ABSTRACT
To further describe the effect of the “fragile population” and their “higher-risk” comorbidities on prognosis among hospitalized Omicron patients, this observational cohort study enrolled hospitalized patients confirmed with SARS-CoV-2 during the 2022 Omicron wave in Shanghai, China. The primary outcome was progression to severe or critical cases. The secondary outcome was viral shedding time from the first positive SARS-CoV-2 detection. A total of 847 participants were enrolled, most of whom featured as advanced age (>70 years old: 30.34%), not fully vaccinated (55.84%), combined with at least 1 comorbidity (65.41%). Multivariate cox regression suggested age >70 years old (aHR[95%CI] 0.78[0.61–0.99]), chronic kidney disease (CKD) stage 4–5 (aHR[95%CI] 0.61[0.46–0.80]), heart conditions (aHR[95%CI] 0.76[0.60–0.97]) would elongate viral shedding time and fully/booster vaccination (aHR[95%CI] 1.4 [1.14–1.72]) would shorten this duration. Multivariate logistic regression suggested CKD stage 4–5 (aHR[95%CI] 3.21[1.45–7.27]), cancer (aHR[95%CI] 9.52[4.19–22.61]), and long-term bedridden status (aHR[95%CI] 4.94[2.36–10.44]) were the “higher” risk factor compared with the elderly, heart conditions, metabolic disorders, isolated hypertension, etc. for severity while female (aHR[95%CI] 0.34[0.16–0.68]) and fully/booster Vaccination (aHR[95%CI] 0.35[0.12–0.87]) could provide protection from illness progression. CKD stage 4–5, cancer and long-term bedridden history were “higher-risk” factors among hospitalized Omicron patients for severity progression while full vaccination could provide protection from illness progression.
Introduction
Over the past two years, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had caused about 574 million confirmed cases and 6.3 million deaths worldwide until 3rd August 2022 [Citation1]. Since first detected in South Africa in November 2021 [Citation2], Omicron, the novel coronavirus variant, had rapidly spread around and become the predominant variant around the world.
Compared with Delta variant, early reports suggested that Omicron is 25–50% more transmissible along with substantially reduced overall hospitalization and severity [Citation3, Citation4], which presents milder clinical manifestation as well as faster symptom resolution [Citation5–7]. A cross-sectional study in Huston, Texas reported that 56.4% of patients infected with Omicron required at least low flow oxygen while this ratio is 81.4% with alpha variants and 80.7% with delta variants [Citation8]. However, the actual threat of omicron was still unignorable considering a huge base of susceptible population, especially those with high risk for severity progression [Citation9–11].
Several previous studies have reported some high-risk factors for COVID-19-related poor outcomes. Underlying diseases including diabetes, chronic liver disease, and chronic kidney disease are the leading risk factors for a severe COVID-19 clinical course [Citation12–14]. A large cross-sectional study suggested that obesity, anxiety and fear-related disorders, and diabetes with complications as well as the total number of conditions are high-risk factors for death [Citation15]. Age is another driving factor for poor outcomes in hospitalized COVID-19 patients [Citation16–18]. Vaccination might provide protection from disease progression and a study in California suggested that receiving an mRNA vaccine booster dose gave over 70% protection against hospitalization or mortality outcomes in Omicron case [Citation19–21].
Despite multiple risk factors reported, most of these evidences were analysed before omicron swept the world. Considering the significantly increased transmissibility and specific characteristic of Omicron, the high-risk condition spectrum might be different from the previous. Besides, considering the huge medical pressure brought by the enormous susceptible population with previously identified risk factors including cardiovascular disease, diabetes, isolated hypertension, etc., it is necessary to redefine the “fragile population” to enable better integration of medical resources during the Omicron pandemic. Therefore, specific higher-risk comorbidities that contribute to increased incidence of severity progression among patients with high-risk comorbidities needed to be further identified. To further describe the effect of the “fragile population” and their “higher-risk” comorbidities on Omicron infection prognosis, we conducted this multicenter study during the 2022 Omicron wave in Shanghai, China.
Methods
Study cohort
This was an observational cohort study to evaluate the higher-risk factors for severity progression and delayed viral clearance among hospitalized patients infected with COVID-19. All patients were diagnosed and treated according to the latest national COVID-19 guideline [Citation22] and this study had no intervention on the treatment course.
