifically, the proportions of PTB, EPTB, and combined TB differ between pediatric and adult TB. The overall proportion of children with extrapulmonary infection (45.76%) was much higher than the overall proportion of adults with extrapulmonary infection (37.44%)[Citation23]. Furthermore, the forms of EPTB differ between pediatric and adult patients. In a study of 19,279 TB patients (6433 EPTB cases) who were hospitalized in Beijing Chest Hospital, the most common types of EPTB were skeletal TB (41.1%) and pleural TB (26.0%). Meningitis TB only constituted 6.8% of the EPTB cases[Citation23]. Another study of 202,998 EPTB inpatients aged ≥ 15 years from 21 hospitals, most of which specialize in TB, showed that the most common type of EPTB was pleural TB (50.15%); only 7.23% of patients exhibited meningitis TB[Citation24]. In our study, the incidence rate of meningitis TB was high in children (34.18%); it was highest in the 1–3-year-old and 4–6-year-old age groups, followed by the > 6-year-old and < 1-year-old age groups.
We found that the diagnostic rate during the first visit was significantly lower for EPTB than for PTB or combined TB (21.16% vs. 34.67% and 44.74%). The low bacterial load in non-respiratory specimens often obscures detection, while sample collection from deep tissues is challenging; thus, EPTB poses a diagnostic challenge[Citation25]. The diagnosis of EPTB is often missed or made at an advanced stage of the disease when complications have already begun [Citation6]. Considering the suboptimal sensitivities of existing immunological and microbiological TB tests in children, the combined use of immune-based tests with culture and nucleic acid amplification tests provides substantially higher positive diagnostic yields; therefore, it should be standard clinical practice in high-resource settings [Citation26]. Additionally, TB diagnostic tests have been developed and largely validated in adults with pulmonary TB [Citation27]. Research focusing on the development and validation of biomarkers or tools in children should be encouraged, particularly concerning tests that may be useful in the diagnosis of EPTB.
The burden of EPTB in children is considerable and requires greater investment of public health effort. Compared with PTB, EPTB and combined TB were associated with higher costs, causing a significant burden for the families of affected patients. The respective mean cost burdens were 35.65% and 34.25% higher for EPTB and combined TB than for PTB ($2486.41 and $2460.76 vs. $1832.98). Moreover, compared with PTB, EPTB and combined TB were associated with higher rates of discharge against medical advice and worse hospitalization plan situation. Therefore, strengthening publicity and management of pediatric EPTB are required to optimize the completion of its standardized treatment.
Among the forms of EPTB in pediatric patients, meningitis TB requires additional consideration. Compared with other forms of EPTB, meningitis TB constitutes a greater proportion (34.18%), is present in younger patients (4.58 years), has higher costs ($3385.54), and carries the highest risk of catastrophic expenditure (8.19-fold). Previous studies showed that young children and people living with human immunodeficiency virus (HIV) have the highest risks of TB meningitis [Citation28–30]; moreover, meningitis TB is the most severe type of TB [Citation31, Citation32]. Therefore, because of the high incidence and generally high risk of death of tuberculous meningitis among Chinese children, the prevention and treatment of meningitis TB in children should be a high-priority public health focus. Further research is needed concerning the diagnosis and treatment [Citation33–35] of meningitis TB.
The current study had three limitations. First, the retrospective design did not involve collection of national surveillance data, potentially limiting the overall relevant scope of the findings. Unfortunately, because EPTB does not substantially contribute to the transmission of TB, it has not been included in China’s National TB Control Programme. Consequently, the acquisition of national surveillance data has been challenging. Second, all children enrolled in our study were HIV-negative. HIV infection has been considered an additional risk factor for EPTB [Citation36, Citation37]. However, children co-infected with TB and HIV were referred to other hospitals that specialize in HIV treatment in China. The absence of patients co-infected with HIV and TB in this study may have led to some bias in our results. Third, the results of Bacillus Calmette–Guérin vaccination and microbiological confirmation were based on single-centre data, and some important data (e.g. tuberculin skin test and interferon-gamma release assay findings) were not available for analysis.
Conclusion
This study described the epidemiological and financial burden of EPTB among patients from nine provincial and 13 municipal hospitals in mainland China. Compared with PTB, pediatric EPTB was associated with a longer LOS and higher cost burden. The most common type of EPTB in children was meningitis TB, which is the most severe type of TB. Pediatric EPTB should receive greater investment of public health effort. Efforts to improve screening approaches for EPTB are needed to reduce the associated diagnostic challenges and financial burden. Therefore, improved screening, enhanced educational efforts, and policy prioritization is essential for adequate management of EPTB. Finally, additional research is needed concerning the diagnosis and treatment[Citation34, Citation38] of meningitis TB.
