ABSTRACT
Pangolins have gained increasing global attention owing to their public health significance as potential zoonotic hosts since the identification of SARS-CoV-2-related viruses in them. Moreover, these animals could carry other respiratory viruses. In this study, we investigated the virome composition of 16 pangolins that died in 2018 with symptoms of pneumonia using metagenomic approaches. A total of eight whole virus sequences belonging to the Paramyxoviridae or Pneumoviridae families were identified, including one human parainfluenza virus 3, one human respiratory syncytial virus A, and six human respiratory syncytial virus B. All of these sequences showed more than 99% nucleotide identity with the virus isolated from humans at the whole-genome level and clustered with human viruses in the phylogenetic tree. Our findings provide evidence that pangolins are susceptible to HPIV3 and HRSV infection. Therefore, public awareness of the threat of pangolin-borne pathogens is essential to stop their human consumption and to prevent zoonotic viral transmission.
Introduction
Human respiratory syncytial virus (HRSV) and human parainfluenza 3 virus (HPIV3) are the most common viral pathogens that cause respiratory infections, especially acute lower respiratory tract infections, in infants and children worldwide[Citation1]. Almost all children are infected with HRSV at least once by the age of 24 months[Citation2]. HRSV belongs to the Orthopneumovirus genus of the Pneumoviridae family and HPIV3 belongs to the genus Respirovirus of the Paramyxoviridae family. They are filamentous, enveloped, negative-sense, single-stranded RNA viruses of the order Mononegavirales, discovered in the 1950s, and are currently thought to be human-specific[Citation3,Citation4].
Closer human contact with wildlife has increased the risk of zoonotic disease transmission to humans. Pangolins are animals that have long been hunted, traded, and trafficked by humans for spiritual use, traditional medicine, and bushmeat consumption[Citation5]. They are valued in different cultures. Owing to ongoing illegal trade, pangolins are now at the risk of extinction. Pangolins are the animals other than bats found to be infected with SARS-CoV-2-related coronaviruses[Citation6–8], and have a high risk of transmitting zoonotic diseases. Therefore, the identification of potential zoonotic pathogens carried by pangolins is of great significance to human health. Viruses belonging to more than 30 families have been identified from pangolins including Sendai virus, parainfluenza virus 5, Dongyang pangolin virus, and Lishui pangolin virus.[Citation9–14].
In this study, we used the metagenomic approach to detect the 16 pangolins that had displayed similar clinical symptoms of pneumonia, and identified the human respiratory viruses in pangolins.
Methods
Sample collection
Sixteen Malayan pangolins were rescued by the Guangxi Zhuang Autonomous Region Terrestrial Wildlife Medical-aid and Monitoring Epidemic Diseases Research Center during their routine anti-smuggling operations between July and September 2018. The pangolins however, died between September and October. The animals were dissected and partial lung tissues were collected for sequencing. Lung tissue samples from three pangolins were collected for pathological examination. The collected tissue samples were fixed with 10% formalin solution for 24 h, embedded in paraffin, sectioned at 4–6 μm, and stained with hematoxylin–eosin followed by microscopic observation (Nikon ECLIPSE TS100).
Viral RNA extraction and reverse-transcription polymerase chain reaction
Viral RNA was extracted from 200 μL of lung homogenate supernatant and eluted in 50 μL nuclease-free water using a High Pure viral RNA Kit (Cat. No. 11858882001 Roche, Switzerland) according to the manufacturer’s instructions. Reverse-transcription polymerase chain reaction (RT–PCR) was performed in a 20 μL reaction system using a First-Strand Synthesis System Kit (Invitrogen, Thermo Fisher Scientific, USA) following the manufacturer’s instructions. The reactions were conducted in a Veriti 96-well thermal cycler (Applied Biosystems, USA) programmed as follows: 25 °C for 10 min, 50 °C for 50 min, and 85 °C for 5 min, followed by a final step at 4 °C for 5 min. Finally, 1 μL RNase H was added to the reaction tube and incubated for 20 min at 37 °C.