Eligible patients were required to be at least 18 years old with confirmed SARS-CoV-2 infection by real-time PCR between 20th March and 10th May 2022 at Fudan University Affiliated Huashan Hospital, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai Jiao Tong University Affiliated Shanghai Ninth People’s hospital and Tongji University Affiliated Shanghai Forth People’s hospital in Shanghai, China. Patients who were unnecessary for hospitalization according to the latest national COVID-19 guideline [Citation22] and the WHO guideline were excluded.
Informed consent was obtained from all participants and guardians, and the study protocol was approved by institutional review boards (2022-KY-069).
Data collection and definition
Data on all enrolled participants were obtained from the hospital information system. We collected baseline characteristics, vaccination, nursing records, laboratory findings, lung CT scan and treatment from electronic medical records. Two researchers double-checked the collected data and sorted it.
Chronic kidney disease stage 4–5 was defined on glomerular filtration rate <30 ml/(min*1.73 m2) or who required dialysis. Heart conditions were defined as patients who had coronary atherosclerotic heart disease, cardiomyopathy, arrhythmia, etc. except isolated hypertension. Cerebral vascular disease included conditions with stenosis, infarction, embolism, hemorrhage, aneurysms, or vascular malformations of the brain blood vessels. Metabolic diseases included diabetes, hyperlipidemia, or hyperuricemia. Long-term bedridden was defined as patients who were bedridden for at least 24 h after admission and required additional nutritional support during hospitalization. Omicron-infected patients without known comorbidities or bedridden history were selected as the control group.
Primary outcome
The primary outcome of the study was severity progression, which was defined as participants progressing to severe or critical COVID-19 during follow-up as represented by WHO guidelines. Severe covid-19 was defined by at least one of the following items: (1) Oxygen saturation <93% on room air. (2) Sign of pneumonia. (3) Signs of severe respiratory distress. Critical COVID-19 was defined by the criteria for acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation (invasive or non-invasive) or vasopressor therapy.
Secondary outcome
The secondary outcome was COVID-19 nucleic acid viral shedding. Viral shedding time was defined as the time interval between the first positive nucleic acid test and the first of two consecutive negative results of both the ORF1ab and N gene. SARS-CoV-2 viral load was detected and quantified by RT–PCR using nasopharyngeal swabs every day after admission. Ct value >35 for both ORF1ab and N gene was considered as negativity.
Statistical analysis
Continuous variables were expressed as median (IQR) and compared with the t-test or Wilcox test; categorical variables were expressed as number (%) and compared by the χ² test or Fisher’s exact test. Cox regression was used to calculate the hazard ratio (HR) and 95% confidence interval (95%CI) to analyse the factors affecting viral shedding time. Kaplan Meier Curves were drawn to describe viral shedding time. Univariate and Multivariate Logistics Regression were used to estimate the factors Associated with Severity Progression. Hazard ratio/odd ratio indicated how often a particular event happens in one group compared to how often it happens in another group over time (hazard ratio) or not over time (odds ratio). Statistical analyses and calculations were performed using the SPSS version 20 and R 3.4.0 (R Foundation for Statistical Computing) software environment. All tests were two-tailed, and P-values <0.05 were considered statistically significant.
Results
General characteristics
A total of 1090 participants admitted to hospital between 20 March 2022 and 10 May 2022 were screened. 56 people were excluded due to insufficient diagnosis, 187 people were excluded because of their age (less than 18 years old), and 847 were finally enrolled (). The general characteristics of the study population were advanced age (>70 years old: 257 [30.34%]), not fully vaccinated (339 [55.84%]), combined with at least 1 comorbidity (564 [65.41%]). The 5 most common comorbidities were heart conditions (257 [30.34%]), metabolic disease (157 [18.54%]), chronic kidney disease stage 4–5 (156 [18.42%]), isolated hypertension (145 [17.12%]) and cancer (106 [12.51%]). 159 [18.77%] had a history of long-term bedridden. The overall rate for severe and critical cases was 10.98% and the median viral shedding time since diagnosis was 13 (IQR: 10–16) days.
Table 1. Baseline characteristics of participants infected with Omicron.
Risk analysis for viral shedding time
Multivariate cox regression on viral shedding time was performed among data with significant difference during univariate regression (). Viral shedding time among patients who had advanced age (aHR [95%CI] 0.78 [0.61–0.99], P = 0.037), combined with chronic kidney disease stage 4–5 (aHR [95%CI] 0.61 [0.46–0.80], P < 0.001) or heart conditions (aHR [95%CI] 0.76 [0.60–0.97], P = 0.030) were enlengthened, while full or booster vaccination could shorten this duration (aHR [95%CI] 1.40 [1.14–1.72], P = 0.001) (, ).