List of investigators and members of Futang Research Centre of Pediatric development (FRCPD)
Yueping Zeng, Jian Tian, Fei Song, Xin Xu — Beijing Children's Hospital, Capital Medical University, National Centre for Children's Health, Beijing, China; Mei Wu — Medical Record Department, Anhui Children's Hospital, Hefei, China; Guosong Wang — Information Centre, Anhui Children's Hospital, Hefei, China; Li Li — Medical Record Department, Dalian Children's Hospital, Dalian, China; Hongjie Sun — Information Department, Dalian Children's Hospital, Dalian, China; Zhenqiang Da — Medical Record Department, Gansu Provincial Maternity and Child-care Hospital, Lanzhou, China; Wenjuan Wang — Information Department, Gansu Provincial Maternity and Child-care Hospital, Lanzhou, China; Qinghong He — Medical Record Department, Guiyang Children's Hospital, Guiyang, China; Shaoqian Liu — Information Centre, Guiyang Children's Hospital, Guiyang, China; Ling Dai — Information Department, Hangzhou Children's Hospital, Hangzhou, China; Waiguang Hu — Information Centre, Hunan Children's Hospital, Hangzhou, China; Xiaomei Chen — Medical Record Department, Inner Mongolia Children's Hospital, Hohhot, China; Xiaoqin Wang, Jian Du — Information Department, Inner Mongolia Children's Hospital, Hohhot, China; Chunxiang Wang — Medical Record Department, Jinan Children's Hospital, Jinan, China; Yuanyi Qu — Information Department, Jinan Children's Hospital, Jinan, China; Daqiao Zhu — Medical Record Department, Kunming Children's Hospital, Kunming, China; Jian Ding — Information Department, Kunming Children's Hospital, Kunming, China; Haibin Zhou — Medical Record Department, Liaocheng Children's Hospital, Liaocheng, China; Jinchi Shi — Information Department, Liaocheng Children's Hospital, Liaocheng, China; Zhijun Pan — Medical Record Department, Liuzhou Maternity and Child Healthcare Hospital, Liuzhou, China; Lei Yang — Information Department, Liuzhou Maternity and Child Healthcare Hospital, Liuzhou, China; Tingting Zhang — Medical Record Department, Nanjing Children's Hospital, Nanjing, China; Jin Xu — Information Department, Nanjing Children's Hospital, Nanjing, China; LianjunRuan — Medical Record Department, Qinghai Children's Hospital, Xining, China; Shu Mai — Information Department, Qinghai Children's Hospital, Xining, China; Fengmei Ma — Department of quality control, Qinghai Children's Hospital, Xining, China; Li Gao — Medical Record Department, Shanxi Children's Hospital, Taiyuan, China; Hongcheng Liu — Information Centre, Shanxi Children's Hospital, Taiyuan, China; Xirong Chen — Medical Record Department, Shenzhen Children's Hospital, Shenzhen, China; Yuzheng Zhang — Information Department, Shenzhen Children's Hospital, Shenzhen, China; Jun Zhou — Medical Record Department, Urumqi Children's Hospital, Urumqi, China; Chunxiang Yan — Medical Record Department, Wuhan Children's Hospital, Wuhan, China; Jian Fang — Information Centre, Wuhan Children's Hospital, Wuhan, China.
Supplemental Material
Download Zip (402.5 KB)Acknowledgements
The authors thank the staff members of Futang Research Centre of Pediatric Development (FRCPD) for their help and collaboration. The authors have declared that no conflict of interest exists.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. Global tuberculosis report 2020. Geneva, Switzerland: WHO; 2020.
- Floyd K, Glaziou P, Zumla A, et al. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. The Lancet Respir Med. 2018;6(4):299–314.
- Jenkins HE, Yuen CM, Rodriguez CA, et al. Mortality in children diagnosed with tuberculosis: a systematic review and meta-analysis. The Lancet Infectious Dis. 2017;17(3):285–295.
- Dodd PJ, Yuen CM, Sismanidis C, et al. The global burden of tuberculosis mortality in children: a mathematical modelling study. The Lancet Global Health. 2017;5(9):e898–e906.
- United Nations. United to end tuberculosis: an urgent global response to a global epidemic. UN General Assembly High-Level Meeting on the fight against tuberculosis; 2018. New York: United Nations; 2018.
- Sharma SK, Mohan A, Kohli M. Extrapulmonary tuberculosis. Expert Rev Resp Med. 2021;15:931–948.
- Rodriguez-Takeuchi SY, Renjifo ME, Medina FJ. Extrapulmonary tuberculosis: pathophysiology and imaging findings. Radiographics. 2019;39(7):2023–2037.
- Pavan KN, Anuradha R, Andrade BB, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20(5):704–711.
- Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. The Lancet. 2014;383(9934):2057–2064.
- Huang F, Cheng S, Du X, et al. Electronic recording and reporting system for tuberculosis in China: experience and opportunities. J Am Med Inform Assn. 2014;21(5):938–941.
- Li T, Shewade HD, Soe KT, et al. Under-reporting of diagnosed tuberculosis to the national surveillance system in China: an inventory study in nine counties in 2015. BMJ Open. 2019;9(1):e21529.
- Wu XR, Yin QQ, Jiao AX, et al. Pediatric tuberculosis at Beijing Children's Hospital: 2002-2010. Pediatrics. 2012;130(6):e1433–e1440.
