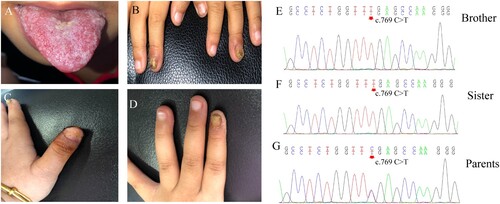
A 2-year-old boy was admitted to the hospital because of recurrent thrush since the age of 6 months, along with yellowish discoloration and thickening of his nails. Physical examination revealed oral mucosal erosion and white patches on the tongue with a pseudomembrane ((A)). Two of his fingernails were thick and discolored with erythema around ((B)). He also experienced progressively impaired vision, which was diagnosed as retinitis pigmentosa, causing no light perception in one eye and light perception up to 0.1 in the other. His 4-year-old sister had similar dermatological manifestations with 3 years of recurrent thrush and fingernail involvement ((C, D)). Direct microscopic examination of their nails and the brother’s tongue showed branched pseudohyphae and yeast cells, and Candida albicans was identified through fungal culture. The rest of the physical examinations did not reveal any other obvious abnormalities. Their parents belonged to the Hui population (a minority population in China) and had a history of consanguineous marriage. Their father had a history of onychomycosis with unknown specific pathogens. Chronic mucocutaneous candidiasis (CMC) was diagnosed, and oral fluconazole was prescribed at a daily dose of 150 mg for the brother and 200 mg for the sister. After continuous fluconazole treatment for 6 months, the nails and the tongue became normal. These patients are still under follow-up.
Due to the recurrent Candida infections and history of consanguineous marriage, genetic susceptibility was suspected. Genomic DNA of all family members was extracted from peripheral blood leukocytes, and whole-exome sequencing (WES) was performed. When we compared the data with all genes reported to be related to CMC, a homozygous mutation in the AIRE gene was noted (c. 769 C > T, p. Arg257Ter) in both patients ( (E, F)). The parents were heterozygous carriers of the variant ( (G)).
CMC is a unique type of candidiasis that manifests as severe and recurrent or persistent infections of the skin, nails, and mucosa by Candida species. In the past decade, various gene mutations, including STAT1, STAT3, AIRE, CLEC7A, CARD9, RORC, ACT1, IL-17RA, IL-17RC, and IL-17F, have been reported to predispose individuals to CMC [Citation1]. STAT1 mutations among these mutations are considered the most common cause of isolated CMC worldwide, whereas mutations in the AIRE gene usually cause a syndrome named autoimmune polyendocrinopathy candidiasis and ectodermal dystrophy (APECED) mostly in northern European Caucasians [Citation2–4]. Clinical manifestations of APECED vary greatly, usually starting in infancy with CMC, followed by autoimmune attacks on the parathyroid glands, adrenal cortex, thyroid, and other organs, including the retina [Citation5]. The deficiency of AIRE impairs central immune tolerance, generating pathogenic autoreactive T cells and autoantibodies directed against many specific cytokines, such as IL-17 and IL-22 [Citation6]. Pigmented retinitis is considered as one of the APECED manifestations, affecting 3.6% of APECED patients [Citation7, Citation8]. It destroys the visual field in the early stages and affects vision, leading to blindness in the later stages [Citation9, Citation10]. Patients visiting our clinic with AIRE mutations presented only CMC and pigmented retinitis, whereas other manifestations of APECED might still appear with age and should be closely monitored and followed up. These patients remind us of the importance of genetic analysis, which helps to adjust the time of treatment, as well as predict and detect related complications early in CMC management.
Figure 1. Clinical features and AIRE mutations in two Chinese patients with chronic mucocutaneous candidiasis. A-B. Thrush and fingernail changes of the brother. C-D. Fingernail involvement of the sister causes shedding of the nail plate and oozing scabs of the nail bed. E-F. DNA sequencing chromatograms demonstrate homozygous AIRE mutation in exon6: c769 C > T, p. Arg257Ter in the brother and sister. G. The heterozygous carriers of the variant in exon6: c769 C > T of their parents.
Ethical approvals
This study was approved by the Clinical Research Ethics Committee of the Peking University First Hospital. We obtained blood samples from the patients and their parents, after obtaining informed consent. The patients gave permission to publish their images and medical information.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Puel A. Human inborn errors of immunity underlying superficial or invasive candidiasis. Hum Genet. 2020;139:1011–1022.
- Ahonen P, Myllarniemi S, Sipila I, et al. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829–1836.
- Yan Z, Gang X, Xie X, et al. A case report and literature review. Medicine (Baltimore). 2020;99:e20000.
- Fierabracci A, Bizzarri C, Palma A, et al. A novel heterozygous mutation of the AIRE gene in a patient with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome (APECED). Gene. 2012;511:113–117.
- Kisand K, Link M, Wolff AS, et al. Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes. Blood. 2008;112:2657–2666.
- Break TJ, Oikonomou V, Dutzan N, et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science. 2021;371:252–25+.
- Orlova EM, Sozaeva LS, Kareva MA, et al. Expanding the phenotypic and genotypic landscape of autoimmune polyendocrine syndrome type 1. J Clin Endocrinol Metab. 2017;102:3546–3556.
- Culp CJ, Pappas CM, Toso M, et al. Clinical, histological and genetic findings in a donor with a clinical history of type 1 autoimmune polyendocrinopathy syndrome. Am J Ophthalmol Case Rep. 2022;25:101266.
- Busskamp V, Picaud S, Sahel JA, et al. Optogenetic therapy for retinitis pigmentosa. Gene Ther 2012;19:169–175.
- Morizane Y, Morimoto N, Fujiwara A, et al. Incidence and causes of visual impairment in Japan: the first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn J Ophthalmol. 2019;63:26–33.