Figure 1. Kaplan–Meier Curve for COVID-19 Nucleic Acid Viral Shedding Time. A. Viral shedding time among participants more or less than 70 years old; B. Viral shedding time between participants with and without chronic kidney disease stage 4–5; C. Viral shedding time between participants with and without heart conditions; D. Viral shedding time between participants not fully vaccinated and fully vaccinated or booster.
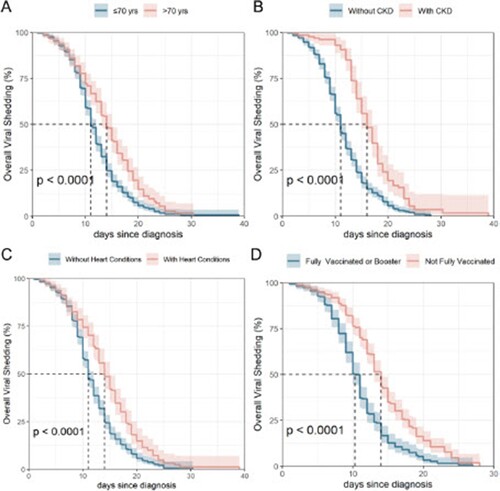
Table 2. Multivariate Cox regression of factors associated with COVID-19 nucleic acid viral shedding time.
Identification of higher-risk factors for COVID-19 severity progression
Logistics regression on whether participants progressed to severe and critical cases was performed among data with significant difference during univariate regression (). Comorbidities of CKD stage 4–5 (aHR [95%CI] 3.21 [1.45–7.27], P = 0.004), cancer (aHR [95%CI] 9.52 [4.19–22.61], P < 0.001), and long-term bedridden (aHR [95%CI] 4.94 [2.36 −10.44], P < 0.001) were higher-risk factors for severity progression, while female (aHR [95%CI] 0.34 [0.16–0.68], P = 0.003) and fully/booster vaccination (aHR [95%CI] 0.35 [0.12–0.87], P = 0.034) were protective factors.
Table 3. Multivariate logistics regression of factors associated with severity progression.
We then compared participants combined with CKD stage 4–5 (B group), cancer (C group), or long-term bedridden (D group) with participants with no comorbidities (A group) (). Compared with the control group, patients with high-risk comorbidities were older and not fully vaccinated (P < 0.0001 in B, C, and D groups). The most common comorbidities in the long-term bedridden group were heart conditions (93 [57.76%]), cerebral vascular disease (62 [38.51%]), chronic kidney disease stage 4–5 (50 [31.06%]), and metabolic disease (50 [31.06%]).
Table 4. Characteristics of participants with high-risk comorbidities for severity progression.
The proportion of severe and critical cases were 24.36%, 30.19%, and 32.3%, respectively, in the CKD, cancer and long-term bedridden group while no participants in the control group progressed to severe or critical cases (P < 0.001). The viral shedding time was 17 (15.5–21), 14 (12.5–17), and 16.5 (11.25–20) days in the CKD, cancer, and long-term bedridden group, which was significantly enlengthened compared to the control group (11 [8.5–13.5] days).
Full vaccination or booster shot provided protection for patients with long-term bedridden history (OR [95%CI] 0.18 [0.03–0.67], P = 0.027). A similar phenomenon was not observed in patients with cancer (OR [95%CI] 0.55 [0.14–1.75], P = 0.34) or chronic kidney disease stage 4–5 (OR [95%CI] 2.33 [0.29–14.87]), which could be possibly due to the limited percentage of vaccinations among these groups.
Dynamic laboratory examination surveillance
Further analysis on the laboratory examination result during hospitalization was performed among the participants with higher-risk comorbidities and the control group (). There was consistent low level of lymphocyte and high level of inflammation parameters (CRP, PCT, and IL-6) in participants with high-risk comorbidities. A significant elevation of hsTnI was observed in patients with chronic kidney disease during the hospitalization.
Discussion
We tried to identify “higher-risk” factors for severity progression and virus clearance among hospitalized patients to further define the specific “fragile population” during the Omicron pandemic. Patients with advanced age or CKD stage 4–5 had a longer viral shedding time, while vaccination of over 2 doses could shorten this duration. CKD stage 4–5, cancer, and long-time bedridden history were the “higher-risk” factors for severity progression compared with the elderly, heart conditions, metabolic disorders, or isolated hypertension, etc. while vaccination of over 2 doses provided protection. Persistent high level of inflammation factor, low level of lymphocyte as well as potential cardiac injury was observed among these fragile population.