- Wang M, Wang J, Liu X. Epidemiological Trends in the Form of Childhood Tuberculosis in a Referral Tuberculosis Hospital in Shandong, China. Biomed Res Int. 2020;2020:1–5.
- Feng G, Zeng Y, Tian J, et al. Disease spectrum analysis of hospitalized children in China: A study of 18 tertiary children's hospitals. Pediatric Invest. 2019;3(3):159–164.
- World Health Organization. Definitions and reporting framework for tuberculosis–2013 revision. Geneva, Switzerland: WHO; 2020.
- Su TT, Kouyate B, Flessa S. Catastrophic household expenditure for health care in a low-income society: a study from Nouna District, Burkina Faso. Bull World Health Organ. 2006;84(1):21–27.
- Abubakar I, Laundy MT, French CE, et al. Epidemiology and treatment outcome of childhood tuberculosis in England and Wales: 1999-2006. Arch Dis Child. 2008;93(12):1017–1021.
- Sepulveda EVF, Yunda LFI, Herrera KCM, et al. Extrapulmonary tuberculosis in colombian children: Epidemiological and clinical data in a reference hospital. Int J Mycobacteriology. 2017;6(2):132–137.
- Galli L, Lancella L, Tersigni C, et al. Pediatric Tuberculosis in Italian Children: Epidemiological and Clinical Data from the Italian Register of Pediatric Tuberculosis. Int J Mol Sci. 2016;17(6.
- Devrim I, Akturk H, Bayram N, et al. Differences between pediatric extra-pulmonary and pulmonary tuberculosis: a warning sign for the future. Mediterr J Hematol Infect Dis. 2014;6(1):e2014058.
- Banta JE, Ani C, Bvute KM, et al. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998–2014]. J Infect Public Heal. 2020;13(1):131–139.
- Santiago-Garcia B, Blazquez-Gamero D, Baquero-Artigao F, et al. Pediatric Extrapulmonary Tuberculosis: Clinical Spectrum, Risk Factors and Diagnostic Challenges in a Low Prevalence Region. Pediatr Infect Dis J. 2016;35(11):1175–1181.
- Pang Y, An J, Shu W, et al. Epidemiology of Extrapulmonary Tuberculosis among Inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–464.
- Kang W, Yu J, Du J, et al. The epidemiology of extrapulmonary tuberculosis in China: A large-scale multi-center observational study. Plos One. 2020;15(8):e237753.
- Kanade SR, Nataraj G, Mehta PR. Improved Case Detection Using Xpert Mycobacterium tuberculosis/Rifampicin Assay in Skeletal Tuberculosis. Indian J Med Microbi. 2018;36(4):590–593.
- Basu RR, Thee S, Blazquez-Gamero D, et al. Performance of immune-based and microbiological tests in children with tuberculosis meningitis in Europe: a multicentre Paediatric Tuberculosis Network European Trials Group (ptbnet) study. Eur Respir J. 2020;56(1.
- Manyelo CM, Solomons RS, Walzl G, et al. Tuberculous Meningitis: Pathogenesis, Immune Responses, Diagnostic Challenges, and the Potential of Biomarker-Based Approaches. J Clin Microbiol. 2021;59(3).
- Bahr NC, Marais S, Caws M, et al. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clin Infect Dis. 2016;62(9):1133–1135.
- Vinnard C, King L, Munsiff S, et al. Long-term Mortality of Patients With Tuberculous Meningitis in New York City: A Cohort Study. Clin Infect Dis. 2017;64(4):w763.
- Chaya S, Dangor Z, Solomon F, et al. Incidence of tuberculosis meningitis in a high HIV prevalence setting: time-series analysis from 2006 to 2011. Int J Tuberculosis and Lung Dis. 2016;20(11):1457–1462.
- Davis AG, Rohlwink UK, Proust A, et al. The pathogenesis of tuberculous meningitis. J Leukocyte Biol. 2019;105(2):267–280.
- Thuong NT, Heemskerk D, Tram TT, et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis. J Infectious Dis. 2017;215(7):1020–1028.
- Arshad A, Dayal S, Gadhe R, et al. Analysis of Tuberculosis Meningitis Pathogenesis, Diagnosis, and Treatment. J Clin Med. 2020;9(9):2962.
- Buonsenso D, Serranti D, Valentini P. Management of central nervous system tuberculosis in children: light and shade. Eur Rev Med Pharmacol Sci. 2010;14(10):845–853.
- Huo Y, Zhan Y, Liu G, et al. Tuberculosis meningitis: Early diagnosis and treatment with clinical analysis of 180 patients. Radiol Infectious Dis. 2019;6(1):21–25.
- Kingkaew N, Sangtong B, Amnuaiphon W, et al. HIV-associated extrapulmonary tuberculosis in Thailand: epidemiology and risk factors for death. Int J Infect Dis. 2009;13(6):722–729.
- Shafer RW, Kim DS, Weiss JP, et al. Extrapulmonary tuberculosis in patients with human immunodeficiency virus infection. Medicine (Baltimore). 1991;70(6):384–397.
- Marx GE, Chan ED. Tuberculous meningitis: diagnosis and treatment overview. Tuberculosis Res Treat. 2011;2011:1–9.