Library preparation and RNA sequencing
First-strand cDNA fragments were used to prepare a sequencing library using the NEBNext® Ultra II Directional RNA Library Prep Kit for Illumina® (NEB, Germany) according to the manufacturer’s instructions. The sequencing library construction mainly consisted of seven steps: first-strand cDNA synthesis, second-strand cDNA synthesis, DNA fragmentation, end-repair/dA-tailing, adaptor ligation, USER-enzyme digestion, and library enrichment using PCR. Purification was performed at every step using AMPure® XP beads (Beckman Coulter, USA). The constructed library was qualified using qRT-PCR (Applied Biosystems, Thermo Fisher Scientific, USA). Finally, the prepared libraries were sequenced using NovaSeq S2 reagent kits on a NovaSeq 6000 instrument (Illumina, USA).
Bioinformatic analysis
For sequencing reads, Fastp (v0.20.0)[Citation15] and BBNorm[Citation16] were used for quality control and repetition. The remaining reads were compared against viral genome databases using diamond BLASTX to identify virus-associated reads. To rule out false positives, viral reads were clustered using Cd-hit[Citation17] and compared against the non-redundant nucleotide (nt) database, and taxonomic lineage information was obtained from the top blast hit of each read. The complete genome was assembled using CLC 12.0, and the reference genome was selected based on BLAST results. A read mapping tool in CLC 12.0 was used to estimate the read number of pangolin viruses. Sequences were first aligned with related viral sequences within the clade using the mafft v7.037 b programme to determine the relatedness of each virus[Citation18]. Maximum likelihood trees were subsequently reconstructed based on sequence alignment using PhyML 3.0, employing the GTR substitution model[Citation19,Citation20].
Ethics statement
The animals (pangolins) were rescued and treated by the Guangxi Zhuang Autonomous Region Terrestrial Wildlife Medical-aid and Monitoring Epidemic Diseases Research Center under ethics approval (Wild Animal Treatment Regulation No. [2011] 85). The sample collection followed the guidelines listed in Pangolins Rescue Procedure, November 2016.
Results
Study animals
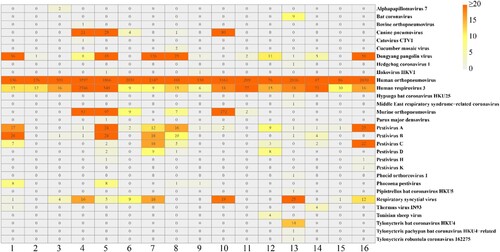
The rescued pangolins were divided into six groups labelled as A-F according to the time of rescue (). The different groups were placed in different rooms to prevent the spread of infectious diseases. All of them showed signs of pneumonia, including varying degrees of lethargy, loss of appetite, and breathing difficulties, and died between September and October 2018 (). Three pangolins from different groups were sampled for histological examination, and lung tissue samples from all 16 pangolins were collected for sequencing. Autopsy photographs of the three pangolins revealed pulmonary edema with hemorrhage (Figure S1-S3, Panels A and B). In addition, interstitial lung congestion and inflammatory cell infiltration were observed, and edema fluid, fibrin, and red blood cells were present in the alveolar cavity (Figure S1-S3, Panels C and D).
Table 1. General information of the rescued Malayan pangolins.
Metagenomic analysis
The proportion of bacterial data in the pangolin sequencing data is very small, and most of them seem to be normal bacteria in the environment. Bacterial infection doesn't seem to explain that the 16 pangolins showed similar signs of illness; furthermore, considering the limited ability of bacteria to spread, we speculated a possible viral spread occurring before or after the rescue through unexpected transmission route. Based on the sequencing results of lung tissue samples from all 16 pangolins, we characterized viruses in the samples at the species level, and all viruses, such as phage, endogenous viruses, and plant viruses, normally carried by mammals were excluded. HRSV and HPIV3 were detected in all the samples (). Dongyang pangolin virus, canine pneumovirus, murine orthopneumovirus, and pestivirus were present in several samples. In addition, a small number of reads associated with HKU4, HKU5, and MERS-CoV coronavirus belonging to Merbecovirus, were detected in sample MJ13.