Several studies had reported the high-risk factors for illness progression among the overall population infected with SARS-CoV-2 [Citation7,Citation18,Citation23], which were listed as indication for hospitalization by WHO living guidelines [Citation22]. However, the medical resources in most countries still could not cover all patients evenly when the virus kept mutating and spreading around. The medical resource should be arranged scientifically and identification of “higher-risk” factors in hospitalized patients might provide evidence for more reasonable health monitoring, treatment, as well as prevention.
Patients with end stage renal disease were featured as low vaccinated rate, advanced age, and high severity rate. Research has demonstrated that CKD was a risk factor in severe COVID-19 and COVID-19-related mortality [Citation24,Citation25]. The study by Williamson et al. claimed that patients with severe CKD have a very high risk of COVID19 mortality, which is even higher than that of other known high-risk groups, including patients with hypertension, chronic heart disease, or lung disease [Citation26]. The probable reason could be the dysfunctional immune system with a heightened inflammatory state and impaired innate immunity in CKD patients [Citation27,Citation28], resulting in more severe inflammation in lungs and more damage to the tissue. Based on our data, omicron-infected CKD patients were prone to cardiac damage, consistent with report from NHC that about 10 percent of patients who died from COVID-19 suffered from heart disease in the absence of CKD [Citation29]. However, the mechanisms between Omicron and acute myocardial damage are not entirely clear, probably associated with downregulation of ACE2, cytokine storm, and hypoxaemia caused by COVID-19.
Long-term bedridden is another significant high-risk factor for COVID-19 progression. Despite the fact that patients who required bedridden were combined with a wide spectrum of comorbidities and few studies have analysed long-term bedridden separately, they were an unignorable huge population both in Shanghai and worldwide. Previous studies suggested that bedridden patients were prone to be infected by COVID-19 and in this study, this population were prone to progress to severe illness [Citation30]. It could be explained by the fact that patients were more likely to have malnutrition, weight loss, and immunodeficiency, which was correlated with severe inflammation, impaired renal function status, and longer duration of COVID-19 disease [Citation31,Citation32].
In meta-analysis research, Tian et al. have concluded that the patients with cancer or cancer survivors are at an elevated risk of suffering from severe or critical COVID-19, which are 2- or 3-fold likelihood of being severely ill and death 7084 patients with COVID-19 [Citation33]. Another research conducted in Wuhan also reported a significant association between cancer and severe cases, as patients with cancer were more likely to have severe COVID-19 than patients without cancer (OR = 3.61 [95%CI 2.59–5.04]; p < 0.0001) [Citation34]. Patients with cancer may have dysregulated immune systems, as it can be either suppressed or hyperactivated due to the disease itself or treatment therapies [Citation35,Citation36].
Several previous studies list advanced age as an unignorable high-risk factor [Citation23]. Adriana et.al summarized that the immune alterations among the elderly might be the reason for susceptibility to illness progression [Citation37], which could be possible due to comorbidities or the natural aging. Our study suggested that the elderly was not as high risk as those three identified comorbidities, which could be possibly explained that comorbidities in the elderly contribute more to the susceptibility to infection.
Our studies suggested that vaccination over 2 doses could provide protection against illness progression among all hospitalized patients, which was consistent with several previous studies [Citation38,Citation39]. However, vaccination efficacy among specific populations required more data to draw the conclusion. Despite several studies claimed satisfying safety, tolerability, and immunogenicity among patients with high-risk comorbidities [Citation40], there still lacked real-world evidence. In this study, vaccination showed protection in patients with long-term bedridden history, while not in patients with chronic kidney disease or cancer, which could be possibly due to the low vaccination rate or the severity of these comorbidities.
There are several limitations in this study. First, we only included hospitalized patients, who cannot represent characteristics of all patients infected with Omicron. Second, the vaccination rate was low in some specific comorbidities including chronic kidney disease, which lead to inaccuracy when discussing the protection of vaccination among these patients. Further studies on vaccination efficacy among these fragile population were needed in the future. Also, some other underlying diseases including hematological malignancy, HIV infection, organ transplantation, etc., which were previously reported to cause significantly higher risk of severe COVID-19 cases, were not studied in this manuscript due to the limited sample size.
Acknowledgements
We thank all the patients who volunteered for this trial and the study site personnel for their contributions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Weekly epidemiological update on COVID-19 – 3 August 2022. [cited 2022 Aug 9]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—3-august-2022
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. [cited 2022 June 12]. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- Butt AA, Dargham SR, Loka S, et al. COVID-19 disease severity in children infected with the Omicron variant. Clin Infect Dis. 2022;75(1):e361–e367.
- Fan Y, Li X, Zhang L, et al. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:141.
- Menni C, Valdes AM, Polidori L, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID study. Lancet. 2022;399:1618–1624.
- Tiecco G, Storti S, Degli Antoni M, et al. Omicron genetic and clinical peculiarities that may overturn SARS-CoV-2 pandemic: a literature review. Int J Mol Sci. 2022;23:1987.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062.
- Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the Omicron variant of severe acute respiratory syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192:642–652.
- Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle Region – case series. N Engl J Med. 2020;382:2012–2022.
- Cai Q, Chen F, Wang T, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398.
- Stefan N, Birkenfeld AL, Schulze MB, et al. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342.
- Solera JT, Árbol BG, Alshahrani A, et al. Impact of vaccination and early monoclonal antibody therapy on COVID-19 outcomes in organ transplant recipients during the Omicron wave. Clin Infect Dis. 2022:ciac324.
- Yonekawa A, Shimono N. Clinical significance of COVID-19 and diabetes: in the pandemic situation of SARS-CoV-2 variants including Omicron (B.1.1.529). Biology (Basel). 2022;11:400.
- Cornberg M, Buti M, Eberhardt CS, et al. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944–951.
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev Chronic Dis. 2021;18:E66.
- Kahn F, Bonander C, Moghaddassi M, et al. Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities – surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill. 2022;27; doi:10.2807/1560-7917.ES.2022.27.9.2200121.
- Ahmad FB, Cisewski JA, Miniño A, et al. Provisional mortality data – United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:519–522.
- Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19-United States, March–September 2020. Open Forum Infect Dis. 2021;8:ofaa638.
- Report 50 – Hospitalisation risk for Omicron cases in England. Imperial College London. [cited 2022 Jun 12]. https://www.imperial.ac.uk/medicine/departments/school-public-health/infectious-disease-epidemiology/mrc-global-infectious-disease-analysis/covid-19/report-50-severity-omicron/.
- Ma A, Parry J. When Hong Kong’s ‘dynamic zero’ covid-19 strategy met omicron, low vaccination rates sent deaths soaring. BMJ. 2022;377:o980.
- Modes ME, Directo MP, Melgar M, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance – One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:217–223.
- Agarwal A, Rochwerg B, Lamontagne F, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379.
- Bakouny Z, Hawley JE, Choueiri TK, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646.
- Chung EYM, Palmer SC, Natale P, et al. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78:804–815.
- ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94.
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436.
- Callender LA, Curran M, Bates SM, et al. The impact of pre-existing comorbidities and therapeutic interventions on COVID-19. Front Immunol. 2020;11:1991.
- D’Marco L, Puchades MJ., Romero-Parra M et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J. 2020;13:297–306.
- Azevedo RB, Botelho BG, Hollanda Jde, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35:4–11.
- Iio R, Kaneko T, Mizuno H, et al. Clinical characteristics of COVID-19 infection in a dialysis center during a nosocomial outbreak. Clin Exp Nephrol. 2021;25:652–659.
- Anker MS, Landmesser U, von Haehling S, et al. Weight loss, malnutrition, and cachexia in COVID-19: facts and numbers. J Cachexia Sarcopenia Muscle. 2021;12:9–13.
- Goldman JD, Robinson PC, Uldrick TS, et al. COVID-19 in immunocompromised populations: implications for prognosis and repurposing of immunotherapies. J Immunother Cancer. 2021;9:e002630.
- Tian Y, Qiu X, Wang C, et al. Cancer associates with risk and severe events of COVID-19: A systematic review and meta-analysis. Int J Cancer. 2021;148:363–374.
- Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903.
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168.
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–668.
- Pedreañez A, Mosquera-Sulbaran J, Muñoz N. SARS-CoV-2 infection represents a high risk for the elderly: analysis of pathogenesis. Arch Virol. 2021;166:1565–1574.
- Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088.
- Al Kaabi N, Oulhaj A, Al Hosani FI, et al. The incidence of COVID-19 infection following emergency use authorization of BBIBP-CORV inactivated vaccine in frontline workers in the United Arab Emirates. Sci Rep. 2022;12:490.
- Sanders J-SF, Bemelman FJ, Messchendorp AL, et al. The RECOVAC immune-response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplantation. 2022;106:821–834.