Complete sequences of human orthopneumovirus and human respirovirus
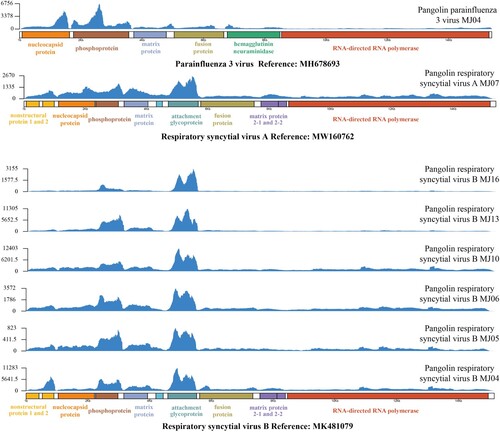
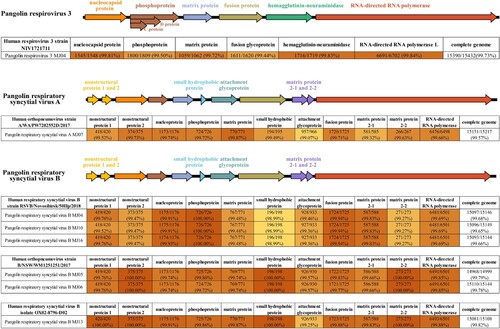
We constructed the complete genomes of several viruses in some samples with sufficient data and obtained one complete sequence of HPIV3 from sample MJ04, one complete sequence of HRSV subtype A (HRSVA) from sample MJ07, and six complete sequences of HRSV subtype B (HRSVB) from samples MJ04, MJ05, MJ06, MJ10, MJ13, and MJ16 (). Additionally, we also observed that genes associated with virus-host interactions and viral RNA replication had high coverage of sequencing reads in these viruses, including nucleocapsid protein and phosphoprotein in HPIV3, and attachment glycoprotein and phosphoprotein in HRSVA and HRSVB (). These complete sequences showed >99% nucleic acid identity to those of viruses isolated from humans ().
Phylogenetic analysis of the novel pangolin viruses
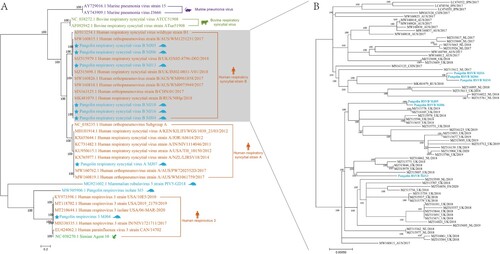
Phylogenetic analysis based on the complete genomes revealed that these viruses were most closely related to viruses found in humans (A). In addition to pangolin coronaviruses, which are associated with SARS-CoV-2[Citation6,Citation7], several viruses previously discovered in pangolins are closely related to animal viruses in the phylogenetic tree. For example, the pangolin respirovirus isolate M5 is most closely related to a murine respiratory virus[Citation21]; and mammalian rubulavirus 5 strain PIV5-GD18 shares more than 99% similarity with mammalian rubulavirus 5 of Panthera tigris, lesser panda, and pig[Citation11]. Additionally, Sendai viruses identified in pangolins are most closely related to mouse Sendai virus[Citation9]. In this study, we observed that the complete sequences of pangolin viruses clustered with those of human viruses in the phylogenetic tree (A). The complete genomes of HRSVB were obtained from several samples; therefore, we investigated whether these pangolin HRSVBs had a similar source of infection or intraspecific transmission. The results showed that six strains of HRSVB belonged to three separate clades in the phylogenetic tree, suggesting that several human-to-pangolin transmissions may have occurred. However, it is also possible that pangolins naturally carry HRSVB (B).
Discussion
In the present study, we sequenced lung tissue samples of 16 Malayan pangolins that died in 2018. These pangolins were infected with HPIV3, HRSVA, and HRSVB to varying degrees, with HRSVB being more prevalent. A total of one HPIV3, one HRSVA, and six HRSVB complete sequences were obtained from high-throughput sequencing data and all sequences had over 99% similarity to known human viruses. HRSV and HPIV3 have previously been identified only in humans[Citation22,Citation23]; herein, we present evidence that these viruses can also infect pangolins.
Cell surface receptors are key factors determining the host range of viruses[Citation24]. The similarity of viral receptors between pangolins and humans greatly increases the risk of cross-species transmission of potentially natural viruses from pangolins to humans. Pangolin angiotensin-converting enzyme 2 (ACE2) shares approximately 84% amino acid similarity with human ACE2, which has been identified as a receptor for SARSr-CoV-2. Additionally, the insulin-like growth factor-1 receptor (IGF1R) is involved in the binding of HRSV to human cells[Citation25]. Pangolin IGF1R shares more than 97% amino acid similarity with human IGF1R, which may account for HRSV infections in pangolins. In humans, α2,3/α2,6-linked sialic acid receptors are required for binding of HPIV3 to respiratory epithelial cells; therefore, HPIV3 may use structurally similar receptors to infect pangolins. The presence of these two receptors is also associated with the spread of influenza in human[Citation26–28]. In addition, we observed that the pathological changes in lung tissue were closely related to pneumonia. Further, increased expression of genes related to viral genome replication was detected indicating increased viral load, which may have increased disease severity. Unfortunately, we failed to obtain any samples for sero-antibodies testing. The conduct of animal infection experiments of healthy pangolins with HRSV and/or HPIV3 would provide more information; however, it is difficult to carry out such experiments as pangolins are protected. Although these pangolins were likely infected by these viruses through contact with humans, especially handlers and smugglers, the possibility that they naturally carried the virus cannot be ruled out.
In conclusion, our study demonstrates that pangolins are susceptible to HPIV3 and HRSV infection, suggesting that in addition to direct person-to-person contact and airborne routes, smuggling of pangolins (which may carrying pathogens) is also a potential route of disease transmission. Although pangolins do not carry as many dangerous pathogens as bats, they can still act as vectors for human virus transmission. Therefore, enhancing the protection of wildlife, stopping illegal capture and trading of wildlife, and distancing from the natural habitat of animals are effective ways to reduce the transmission of zoonotic diseases.
Declaration of competing interest
The authors declare that they have no competing interests.
Supplemental Material
Download MS Word (1.4 MB)Data availability statement
The sequences for this study are available in GenBank under the accession number OM141135-OM141143. The raw data of this study have been deposited in the NGDC (National Genomics Data Center) database with accessions CRA006942.
Acknowledgements
This work was financially supported by a project from Ministry of Science and Technology of China (No. 2020YFC0840805), a start-up funding for Dr. Yi-Gang Tong from Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Guangxi Scientific and Technological Research (GUIKEAB20059002) and Guangxi Medical University Training Program for Distinguished Young Scholars.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Histological examination of Pangolin MJ06 (Figure S1), MJ09 (Figure S2) and MJ10 (Figure S3).
Additional information
Funding
Reference
- Ding Q, Xu L, Zhu Y, et al. Comparison of clinical features of acute lower respiratory tract infections in infants with RSV/HRV infection, and incidences of subsequent wheezing or asthma in childhood. BMC Infect Dis. 2020/05/30;2020;20(1):387.
- Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. American Journal of Diseases of Children. 1986 Jun;1960;140(6):543–546.
- Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003 Apr;16(2):242–264.
- Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. isolation, properties and characterization. Am J Hyg. 1957 Nov;66(3):281–290.
- Boakye MK, Pietersen DW, Kotzé A, et al. Ethnomedicinal use of african pangolins by traditional medical practitioners in Sierra Leone. J Ethnobiol Ethnomed. 2014 Nov;20(10):76.
- Xiao K, Zhai J, Feng Y, et al. Isolation of SARS-CoV-2-related coronavirus from Malayan pangolins. Nature. 2020 Jul;583(7815):286–289.
- Lam TT, Jia N, Zhang YW, et al. Identifying SARS-CoV-2-related coronaviruses in Malayan pangolins. Nature. 2020 Jul;583(7815):282–285.
- Peng MS, Li JB, Cai ZF, et al. The high diversity of SARS-CoV-2-related coronaviruses in pangolins alerts potential ecological risks. Zool Res. 2021 Nov 18;42(6):834–844.
- Liu P, Chen W, Chen JP. Viral metagenomics revealed Sendai virus and coronavirus infection of Malayan pangolins (Manis javanica). Viruses. 2019 Oct 24;11(11):979.
- Gao WH, Lin XD, Chen YM, et al. Newly identified viral genomes in pangolins with fatal disease. Virus Evol. 2020 Jan;6(1):veaa020.
- Wang X, Chen W, Xiang R, et al. Complete genome sequence of parainfluenza virus 5 (PIV5) from a sunda pangolin (Manis javanica) in China. J Wildl Dis. 2019 Oct;55(4):947–950.
- Wang SL, Tu YC, Lee MS, et al. Fatal canine parvovirus-2 (CPV-2) infection in a rescued free-ranging Taiwanese pangolin (Manis pentadactyla pentadactyla). Transbound Emerg Dis. 2020 May;67(3):1074–1081.
- Khatri-Chhetri R, Wang HC, Chen CC, et al. Surveillance of ticks and associated pathogens in free-ranging formosan pangolins (Manis pentadactyla pentadactyla). Ticks Tick Borne Dis. 2016 Oct;7(6):1238–1244.
- Koh FX, Kho KL, Panchadcharam C, et al. Molecular detection of anaplasma spp. in pangolins (Manis javanica) and wild boars (Sus scrofa) in peninsular Malaysia. Vet Parasitol. 2016 Aug 30;227:73–76.
- Chen S, Zhou Y, Chen Y, et al. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics (Oxford, England). 2018 Sep 1;34(17):i884–i890.
- Bushnell B. BBMap [cited 2022 March 4]. Available from: sourceforge.net/projects/bbmap.
- Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics (Oxford, England). 2006 Jul 1;22(13):1658–1659.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013 Apr;30(4):772–780.
- Guindon S, Dufayard JF, Lefort V, et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010 May;59(3):307–321.
- Lefort V, Longueville JE, Gascuel O. SMS: smart model selection in PhyML. Mol Biol Evol. 2017 Sep 1;34(9):2422–2424.
- Yang R, Peng J, Zhai J, et al. Pathogenicity and transmissibility of a novel respirovirus isolated from a Malayan pangolin. J Gen Virol. 2021 Apr;102(4):001586.
- Negrey JD, Reddy RB, Scully EJ, et al. Simultaneous outbreaks of respiratory disease in wild chimpanzees caused by distinct viruses of human origin. Emerg Microbes Infect. 2019;8(1):139–149.
- Mazet JAK, Genovese BN, Harris LA, et al. Human respiratory syncytial virus detected in mountain gorilla respiratory outbreaks. EcoHealth. 2020 Dec;17(4):449–460.
- Paulson JC. Interactions of animal viruses with cell surface receptors. The Receptors: Elsevier. 1985;2:131–219.
- Griffiths CD, Bilawchuk LM, McDonough JE, et al. IGF1R is an entry receptor for respiratory syncytial virus. Nature. 2020 Jul;583(7817):615–619.
- Moscona A, Peluso RW. Analysis of human parainfluenza virus 3 receptor binding variants: evidence for the use of a specific sialic acid-containing receptor. Microb Pathog. 1996 Mar;20(3):179–184.
- Suzuki T, Portner A, Scroggs RA, et al. Receptor specificities of human respiroviruses. J Virol. 2001 May;75(10):4604–4613.
- Byrd-Leotis L, Jia N, Dutta S, et al. Influenza binds phosphorylated glycans from human lung. Sci Adv. 2019 Feb;5(2):eaav2554.